Pinus halepensis Mill. in the Mediterranean region: a review of ecological significance, growth patterns, and soil interactions
iForest - Biogeosciences and Forestry, Volume 18, Issue 1, Pages 30-37 (2025)
doi: https://doi.org/10.3832/ifor4566-017
Published: Feb 15, 2025 - Copyright © 2025 SISEF
Research Articles
Abstract
This review synthesizes current knowledge on Pinus halepensis Mill. with a specific emphasis on its ecological importance, growth dynamics, and interactions with soil in the Mediterranean region. As a dominant species in afforestation projects globally, particularly in the Mediterranean Basin, this article offers a concise overview of the distribution, adaptability to arid environments, and diffusion mechanisms of Aleppo pine trees. Emphasis is placed on the characteristics of aboveground growth, specifically examining the factors that affect radial growth based on dendrological analysis. The impact of soil on Pinus halepensis growth is scrutinized, highlighting its capacity to adapt to low fertility and salinity while addressing water retention and erosion control challenges. The information presented here provides valuable insights into Aleppo pine forest management and conservation efforts in the Mediterranean context.
Keywords
P. halepensis Mill., Radial Growth Dynamics, Soil-plant Interactions, Ecological Importance.
Introduction
Pine forests arguably constitute the largest forested areas in the northern hemisphere, with a range expansion from subtropical to subarctic zones, coastal plains, high plateaus, and mountains ([76]). Several biotic and abiotic agents have altered these forests for years, influencing their current cover and distribution worldwide, especially in the Mediterranean region ([77]). Pines are the most used species in afforestation projects, with nearly 20% of planted forests worldwide, due to their commercial value, soil protection, and rehabilitation purposes ([48], [7]). The genus Pinus is among the top five genera in terms of the volume of growing stock in forests, regardless of whether it is composed of native or introduced species. This genus represents the first growing stock in Europe, the second in Asia and North America, and the fifth in Africa ([30]). Given the number of species belonging to the genus, it is the richest genus of conifers, with approximately 115 verified species ([100]). Ten of these species are found in the Mediterranean area, and four of them are considered native to the region in terms of climatic requirements and distributional range: Pinus halepensis Mill., Pinus pinaster Ait., Pinus pinea L. and Pinus brutia Ten ([75], [9]).
This work focuses on the Aleppo pine, Pinus halepensis Mill., particularly in its natural habitat in the Mediterranean region. This paper aims to review recent literature regarding the importance, productivity, and factors affecting the growth of this valuable Mediterranean species.
Aleppo pine
Description and distribution
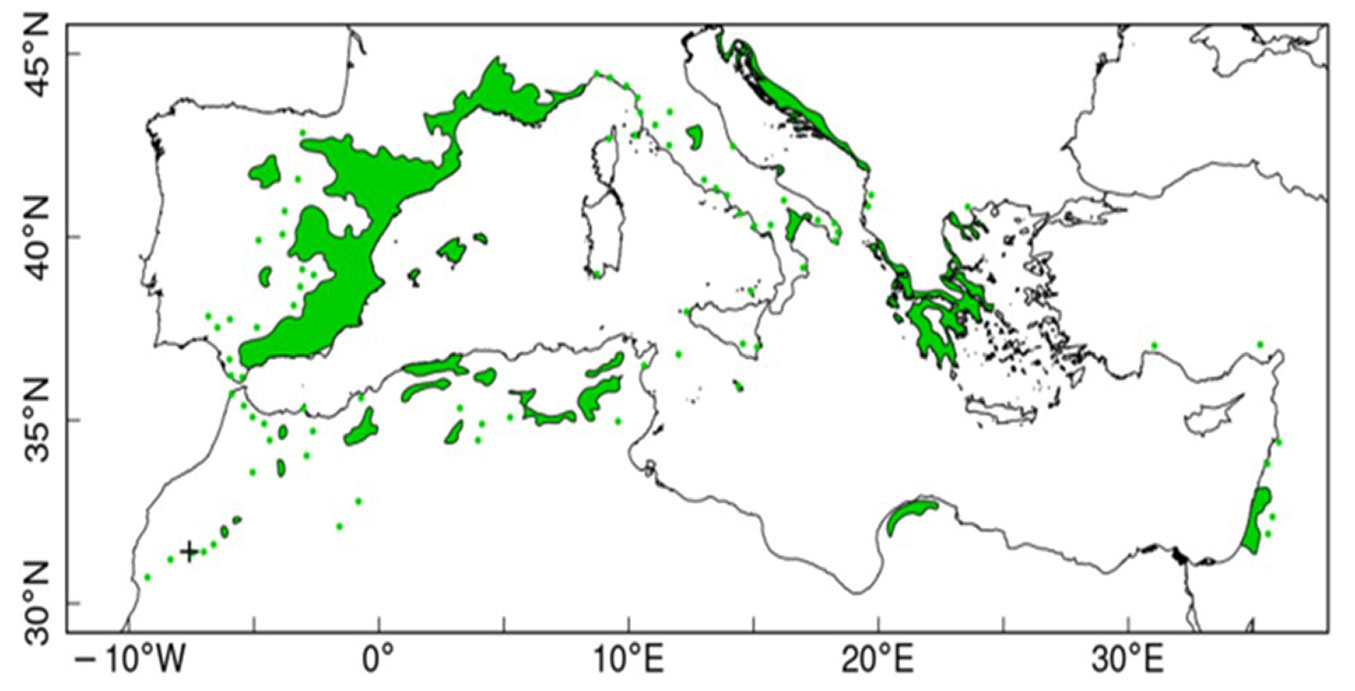
P. halepensis is a circum-Mediterranean species known as the most widely distributed pine species in the region, covering nearly 3.5 million hectares. ([38]). Aleppo pine grows naturally in almost every Mediterranean country but is mainly concentrated in the basin’s western side, in Spain and southern France (Fig. 1) ([20], [96]). It is usually a low-elevation species, but it can grow at higher altitudes up to 1700 m a.s.l. in Morocco ([31]). The plasticity, drought tolerance, and rapid growth rate of the species make it suitable for harsh semi-arid environments ([61]), and it is a favorite candidate in afforestation projects in these areas. Aleppo pine is a perennial