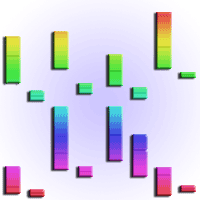
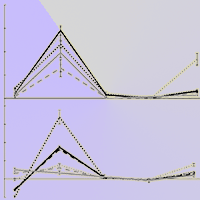
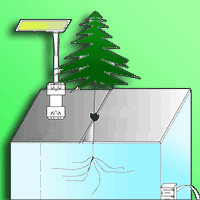
Prolonged droughts are predicted for some parts of the Amazon; however, it is still unclear how Amazonian trees will respond to water stress under the ongoing increase in CO2 concentration. The aim of this study was to assess the effect of elevated CO2 (eCO2) and drought on photosynthetic rates, water-use efficiency, and biomass allocation in andiroba (Carapa surinamensis). The plants were grown in pots at ambient (400 ppm CO2) and eCO2 (700 ppm) at two water regimes, soil at 50% field capacity, FC (drought) and soil at 100% FC for 163 days. We measured light saturated photosynthesis on a mass basis (Asat-mass), stomatal conductance to CO2 on a mass basis (gsCO2-mass), whole-plant water-use efficiency (WUEP), biomass accumulation, specific leaf area (SLA) and total leaf area. At eCO2, Asat-mass increased 28% in well-watered plants and 93% under drought, whereas gsCO2-mass declined 39% in well-watered plants at eCO2, with no effect of drought on gsCO2-mass at eCO2. The total biomass gain improved 73% at eCO2 and over CO2 levels it was reduced (54%) by drought. WUEP improved (188%) at eCO2 in well-watered plants and 262% under drought. SLA declined 23% at eCO2, but the effect of drought on SLA was null. On the contrary, total leaf area was greatly reduced (67%) by drought, but it was not affected by eCO2. The large increase in total biomass and the substantial improvement in WUEP under eCO2, and the sharp decline in leaf area under water stress widen our knowledge on the physiology of this important species for the forest management of large areas in the Amazon region.
Keywords
, , , ,
Citation
Oliveira MF, Marenco RA (2019). Gas exchange, biomass allocation and water-use efficiency in response to elevated CO2 and drought in andiroba (Carapa surinamensis, Meliaceae). iForest 12: 61-68. - doi: 10.3832/ifor2813-011
Academic Editor
Rossella Guerrieri
Paper history
Received: Apr 14, 2018
Accepted: Nov 14, 2018
First online: Jan 24, 2019
Publication Date: Feb 28, 2019
Publication Time: 2.37 months
© SISEF - The Italian Society of Silviculture and Forest Ecology 2019
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.

Breakdown by View Type
(Waiting for server response...)
Article Usage
Total Article Views: 48860
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 39763
Abstract Page Views: 5059
PDF Downloads: 3182
Citation/Reference Downloads: 6
XML Downloads: 850
Web Metrics
Days since publication: 2563
Overall contacts: 48860
Avg. contacts per week: 133.45
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2019): 8
Average cites per year: 1.14
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
(1)
Ainsworth EA, Long SP (2005)What have we learned from 15 years of free-air CO
2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO
2. New Phytologist 165: 351-372.
CrossRef |
Gscholar
(2)
Ainsworth EA, Rogers A (2007)The response of photosynthesis and stomatal conductance to rising [CO
2]: mechanisms and environmental interactions. Plant, Cell and Environment 30: 258-270.
CrossRef |
Gscholar
(3)
Bahar NH, Hayes L, Scafaro AP, Atkin OK, Evans JR (2018)Mesophyll conductance does not contribute to greater photosynthetic rate per unit nitrogen in temperate compared with tropical evergreen wet-forest tree leaves. New Phytologist 218: 492-505.
CrossRef |
Gscholar
(4)
Bellasio C, Quirk J, Beerling DJ (2018)Stomatal and non-stomatal limitations in savanna trees and C4 grasses grown at low, ambient and high atmospheric CO
2. Plant Science 274: 181-192.
CrossRef |
Gscholar
(5)
Bradford KJ, Hsiao TC (1982)Physiological responses to moderate water stress. In: “Physiological Plant Ecology II - Water Relation and Carbon Assimilation” (Lange OL, Nobel PS, Osmond CB, Ziegler H eds). Springer-Verlag, Heidelberg, Germany, pp. 263-324.
CrossRef |
Gscholar
(6)
Brazil-MMA (2016)Nota técnica 01/2016/DEMC/ SMCQ/MMA [Technical note 01/2016/DEMC/ SMCQ-MMA]. Ministério do Meio Ambiente MMA, Brasília, Brazil, pp. 1-6.
