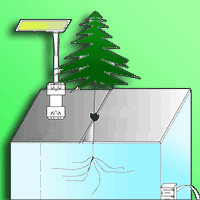
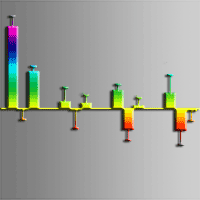
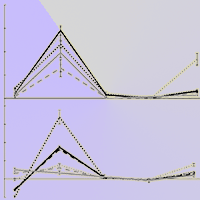
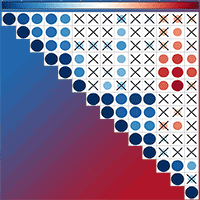
To understand whether Chinese fir (Cunninghamia lanceolata) can conserve energy by reducing root volatiles to maintain growth under low phosphorus (P) conditions, we cultivated two half-sib families of Chinese fir that display high and low P use efficiency under conditions of normal P supply and total P deficiency. Gas chromatography-mass spectrometry analysis was used to determine the content of root volatiles, and the relationships among root volatiles and root growth index, P content, and distribution were analyzed. There were significantly fewer volatile organic compounds (VOCs) in the rhizosphere of these two fir families, No. 25 and No. 32, under P deficiency. Low P supply significantly promoted root growth in No. 25, increasing both average diameter and volume. A negative correlation was found between the volatiles and the increment of root average diameter and surface area. The belowground P distribution and the root to shoot P concentration (Pr/Ps) were higher in No. 25 than in No. 32. The total amount of VOCs, as well as the amount of 18 individual volatiles were positively correlated with P accumulation, aboveground P distribution, and belowground P distribution, but the opposite pattern was seen in Pr/Ps for family No. 25 seedlings. We conclude that the content and types of VOCs differ among the Chinese fir genotypes. Under low-P stress, the roots of Chinese fir reduce the release of VOCs to maintain seedling growth.
Keywords
, , , ,
Citation
Lai H, Wu K, Wang N, Wu W, Zou X, Ma X, Wu P (2018). Relationship between volatile organic compounds released and growth of Cunninghamia lanceolata roots under low-phosphorus conditions. iForest 11: 713-720. - doi: 10.3832/ifor2797-011
Academic Editor
Claudia Cocozza
Paper history
Received: Mar 21, 2018
Accepted: Aug 21, 2018
First online: Nov 06, 2018
Publication Date: Dec 31, 2018
Publication Time: 2.57 months
© SISEF - The Italian Society of Silviculture and Forest Ecology 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.

Breakdown by View Type
(Waiting for server response...)
Article Usage
Total Article Views: 47382
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 40520
Abstract Page Views: 3073
PDF Downloads: 2883
Citation/Reference Downloads: 4
XML Downloads: 902
Web Metrics
Days since publication: 2649
Overall contacts: 47382
Avg. contacts per week: 125.21
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2018): 4
Average cites per year: 0.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
(1)
Achard P, Vriezen WH, Van Der Straeten D, Harberd NP (2003)Ethylene regulates
Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant and Cell 15: 2816-2825.
CrossRef |
Gscholar
(2)
Asensio D, Penuelas J, Filella I, Llusia J (2007)On-line screening of soil VOCs exchange responses to moisture, temperature and root presence. Plant and Soil 291: 249-261.
CrossRef |
Gscholar
(3)
Bruinsma M, Posthumus MA, Mumm R, Mueller MJ, Van Loon JJA, Dicke M (2009)Jasmonic acid-induced volatiles of
Brassica oleracea attract parasitoids: effects of time and dose, and comparison with induction by herbivores. Journal of Experimental Botany 60: 2575-2587.
CrossRef |
Gscholar
(4)
Burton AL, Lynch JP, Brown KM (2013)Spatial distribution and phenotypic variation in root cortical aerenchyma of maize (
Zea mays L). Plant and Soil 367: 263-274.
CrossRef |
Gscholar
(5)
Chen H (2003)Phosphatase activity and P fractions in soils of an 18-year old Chinese fir (
Cunninghamia lanceolata) plantation. Forest Ecology and Management 178: 301-310.