evergreen tree that can grow up to 20 meters in height and reach a diameter of 100 cm at breast height, with a potential lifespan exceeding 150 years. However, it is typically shorter in height and susceptible to disturbances such as wildfires ([1], [56], [2]). The species has thick, cracked bark and pedunculate cones. It is also characterized by delicate, pliable, light green needles ([29]). As early as three years of age, Aleppo pine starts the production of female cones. Notably, this species is distinguished by a well-developed root system featuring a substantial woody taproot and robust lateral roots ([99]).
Fig. 1 - Geographical distribution of Pinus halepensis Mill. in the Mediterranean Basin (source: [96]).
Ecological importance in the Mediterranean region
The ability of P. halepensis to survive in dry environments is characterized by its adaptation to seasonal precipitation fluctuations and isohydric response ([20]). Thus, the species’ potential to grow on poor fertility and rocky calcareous soils makes it a favored candidate in afforestation projects. Aleppo pine is a key species in the Mediterranean ecosystem, contributing to its ecological and economic value. It plays a crucial role in facilitating the establishment of perennial grasses and shrubs, contributing to the overall biodiversity of the region ([52]). However, its allelopathic effects, particularly the release of volatile organic compounds, can inhibit herbaceous species’ germination and root growth ([83]). Regarding habitat, P. halepensis forests have been found to host more diagnostic species of foredune habitats than other pine forests, highlighting their unique ecological characteristics ([8]). Moreover, the ability to adapt to various soil types allows its growth even in heavily polluted lands, such as mine tailing and landfill sites, tolerating high concentrations of heavy minerals like Zinc (Zn), Lead (Pb), and Cadmium (Cd) ([47]). Furthermore, the species is suitable for phytostabilization of metal(loid)-polluted soils in semiarid environments in Southeast Spain ([69]). P. halepensis is a pioneer species ([32], [5]) with enormous potential to expand and colonize nearby degraded and abandoned lands. For example, Lavi et al. ([48]) reported that P. halepensis invaded the surrounding natural vegetation of Quercus ithaburensis Decne. and Pistacia lentiscus L. in Israel. The broad distribution and survival traits of P. halepensis in the Mediterranean Basin can be attributed partly to its reproductive strategy, which involves producing both serotinous and non-serotinous cones ([59]). Aleppo pine trees are typically planted in the region to reduce soil and water losses and restore vegetation cover. They represent a suitable alternative in preventing excessive degradation in susceptible arid regions ([71], [17]).
Reproduction and expansion
Aleppo pine’s diffusion capacity has been thoroughly studied, with seedlings observed as far as 100 meters from the stand edge and even at greater distances, forming “islands” of pine trees amidst the natural vegetation ([48]). Similar findings have been reported in several places in the southern hemisphere, including the Argentine pampas ([87]) and the Ernesto Tornquist Provincial Park in Argentina ([22]). In further investigation, the authors found that the structure and composition of the natural Pampean grasslands may be impacted by indirect consequences linked to the presence of feral horses, which could favor the establishment of P. halepensis. Moreover, Osem et al. ([64]) found that cattle grazing of natural herbaceous vegetation can favor the emergence of Aleppo pine seedlings in Ramat Hanadiv Nature Park in Israel.