Online |
Gscholar
(7)
Brienza S, Pereira JF, Yared JAG, Mourão M, Gonçalves DA, Galeão RR (2008)Recuperação de áreas degradadas com base em sistema de produção florestal energético-madeireiro: indicadores de custos, produtividade e renda [Land reclamation based on production forestry system: cost indicators, productivity and incomes]. Amazônia Ciência e Desenvolvimento 4: 197-219. [in Portuguese]
Gscholar
(8)
Camargo MAB, Marenco RA (2012)Growth, leaf and stomatal traits of crabwood (
Carapa guianensis Aubl.) in central Amazonia. Revista Árvore 36: 07-16.
CrossRef |
Gscholar
(9)
Camargo MAB, Marenco RA (2017)Tree growth over three years in response to monthly rainfall in central Amazonia. Dendrobiology 78: 10-17.
CrossRef |
Gscholar
(10)
Cernusak LA, Winter K, Martínez C, Correa E, Aranda J, Garcia M, Jaramillo C, Turner BL (2011)Responses of legume
versus non legume tropical tree seedlings to elevated CO
2 concentration. Plant Physiology 157: 372-385.
CrossRef |
Gscholar
(11)
Córdoba J, Pérez P, Morcuende R, Molina-Cano JL, Martinez-Carrasco R (2017)Acclimation to elevated CO
2 is improved by low Rubisco and carbohydrate content, and enhanced Rubisco transcripts in the G132 barley mutant. Environmental and Experimental Botany 137: 36-48.
CrossRef |
Gscholar
(12)
Cornic G, Ghashghaie GCJ, Genty B, Briantais JM (1992)Leaf photosynthesis is resistant to a mild drought stress. Photosynthetica 27: 295-309.
Online |
Gscholar
(13)
Curtis PS, Wang X (1998)A meta-analysis of elevated CO
2 effects on woody plant mass, form, and physiology. Oecologia 113: 299-313.
CrossRef |
Gscholar
(14)
Dias DP, Marenco RA (2016)Tree growth, wood and bark water content of 28 Amazonian tree species in response to variations in rainfall and wood density. iForest - Biogeosciences and Forestry 9: 445-451.
CrossRef |
Gscholar
(15)
Duffy PB, Brando P, Asner GP, Field CB (2015)Projections of future meteorological drought and wet periods in the Amazon. Proceedings of the National Academy of Sciences USA 112: 13172-13177.
CrossRef |
Gscholar
(16)
Dunisch O, Schwarz T, Neves EJM (2002)Nutrient fluxes and growth of
Carapa guianensis Aubl. in two plantation systems in the Central Amazon. Forest Ecology and Management 166: 55-68.
CrossRef |
Gscholar
(17)
Evans JR, Seemann JR (1984)Differences between wheat genotypes in specific activity of ribulose-1.5-bisphosphate carboxylase and the relationship to photosynthesis. Plant Physiology 74: 759-765.
CrossRef |
Gscholar
(18)
Fatichi S, Leuzinger S, Körner C (2014)Moving beyond photosynthesis: from carbon source to sink-driven vegetation modeling. New Phytologist 201: 1086-1095.
CrossRef |
Gscholar
(19)
Flexas J, Gallé A, Galmés J, Ribas-Carbo M, Medrano H (2012)The response of photosynthesis to soil water stress. In: “Plant Responses to Drought Stress” (Aroca R ed). Springer-Verlag, Berlin, Germany, pp. 129-144.
CrossRef |
Gscholar
(20)
Fournier LA (2002)Species description
Carapa guianensis. In: “Tropical tree seed manual” (Vozzo JA ed). Agricultural Handbook 72, USDA Forest Service, Washington, DC, USA, pp. 360-362.
Gscholar
(21)
Grace J (2016)The Amazon carbon balance: an evaluation of methods and results. In: “Interactions between Biosphere, Atmosphere and Human Land Use in the Amazon Basin” (Nagy L, Forsberg BR, Artaxo P eds). Springer-Verlag, Berlin, Germany, pp. 79-100.
CrossRef |
Gscholar
(22)
Kauwe MG, Medlyn BE, Zaehle S, Walker AP, Dietze MC, Hickler T, Jain AK, Luo Y, Parton WJ, Prentice IC, Smith B, Thornton PE, Wang S, Wang Y, Warlind D, Weng E, Crous KY, Ellsworth DS, Hanson PJ, Kim H, Warren JM, Oren R, Norby RJ (2013)Forest water use and water use efficiency at elevated CO
2: a model-data intercomparison at two contrasting temperate forest FACE sites. Global Change Biology 19: 1759-1779.
CrossRef |
Gscholar
(23)
Kelly JW, Duursma RA, Atwell BJ, Tissue DT, Medlyn BE (2016)Drought x CO
2 interactions in trees: a test of the low-intercellular CO
2 concentration (Ci) mechanism. New Phytologist 209: 1600-1612.
CrossRef |
Gscholar
(24)
Kenfack D (2011)A synoptic revision of
Carapa (Meliaceae). Harvard Papers in Botany 16: 171-231.