CrossRef |
Gscholar
(6)
Chen ZY, Li Q, Zou XH, Ma XQ, Wu PF (2016)Effect of neighboring competition on photosynthetic characteristics and biomass allocation of Chinese fir seedlings under low phosphorus stress. Chinese Journal of Plant Ecology 40 (2): 177-186.
CrossRef |
Gscholar
(7)
Chiou TJ, Lin SI (2011)Signaling network in sensing phosphate availability in plants. Annual Review of Plant Biology 62: 185-206.
CrossRef |
Gscholar
(8)
Dam NM, Qiu BL, Hordijk Vet CA LEM, Jansen JJ (2010)Identification of biologically relevant compounds in aboveground and belowground induced volatile blends. Journal of Chemical Ecology 36: 1006-1016.
CrossRef |
Gscholar
(9)
Depuydt S (2014)Arguments for and against self and non-self root recognition in plants. Frontiers in Plant Science 5: 1-7.
CrossRef |
Gscholar
(10)
Dudareva N, Negre F, Nagegowda DA, Orlova I (2006)Plant volatiles: recent advances and future perspectives. Critical Reviews in Plant Sciences 25: 417-440.
CrossRef |
Gscholar
(11)
Ens EJ, Bremner JB, French K, Korth J (2009)Identification of volatile compounds released by roots of an invasive plant, bitoubush (
Chrysanthemoides monilifera spp.
rotundata), and their inhibition of native seedling growth. Biological Invasions 11: 275-287.
CrossRef |
Gscholar
(12)
Falik O, Hoffmann I, Novoplansky A (2014)Say it with flowers: flowering acceleration by root communication. Plant Signal and Behavior 9 (3): e28258.
CrossRef |
Gscholar
(13)
Fan MS, Zhu JM, Richards C, Brown KM, Lynch JP (2003)Physiological roles for aerenchyma in phosphorus-stressed roots. Functional Plant Biology 30: 493-506.
CrossRef |
Gscholar
(14)
Fares S, Brilli F, Noguès I, Velikova V, Tsonev T, Dagli S, Loreto F (2008)Isoprene emission and primary metabolism in
Phragmites australis grown under different phosphorus levels. Plant Biology 10 (1): 38-43.
CrossRef |
Gscholar
(15)
Farooq TH, Tigabu M, Ma XQ, Zou XH, Liu AQ, Odén PC, Wu PF (2018)Nutrient uptake, allocation and biochemical changes in two Chinese fir cuttings under heterogeneous phosphorus supply. iForest - Biogeosciences and Forestry 11: 411-417.
CrossRef |
Gscholar
(16)
Frost CJ, Mescher MC, Dervinis C, Davis JM, Carlson JE, De Moraes CM (2008)Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytologist 180: 722-734.
CrossRef |
Gscholar
(17)
He Y, Liao H, Yan XL (2003)Localized supply of phosphorus induces root morphological and architectural changes of rice in split and stratified soil cultures. Plant and Soil 248: 247-256.
CrossRef |
Gscholar
(18)
Lim JH, Chung IM, Ryu SS, Yun SJ (2003)Differential responses of rice acid phosphatase activities and isoforms to phosphorus deprivation. Journal of Biochemistry and Molecular Biology 36: 597-602.
CrossRef |
Gscholar
(19)
Lin C, Owen SM, Peñuelas J (2007)Volatile organic compounds in the roots and rhizosphere of
Pinus spp. Soil Biology and Biochemistry 39: 951-960.
CrossRef |
Gscholar
(20)
Lynch JP (1998)The role of nutrient efficient crops in modern agriculture. Journal of Crop Production 1: 241-264.
CrossRef |
Gscholar
(21)
Lynch JP, Ho MD (2005)Rhizoeconomics: carbon costs of phosphorus acquisition. Plant and Soil 269: 45-56.
CrossRef |
Gscholar
(22)
Lynch JP (2014)Root phenes that reduce the metabolic costs of soil exploration: opportunities for 21st century agriculture. Plant Cell and Environment 38: 1-10.