Aleppo pine has serotinous cones that facilitate regeneration after fires. These cones remain closed on the tree for an extended period, acting as a “canopy seed bank” until they are exposed to intense heat, such as fire, which causes the cones to open, releasing pine seeds ([59]). Pine trees in fire-prone habitats, such as the Mediterranean basin, have a higher proportion of serotinous cones than trees in populations with few fires or no fire history ([79]).
Aboveground growth attributes of P. halepensis: what drives the species radial growth?
Previous studies on the mean annual radial growth of P. halepensis have identified several key factors influencing the ecological performances of the species. P. halepensis exhibits superior growth and carbon sequestration compared to other pine trees in the Mediterranean region ([23], [20]). Tree size, competition indices, and water stress indices are significant predictors of radial growth, with thinning having a positive effect ([43]). The species’ cambial rhythm and cell differentiation vary based on environmental conditions, indicating high adaptability ([74]). The stand structure can modulate the species’ response and long-term vulnerability to drought. Indeed, trees in densely forested stands can face a pronounced water shortage due to the intense competition among individuals for soil moisture. Consequently, these trees may be more susceptible to drought compared to those in more open, widely spaced woodland stands, according to Moreno-Gutiérrez et al. ([58]).
Growth dynamics in response to environmental factors
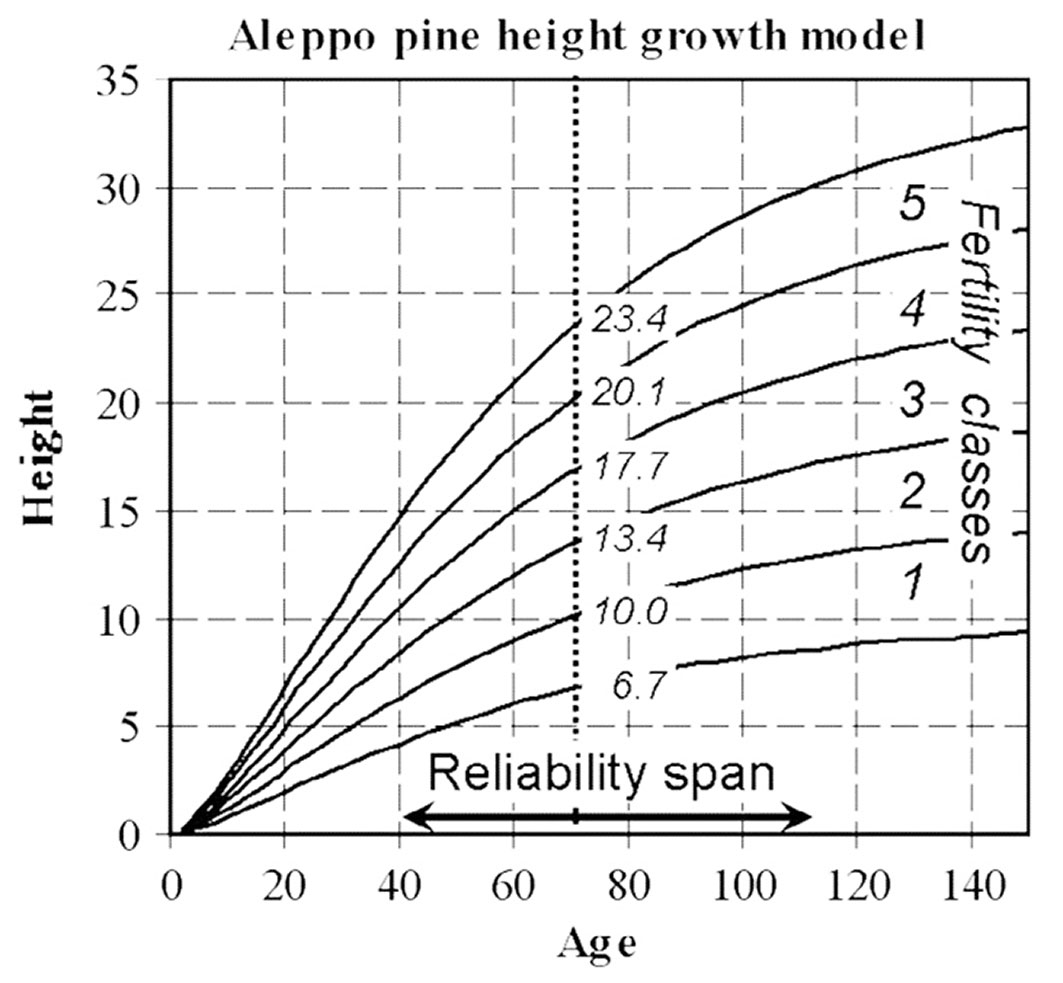
Climate change has negatively impacted pine forests by causing significant growth decline and dieback mortality due to extended drought periods. Moreover, environmental variables such as rainfall and temperature considerably affect radial growth, influencing different stages of growth and rest periods ([54], [14]). P. halepensis exhibits superior growth attributes and has a larger potential for enhancing carbon fixation than other pine species in the Iberian Peninsula ([23]). Mean annual growth varies for P. halepensis trees around the Mediterranean basin. Higher mean annual growth (MAG) rates were recorded from both pure and mixed stands in The Ouarsenis massif in Algeria ([84]), Greece ([68]) and Tunisia ([28]). According to later studies, repeated drought waves can significantly reduce radial growth and increase mortality rates. On the other hand, far lower MAG rates were observed in Morocco ([82]) and South France ([92]). Vennetier et al. ([93]) developed a site index model for height growth using Chapman-Richard’s equations based on local site conditions and climatic factors from 512 plots of P. halepensis across France (Fig. 2). This model accurately applies to all