CrossRef |
Gscholar
(25)
Kitao M, Yazaki K, Kitaoka S, Fukatsu E, Tobita H, Komatsu M, Maruyama Y, Koike T (2015)Mesophyll conductance in leaves of Japanese white birch (
Betula platyphylla var.
japonica) seedlings grown under elevated CO
2 concentration and low N availability. Physiologia Plantarum 155: 435-445.
CrossRef |
Gscholar
(26)
Klimas CA, Cropper WP, Kainer KA, Wadt LHO (2012)Viability of combined timber and non-timber harvests for one species: a
Carapa guianensis case study. Ecological Modelling 246: 147-156.
CrossRef |
Gscholar
(27)
Lambers H, Chapin FS, Pons TL (2008)Plant physiological ecology (2nd edn). Springer, New York, USA, pp. 11-254.
Gscholar
(28)
Lauteri M, Scartazza A, Guido MC, Brugnoli E (1997)Genetic variation in photosynthetic capacity, carbon isotope discrimination and mesophyll conductance in provenances of
Castanea sativa adapted to different environments. Functional Ecology 11: 675-683.
CrossRef |
Gscholar
(29)
Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR (2009)Elevated Longed CO
2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. Journal of Experimental Botany 60: 2859-2876.
CrossRef |
Gscholar
(30)
Leakey ADB, Ainsworth EA, Bernacchi CJ, Zhu X, Long SP, Ort DR (2012)Photosynthesis in a CO
2-rich atmosphere. In: “Photosynthesis: Plastid Biology, Energy Conversion and Carbon Assimilation” (Eaton-Rye JJ, Tripathy BC, Sharkey TD eds). Springer, Dordrecht, The Netherlands, pp. 733-768.
CrossRef |
Gscholar
(31)
Marenco RA, Camargo MAB, Antezana-Vera AS, Oliveira MF (2017)Leaf trait plasticity in six forest tree species of central Amazonia. Photosynthetica 55: 679-688.
CrossRef |
Gscholar
(32)
Marenco RA, Gonçalves JFC, Vieira G (2001)Photosynthesis and leaf nutrient contents in
Ochroma pyramidale (Bombacaceae). Photosynthetica 39: 539-543.
CrossRef |
Gscholar
(33)
Morison JIL (1998)Stomatal response to increased CO
2 concentration. Journal of Experimental Botany 49: 443-452.
CrossRef |
Gscholar
(34)
Nowak RS, Ellsworth DS, Smith SD (2004)Functional responses of plants to elevated atmospheric CO
2 - do photosynthetic and productivity data from FACE experiments support early predictions? New Phytologist 162: 253-280.
CrossRef |
Gscholar
(35)
Pan Y, Birdsey RA, Phillips OL, Jackson RB (2013)The structure, distribution, and biomass of the world’s forests. Annual Review of Ecology, Evolution and Systematics 44: 593-622.
CrossRef |
Gscholar
(36)
Parry MAJ, Andralojc P, Khan S, Lea P, Keys AJ (2002)Rubisco activity: effects of drought stress. Annals of Botany 89: 833-839.
CrossRef |
Gscholar
(37)
Poorter H, Nagel O (2000)The role of biomass allocation in the growth response of plants to different levels of light, CO
2, nutrients and water: a quantitative review. Australian Journal of Plant Physiology 27: 595-607.
CrossRef |
Gscholar
(38)
Saatchi SS, Houghton RA, Alvala RCS, Soares JV, Yu Y (2007)Distribution of aboveground live biomass in the Amazon basin. Global Change Biology 13: 816-837.
CrossRef |
Gscholar
(39)
Salati E, Vose PB (1984)Amazon Basin: a system in equilibrium. Science 225: 129-138.
CrossRef |
Gscholar
(40)
Singsaas EL, Ort DR, Delucia EH (2004)Elevated CO
2 effects on mesophyll conductance and its consequences for interpreting photosynthetic physiology. Plant, Cell and Environment 27: 41-50.
CrossRef |
Gscholar
(41)
Souza CR, Azevedo CP, Rossi L (2008)Desempenho de espécies florestais para uso múltiplo na Amazônia [Efficiency of forest species for multiple use in Amazonia]. Scientia Forestalis 36: 7-14. [in Portuguese]
Gscholar
(42)
Tardieu F, Granier C, Muller B (2011)Water deficit and growth. Co-ordinating processes without an orchestrator? Current Opinion in Plant Biology 14: 283-289.
CrossRef |
Gscholar
(43)
Way DA, Oren R, Kroner Y (2015)The space-time continuum: the effects of elevated CO
2 and temperature on trees and the importance of scaling. Plant, Cell and Environment 38: 991-1007.
CrossRef |
Gscholar
(44)
Yan F, Li X, Liu F (2017)ABA signaling and stomatal control in tomato plants exposure to progressive soil drying under ambient and elevated atmospheric CO
2 concentration. Environmental and Experimental Botany 139: 99-104.
CrossRef |
Gscholar