CrossRef |
Gscholar
(23)
Nagarajan VK, Smith AP (2012)Ethylene’s role in phosphate starvation signaling: more than just a root growth regulator. Plant and Cell Physiology 53: 277-286.
CrossRef |
Gscholar
(24)
Nielsen KL, Eshel A, Lynch JP (2001)The effect of phosphorus availability on the carbon economy of contrasting common bean (
Phaseolus vulgaris L.) genotypes. Journal of Experimental Botany 52: 329-339.
CrossRef |
Gscholar
(25)
Ormeño E, Fernandez C, Mévy J (2007)Plant coexistence alters terpene emission and content of Mediterranean species. Phytochemistry 68: 840-852.
CrossRef |
Gscholar
(26)
Peñuelas J, Asensio D, Tholl D (2014)Biogenic volatile emissions from the soil. Plant Cell and Environment 37: 1866-1891.
CrossRef |
Gscholar
(27)
Pierik R, Ballaré CL, Dicke M (2014)Ecology of plant volatiles: Taking a plant community perspective. Plant Cell and Environment 37: 1845-1853.
CrossRef |
Gscholar
(28)
Plassard C, Dell B (2011)Phosphorus nutrition of mycorrhizal trees. Tree Physiology 30: 1129-1139.
CrossRef |
Gscholar
(29)
Robert CAM, Erb M, Duployer M, Zwahlen C, Doyen GR, Turlings TCJ (2012)Herbivore-induced plant volatiles mediate host selection by a root herbivore. New Phytologist 194: 1061-1069.
CrossRef |
Gscholar
(30)
Strock CF, Morrow LDLR, Lynch J (2018)Reduction in root secondary growth as a strategy for phosphorus acquisition. Plant Physiology 176: 691-703.
CrossRef |
Gscholar
(31)
Wu PF, Ma XQ, Tigabu M, Wang C, Liu AQ, Odén PC (2011)Root morphological plasticity and biomass production of two Chinese fir clones with high phosphorus efficiency under low phosphorus stress. Canadian Journal of Forest Research 41: 228-234.
CrossRef |
Gscholar
(32)
Wu P, Shou HX, Xu GH, Lian XM (2013)Improvement of phosphorus efficiency in rice on basis of understanding phosphate signaling and homeostasis. Current Opinion in Plant Biology 16: 205-212.
CrossRef |
Gscholar
(33)
Wu PF, Wang GY, Farooq TH, Li Q, Zou XH, Ma XQ (2017)Low phosphorus and competition affect Chinese fir cutting growth and root organic acid content: does neighboring root activity aggravate P nutrient deficiency? Journal of Soils and Sediments 17: 2775-2785.
CrossRef |
Gscholar
(34)
Wu PF, Lai HY, Tigabu M, Wu WJ, Wang P, Wang GY, Ma XQ (2018)Does phosphorus deficiency induce formation of root cortical aerenchyma maintaining growth of
Cunninghamia lanceolata? Trees 215 (11): 76.
CrossRef |
Gscholar
(35)
Zhu XF, Zhu CQ, Zhao XS, Zheng SJ, Shen RF (2016)Ethylene is involved in root phosphorus remobilization in rice (
Oryza sativa) by regulating cell-wall pectin and enhancing phosphate translocation to shoots. Annals of Botany 118: 645-653.
CrossRef |
Gscholar
(36)
Zou XH, Wu PF, Chen NL, Wang P, Ma XQ (2015)Chinese fir root response to spatial and temporal heterogeneity of phosphorus availability in the soil. Canadian Journal of Forest Research 45: 402-410.
CrossRef |
Gscholar
(37)
Zou XH, Wei D, Wu PF, Zhang Y, Hu YN, Chen ST, Ma XQ (2018)Strategies of organic acid production and exudation in response to low-phosphorus stress in Chinese fir genotypes differing in phosphorus-use efficiencies. Trees 32: 897-912.
CrossRef |
Gscholar