types of P. halepensis stands in France despite sub-regional differences, indicating that height growth patterns in France are homogeneous, regardless of climatic variability. Additionally, this model holds the potential for adaptation in similar Mediterranean regions. A study on P. halepensis radial growth has revealed its sensitivity to climate variability, particularly precipitation and temperature ([88]), which is further influenced by local site and stand characteristics, such as competition and water stress ([43]). Aleppo pine exhibits a notable ability to exploit soil water during cooler seasons, particularly in winter and autumn, in the absence of other limiting environmental factors ([33]). In the adult stage, Aleppo pine displays resilience and plasticity in response to adverse environmental conditions, modulating its physiological activity based on environmental and seasonal nuances ([36]). Active growth phases are most apparent during spring and autumn when temperatures are favorable and soil water is available, especially in coastal ecosystems compared to continental ones ([66]). Aleppo pine can withstand drought and adverse environmental conditions, enabling it to recover when more favorable conditions occur. For instance, Gazol et al. ([37]) demonstrated the capacity of Aleppo pine to absorb water during rainy seasons, fostering robust growth during autumn and winter. This adaptive strategy enables Aleppo pine to successfully colonize nutrient-poor, abandoned, and arid lands ([18]), thus providing production and protection value to bare lands and forest ecosystems. Both Olivar et al. ([63]) and Sarmoum et al. ([85]) noted the primary influence of rainfall on the radial growth of Aleppo pine. Moreover, supporting findings reported that radial growth is related to water availability in the soil during preceding wet seasons, specifically in spring and winter ([15]).
Fig. 2 - Aleppo pine height growth model and limits of fertility classes in France. Within 40-110 years, the average prediction error on tree SI compared to the real value from stem analysis is always less than 10% (Source: [93]).
Exposure, altitude, and topographic location also impact radial growth ([40]). In a study on P. halepensis trees growing in The Atlas Mountains in Morocco, the authors proposed that recurrent severe drought episodes, which are prevalent in the Mediterranean basin, might potentially result in a dieback phenomenon and subsequent loss of habitat for P. halepensis if they persist ([96]). The strong correlation between radial growth and environmental parameters, such as precipitation, temperature, and seasonal changes, highlights the species’ ability to withstand climate change and demonstrates its usefulness as a measure of tree performance under different circumstances.
Dendroclimatological insights into P. halepensis growth
Several dendrochronological investigations have been carried out in the Mediterranean region to examine growth patterns and past events impacting forest ecosystems. Among different tree species used, P. halepensis was documented as a reliable species for dendrochronological studies in the region ([68], [21], [61], [70], [4], [88], [10], [81]). Most of these studies discussed the annual-rings width relationship with climatic parameters like accumulated annual precipitation and monthly average temperatures. Pine’s wood structure has easily distinguishable annual rings of clear earlywood (EW) and latewood (LW), with a readily detectable gradual transition between both formations ([86]). Variation in tree-ring width could be attributed to specific tree factors like age, location, and applied silvicultural practices or to various climatic factors such as precipitation and temperature ([35]). In low-elevation sites, precipitation is considered the most common factor responsible for variability in width for conifers annual rings ([89], [68]). Pines and other tree species in the arid Mediterranean habitat exhibit distinct seasonal fluctuation. Spring and autumn precipitation are responsible for bimodal growth patterns for pine species in the region rather than the cold winter and hot summer seasons ([21]). These bimodal patterns are related to the start of cambial activity, which typically begins in spring under Mediterranean climatic conditions due to increasing temperature and prolonged photoperiod duration ([95]). In a dendroclimatological study on 32 P. halepensis Mediterranean forests, Camarero et al. ([15]) revealed a significant augmentation in the growth of Aleppo pine within more humid sites, demonstrating the species’ adaptability to varying climatic conditions. The tree-ring width of P. halepensis was positively correlated with winter and spring precipitation and negatively correlated with summer temperature, as Vieira et al. ([96]) reported in their study on the growth decline of the species in the Atlas Mountains in Morocco. In a study on the growth of mixed Quercus ilex and Pinus halepensis Mediterranean forest near Barcelona, Spain, Campelo et al. ([16]) used the Vaganov-Shashkin (VS)-Lite model to analyze the species annual growth patterns. Both species exhibited bimodal growth curves, with two growth peaks occurring during the spring and fall seasons, coinciding with the greatest precipitation periods. The VS model has been proven sufficient to analyze growth bimodality in tree species under Mediterranean environments, with better performance in dry-arid sites rather than humid and sub-humid sites ([15]). Moreover, Valeriano et al. ([90]) found robust bimodal growth among pine species compared to junipers in their study on four pine and four juniper stands in eleven sites across Spain. They concluded that the pine growth rate improved in wet winter-spring seasons in earlywood (EW) formation and wet autumn conditions in latewood (LW) formation in most coastal and less continental pine sites. Variations in P. halepensis ring width significantly correlate with periods of experienced drought seasons, and this may negatively alter the species’ seasonal wood formation as a response to such severe events ([70]). Higher proportions of EW were observed in habitats with wetter conditions, while in contrast, LW proportions were higher in areas with more xeric conditions in the Iberian Peninsula ([81]). Precipitation, particularly autumn rainfall, can improve P. halepensis growth and its cambial activity after a long dry summer ([88], [13]). Temperature, on the other hand, is expected to have a variable impact on the growth of P. halepensis ([94]). High temperatures often lead to water stress and high rates of evapotranspiration, which negatively impact soil moisture availability. Variations in temperature during rainy seasons and soil moisture during dry seasons are the main drivers for increasing the growth rate of P. halepensis in southern Italy, according to Attolini et al. ([3]). The role of winter temperature as a crucial factor affecting annual ring growth is more evident in sites with colder winter seasons ([20]). On the contrary, high-temperature records during the summer season negatively affected the width of P. halepensis tree rings, and the species growth was highly dependent on the resumption of favored climatic conditions during the summer-early autumn period, as reported in a dendrochronological study in Albania ([88]). It is well concluded that soil water availability is the most crucial factor responsible for pine growth in temperate and semi-arid regions such as the Mediterranean region ([39]).
Forest management approaches, including applying proper silvicultural treatments such as thinning, may enhance the radial growth of P. halepensis in vulnerable water-stressed populations. In a study on a 60-year-old P. halepensis plantation in one of Spain’s driest regions, Olivar et al. ([62]) found that thinning, which reduced stand density by 30%-45%, significantly enhanced radial growth in both dominant and suppressed trees by improving overall growth conditions. This trend of P. halepensis ring-width response to climatic factors appears steady throughout the region and not limited only to specific areas or countries. De Luis et al. ([20]) constructed a series of tree-ring chronologies from over 60 sites representing several Mediterranean countries (Algeria, France, Greece, Israel, Italy, Jordan, Slovenia, Spain, and Turkey). Their findings highlighted the importance of precipitation as the primary climatic factor influencing the variability in annual ring width. However, radial growth patterns exhibited distinct variations across Mediterranean countries located in the northern and southern regions. These variations appear to represent P. halepensis trees’ adaptive responses to the dry and hot conditions in the southern half and the cool and moist conditions in the northern part of the Mediterranean basin.
The impact of soil on P. halepensis growth
Soil plays a critical role in supporting the growth of forest trees and preserving fertility and stability throughout different stages of forest development ([17]). Conversely, vegetation plays a crucial role in modifying soil properties, enhancing the functioning and dynamics of ecosystems ([91]). P. halepensis has been shown to exert varying effects on soil properties. For instance, Hedo et al. ([42]) reported that the tree’s canopy coverage had little influence on altering soil characteristics, but the climate had a more substantial effect. In contrast, studies by Jeddi & Chaieb ([46]) and Romeo et al. ([78]) reported positive effects, including increased organic carbon, total nitrogen, and available phosphorus, as well as enhanced soil moisture content beneath the tree canopy. Furthermore, these studies also noted the facilitation of understory vegetation and enhanced seedling regeneration. Hence, the correlation between the development of Aleppo pine trees and soil characteristics is complex, and understanding soil’s physiological and chemical effects on growth patterns is essential for improved forest management.
Physical soil attributes of Aleppo pine forests
The physical properties of forest soils are influenced by a range of factors, including the presence of inorganic and organic solids, solutes, liquids, and gases ([65]). These properties can vary significantly between forest and arable soils, with differences in bulk density, water content, and soil matrix potential ([67]). The physical and chemical properties of forest soils play a crucial role in supporting forest productivity (Tab. 1), with soil texture being a particularly important factor ([55]). The formation of dense surface horizons in forest soils is linked to the active eluviation of iron under reducing conditions ([34]). Various factors influence the effect of P. halepensis on soil physical properties. Del Río et al. ([24]) reported that higher Site Index (SI) values are correlated with clay or loamy soil textures, while, on the other hand, lower SI values are common with soils with low clay contents. Moreover, Eldiabani et al. ([27]) found that silt loam is the most common soil texture in burned and unburned pine plantation soils in Northeast Libya, with clay representing only a small fraction of the soil profile. They also noted that the soil in this region is deficient in organic matter, with higher concentrations confined to the surface layers (0-15 cm). Depending on fire severity, burned soils typically exhibit more sand particles than clay at the surface ([50]). Additionally, soil bulk density and electrical conductivity (EC) are reported to increase in burned areas compared to unburned lands. However, in comparing burned and unburned forests in Libya, fire did not significantly impact physical soil attributes ([26]). P. halepensis exhibits a strong correlation between its electrical signal, which refers to a continuous electrical potential between the electrodes inserted in the tree’s phloem and the surrounding soil, and environmental factors such as rainfall and temperature ([98]). Fires of low intensity and severity tend to have minimal to low impact on both organic matter and mineral content, particularly at the uppermost surface soil layers under P. halepensis stand canopies in the NE Iberian Peninsula ([97]). Hedo et al. ([42]) found that the canopy coverage of this species did not significantly impact soil properties, with climate and season playing a more significant role. However, Maestre & Cortina ([53]) underscored that the planting methods for P. halepensis may exacerbate runoff and soil erosion while offering only marginal benefits to physio-chemical properties. Nevertheless, pine plantations are widely used as a restoration strategy to mitigate soil erosion and manage water runoff in arid and semi-arid regions. For instance, P. tabulaeformis plantations in the Anjiagou watershed of the Gansu region in China have proven effective in reducing erosion and runoff, achieving a 6%-17% decrease in runoff and a 48%-72% reduction in erosion rates, as demonstrated by long-term experimental plots compared to agricultural lands planted with wheat ([45]). Similarly, the average annual soil loss in planted areas of Anatolian Black pine (Pinus nigra) was found to be around 5.5 times less than that of barren areas at soil depths of 0-50 cm in a study conducted in Ankara province, Turkey ([41]). Although Aleppo pine is widely planted in semi-arid regions for surface runoff and erosion control, Cerdà et al. ([17]) found that soil and water loss in these plantations were ten times higher than in Holm oak forests growing in the same area in eastern Spain. Other site factors, such as hillside orientation and length, but not the slope’s steepness, can accelerate erosion rates in addition to P. halepensis cover ([72]). Furthermore, the introduction of P. halepensis has been shown to negatively affect soil moisture and hinder the growth of native shrubs, especially at higher tree densities ([5]). Afforestation projects using Aleppo pine trees in semi-arid regions, particularly those with impoverished and shallow soils, may not inherently enhance water retention and reduce soil erosion rates, and their impacts may be reversible ([80]).
Tab. 1 - Macronutrients soil elements (N, P, K), organic matter (OM) content, and soil texture for unburned natural/planted Aleppo pine forests in several Mediterranean regions, according to recent literature.
| Study Area | Stand type |
Soil Texture |
Organic matter (%) |
N content (%) |
P content |
K content |
Source |
|---|---|---|---|---|---|---|---|
| Cerros Concejiles and Cerro Palomero (Central Spain) |
Plantations | Sand-silty/clay (1:2) |
0.81 ± 0.44 | 0.19 | 13.2 ± 1.9 ppm |
22.4 ± 8.5 ppm |
Morcillo et al. ([57]) |
| Beni Sohane (Morocco) | Plantations | Clay- silty/clay (1:1) |
1.51 | 0.14 | 2.43 ppm |
152.4 mg kg-1 |
Benarchid et al. ([6]) |
| Castile and Leon region (N Spain) |
Plantations | Clay/loam | 1.54 | 0.13 | 2.23 mg/kg |
0.70 cmol+ kg-1 |
Bueis et al. ([11]) |
| Calasparra, Murcia (SE Spain) |
Plantation |
Loamy |
5.50 |
0.2 |
1.96 mg kg-1 |
- |
Hedo et al. ([42]) |
| Serra de Senadelles (NE Spain) |
Natural forest | Clay-silt-sandstone | 5.58 | 0.29 | - | 260.05 ppm |
Xifré-Salvadó et al. ([97]) |
| Castilla-La Mancha (Spain) |
Mixed natural forest |
Sand/silt/clay (1:1:2) |
2.44 ± 0.64 | 0.21 | 1.27 ppm |
541.17 ppm |
Plaza-Álvarez et al. ([73]) |
| Al Jabal Al-Akhdar NE Libya) |
Plantation | Sand/silt/clay (1:3:1) |
1.15 | 0.15 | 2.57 ppm |
0.42 meq. 100 g soil-1 |
Eldiabani et al. ([27]) |
Chemical soil attributes of Aleppo pine forests
Soil fertility is crucial for growth and stability throughout forest developmental stages ([17]). P. halepensis is well-adapted to low soil fertility and salinity, making it suitable for phytostabilization of metal(loid)-polluted soils ([69]). In the southern Mediterranean countries, P. halepensis can improve soil fertility and enhance growth conditions, although its effectiveness is significantly less than that of native sclerophyllous shrubs ([6]). In southern Tunisia, newly established stands of P. halepensis have been shown to improve total carbon, nitrogen, phosphorus, and soil moisture content compared to nearby open areas. These enhancements become more significant as the stands mature, creating favorable conditions for the growth of diverse understory vegetation ([46]). On the contrary, using this species in reforestation projects was found to have a negative impact on total soil nutrients, especially phosphorus and boron ([6]). In dry and fire-susceptible ecosystems, the nutrient content in P. halepensis foliar parts showed resilience and adaptability, decreasing during spring and increasing during autumn ([19]). Most of the literature investigating the impact of P. halepensis on soil (and vice versa) primarily examined changes in soil attributes following disturbance events, particularly fires ([49], [51], [78]). Low-intensity fires in a P. halepensis forest increased total carbon, organic carbon, total nitrogen, electrical conductivity, and potassium, indicating a short-term positive impact on soil ([97]). Wildfires and logging have been found to have short-term impacts on soil functionality in P. halepensis forests, with burned plots showing higher organic matter and total nitrogen content ([51]). The common belief that conifers usually lower soil pH, resulting in increased acidity, may not be as significant as previously thought. Moya et al. ([60]) observed that pH levels increased shortly after severe fires in their study of burned P. halepensis forests in the southeastern Iberian Peninsula. The pH increase was attributed to the combustion of organic matter and the subsequent release of inorganic ions. Knowing the acidity level is crucial for understanding microbial activities in the soil profile. For instance, soil pH and water availability are major constraints for enzyme activities in acidic and calcareous soil underneath pine plantations ([11]). Relevant findings on pH levels were reported in a conducted mulching treatment experiment using Pinus halepensis and Olea europaea leaves in Croatia ([25]). Additionally, Perdomo-González et al. ([71]) observed that soils under newly established P. halepensis stands in the Canary Islands had a saline nature with a pH value of 7.9. Further, Burgess-Conforti et al. ([12]) reported no significant differences in soil acidity between coniferous and deciduous stands in an Ozark National Forest in Arkansas experiment. In acidic Mediterranean soils in northern Spain, the carbon content was higher in the forest floor of pine stands compared to oak stands ([44]). However, Maestre & Cortina ([53]) highlighted potential negative effects of P. halepensis plantations, such as enhanced runoff and soil losses, limited improvement in physio-chemical properties, and reduced biodiversity.
Conclusion
This review sheds light on the multifaceted ecological importance of P. halepensis in the Mediterranean region. As a dominant species in afforestation projects, Aleppo pine stands out as a resilient and versatile species, well-suited to the challenging environmental conditions of the Mediterranean region. Its ecological importance, adaptability, and growth potential make it a critical component of forest management and conservation efforts. As the Mediterranean region faces increasing pressures from climate change and human activities, the role of P. halepensis in maintaining ecosystem stability and supporting biodiversity will become more significant.
The aboveground growth attributes, explored in detail through dendrological analysis, reveal the species’ dynamic responses to environmental variability, with factors like rainfall, temperature, and soil conditions influencing radial growth patterns. The species’ growth response to climatic factors, particularly its ability to capitalize on favorable conditions during cooler and wetter periods, highlights its potential for carbon sequestration and its role in mitigating the impacts of climate change in the region. The impact of P. halepensis on soil characteristics is a key focus, highlighting both positive and negative effects on soil fertility and stability. While the species is adaptable to low soil fertility and salinity, its introduction in specific contexts may lead to changes in the soil nutrient balance. The intricate interplay between Aleppo pine and soil conditions underscores the need for careful forest management practices, considering both the edaphic conditions and the species’ ecological requirements.
Overall, this review contributes to the current knowledge base on Aleppo pine in the Mediterranean, offering insights crucial for informed decision-making in forest conservation, afforestation strategies, and sustainable land management in the Mediterranean region.
References
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Hazandy Abdul-Hamid 0000-0002-3525-556x
Johar Mohamed 0000-0001-7330-8282
Moussa Masoud 0000-0002-6921-6016
Department of Forestry Science and Biodiversity, Faculty of Forestry and Environment, Universiti Putra Malaysia - UPM, Serdang 43400, Selangor (Malaysia)
Moussa Masoud 0000-0002-6921-6016
Department of Forests and Rangelands, Faculty of Natural Resources and Environmental Sciences, Omar Al-Mukhtar University, Albayda (Libya)
Institute of Tropical Forestry and Forest Products, Universiti Putra Malaysia, Serdang 43400, Selangor (Malaysia)
Corresponding author
Paper Info
Citation
Alsanousi AA, Abdul-Hamid H, Mohamed J, Masoud M (2025). Pinus halepensis Mill. in the Mediterranean region: a review of ecological significance, growth patterns, and soil interactions. iForest 18: 30-37. - doi: 10.3832/ifor4566-017
Academic Editor
Marco Borghetti
Paper history
Received: Jan 25, 2024
Accepted: Oct 23, 2024
First online: Feb 15, 2025
Publication Date: Feb 28, 2025
Publication Time: 3.83 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2025
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 20574
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 10187
Abstract Page Views: 6602
PDF Downloads: 3542
Citation/Reference Downloads: 5
XML Downloads: 238
Web Metrics
Days since publication: 361
Overall contacts: 20574
Avg. contacts per week: 398.94
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
(No citations were found up to date. Please come back later)
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Review Papers
Should the silviculture of Aleppo pine (Pinus halepensis Mill.) stands in northern Africa be oriented towards wood or seed and cone production? Diagnosis and current potentiality
vol. 12, pp. 297-305 (online: 27 May 2019)
Review Papers
Biomass, radial growth and regeneration capacity of Aleppo pine, and its possible use as rootstock in arid and degraded areas
vol. 15, pp. 213-219 (online: 16 June 2022)
Research Articles
Species interactions in pure and mixed-species stands of silver fir and European beech in Mediterranean mountains
vol. 14, pp. 1-11 (online: 02 January 2021)
Research Articles
Controlled-release fertilizers combined with Pseudomonas fluorescens rhizobacteria inoculum improve growth in Pinus halepensis seedlings
vol. 8, pp. 12-18 (online: 12 May 2014)
Research Articles
Growth dynamics and productivity of pure and mixed Castanea sativa Mill. and Pseudotsuga menziesii (Mirb.) Franco plantations in northern Portugal
vol. 7, pp. 92-102 (online: 18 December 2013)
Research Articles
Comparison of alternative harvesting systems for selective thinning in a Mediterranean pine afforestation (Pinus halepensis Mill.) for bioenergy use
vol. 14, pp. 465-472 (online: 16 October 2021)
Research Articles
The impact of post-defoliation foliage of Pinus halepensis Mill. on the larval performance of Thaumetopoea pityocampa and its relationship with the tree-induced defense
vol. 18, pp. 186-193 (online: 01 July 2025)
Research Articles
Tree-oriented silviculture: a new approach for coppice stands
vol. 9, pp. 791-800 (online: 04 August 2016)
Research Articles
Stand dynamics and natural regeneration in silver fir (Abies alba Mill.) plantations after traditional rotation age
vol. 7, pp. 313-323 (online: 08 April 2014)
Research Articles
Seasonal variations in monoterpene profiles and ecophysiological traits in Mediterranean pine species of group “halepensis”
vol. 1, pp. 65-74 (online: 28 February 2008)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword