Plant phenotype affects oviposition behaviour of pine processionary moth and egg survival at the southern edge of its range
iForest - Biogeosciences and Forestry, Volume 11, Issue 5, Pages 572-576 (2018)
doi: https://doi.org/10.3832/ifor2675-011
Published: Sep 01, 2018 - Copyright © 2018 SISEF
Research Articles
Abstract
Morphological traits of Aleppo pine (Pinus halepensis) needles in native and planted stands at the southern edge of its range influence oviposition behaviour of the pine processionary moth (Thaumetopoea pityocampa). Extreme environmental conditions result in a reduction in needle size of the host plant which corresponds to a lower rate of fecundity in the moth. Our results showed that egg batches were laid closer to the needle buds, especially on native trees with short needles, and this resulted in increased egg mortality. Number of eggs laid by the female moths did not vary between native and planted stands, nor did the number of parasitized eggs of the two common Hymenopteran parasitoids, Baryscapus servadeii and Ooencyrtus pityocampae. The observed differences in egg mortality are likely due to abiotic factors associated with the position of the egg batch on the needles. Thaumetopoea pityocampa eggs require a thermal niche for optimal development, and further measurements are required to determine the thermal threshold of these eggs. Understanding the role of climate in T. pityocampa populations will be an important factor for the survival of the Aleppo pine forests and protecting it from desertification.
Keywords
Algeria, Egg Parasitoid, Pinus halepensis, Plantation, Thaumetopoea pityocampa
Introduction
Aleppo pine forests (Pinus halepensis Mill.) are widely distributed in the Mediterranean region ([3]) and are highly susceptible to periodic attacks by the pine processionary moth Thaumetopoea pityocampa (Denis & Schiffermüller 1775, Lepidoptera: Notodontidae). This species is considered the most important defoliator of both native and artificial stands of pines and other conifers such as cedars ([8]); causing significant economic loss from attacked stands. Larvae of T. pityocampa also have urticating hairs which cause serious allergic reactions to humans and animals ([2]). Despite a number of studies on the biology and ecology of this species, its oviposition behaviour relating to the morphology of the host plant is poorly understood.
An earlier study determined that T. pityocampa selects pines with rapid growth, long needles and low content of secondary metabolites such as limonene, to ensure the offspring are provided with the best available resources ([13]). In this perspective, Aleppo pine offers better oviposition conditions for the processionary moth compared to the maritime pine Pinus pinaster Ait. ([14]). Thompson & Pellmyr ([22]) stated that the host trees selected by females should offer the highest nutritional content for the offspring, and this factor was important for the expansion of processionary moth populations in the Alps ([21]). Furthermore, olfactory and visual cues played an important role in the preference for oviposition on edge trees, as observed in pine plantations by Paiva et al. ([12]) and Dulaurent et al. ([5]). Additionally, Zhang et al. ([24]) studied the role of terpenes in host selection by female moths and determined that female antennae responded strongly to four monoterpenes, i.e., myrcene, b-phellandrene, trans-b-ocimene, and terpinolene. Another study stated that physical and chemical traits of the host plant can influence the selection of oviposition sites ([17]). Therefore, these findings may be important factors for the population dynamics of T. pityocampa ([7]).
Oviposition choice by female T. pityocampa moths may indirectly affect the activity of egg parasitoids. Two principal egg parasitoids are known for T. pityocampa, the specialist Baryscapus servadeii (Domenichini 1965, Hymenoptera: Eulophidae) and the generalist Ooencyrtus pityocampae (Mercet 1921, Hymenoptera: Encyrtidae - [1]). The generalist O. pityocampae has a more flexible host selection, it can adapt its development to hosts of different origins and size ([18]), while the specialist B. servadeii is more dependent on the quality of the host plant ([1]). Thus, the differences in the quality of host plant available for oviposition may influence the performance of parasitoids ([9]).
The hatching of pine processionary moth larvae may be affected by temperature and exposure to solar radiation ([10]). The temperature of egg batches with and without scales differed strongly in relation to the intensity of solar radiation in a pre-alpine valley located in Friuli, North-East of Italy, with higher temperature in egg batches with scales and exposed to the sun. In addition, the temperature of egg batches was remarkably higher than the temperature of the pine needle where the eggs were oviposited ([10]).
The aim of this study was to explore the effect of host plant needle traits on the oviposition of female pine processionary moths in native and artificial stands of Aleppo pine in Algeria. As pine needles under these extreme conditions are generally shorter than normal, we wanted to test if the needle traits affect the oviposition behaviour of the female moths; this was based on the hypothesis developed by Demolin ([4]) about the optimal thickness for oviposition. We also tested if oviposition choice had consequences on hatching performance, based on the study by Milani ([10]) about the role of temperature, solar radiation, and parasitism by the two major egg parasitoids.
Materials and methods
Study site
This study was conducted between 2012 and 2014 in a semi-arid area located in the Saharian Atlas Mountains (Algeria), around the town of Djelfa, 300 km south of Algiers, at an elevation of about 1250-1344 m a.s.l. (Tab. 1). This area is characterized by an annual average temperature of 8.4 °C and annual average precipitation of 324 mm (data from the national office of meteorology, Djelfa, Algeria; elevation 1144 m a.s.l., period 1974-1995). Soil is generally shallow in this topography and the bedrock is limestone. In this environment, P. halepensis grows naturally in isolated patches and plantations have been developed close to relic native stands. Stands are part of the “Barrage vert” afforestation plan, established from 1965 to stop desertification ([19]). Tree height from the stands varied between 3 and 4 m. The sites were severely attacked by T. pityocampa in the last decades ([23]). The population is genetically related to the pityocampa clade and shares mitochondrial DNA haplotypes with the populations of south Morocco, whereas the nuclear DNA is closer to the one of ENA clade of the eastern Maghreb ([6]). This T. pityocampa population is located at the most southern edge of its species range and has the lowest average fecundity within this species ([23]).
Tab. 1 - Location coordinates of the study sites.
| Stands | Sites | Elevation (m a.s.l.) |
Coordinates | |
|---|---|---|---|---|
| Longitude E | Latitude N | |||
| Planted | S1 | 1255 | 3° 23′ 18.79″ | 34° 36′ 39.22″ |
| S2 | 1344 | 3° 01′ 24.85″ | 34° 31′ 12.47″ | |
| S3 | 1257 | 3° 15′ 35.86″ | 34° 51′ 00.77″ | |
| Native | S4 | 1333 | 3° 08′ 11.25″ | 34° 38′ 07.19″ |
| S5 | 1339 | 2° 46′ 40.31″ | 34° 32′ 59.56″ | |
| S6 | 1291 | 3° 23′ 30.96″ | 34° 55′ 56.85″ | |
Egg batches collection
Egg batches of T. pityocampa were collected from trees growing in three native and three planted stands (Tab. 1) between the end of August and beginning of September each year for 2 years. Eggs started hatching in the laboratory from around mid-September. In total, 964 needle pairs carrying egg batches were randomly collected at an accessible height from P. halepensis distributed throughout the stands, with a maximum of three egg batches collected per tree. In 2012, a total of 242 egg batches were collected (32 from S1, 106 from S2, 34 from S4, and 70 from S5). In 2014, 842 egg batches were collected (217 from S1, 176 from S2, 60 from S3, 160 from S4, 169 from S5, and 62 from S6). At the laboratory, egg batches on the needle pairs were removed and put in individual test tubes of 10 cm length and 1 cm diameter. Laboratory temperature was set at 26 ± 2 °C, and the emergence of egg parasitoids from these egg batches were closely monitored. Ninety needle pairs per site without egg batches from the same trees were collected as a control for analysing the morphology of the needles.
Analysis of needles
Position of the egg batch on the needle pair may vary from basal to central or apical. To assess the needle traits in each section, the needles were divided into three parts. Basal part was characterised as the leaf bud to the first egg of the egg batch, medium part was the area covered by the whole egg batch, and apical part was the area above the egg batch. Each part was measured for length, diameter, and dry weight. Length and diameter of randomly chosen control needles were also measured.
Analysis of egg batches
At the end of the parasitoids emergence (spring from the following year of collection), all egg batches were analysed to determine the percentage of hatched and unhatched T. pityocampa eggs, and rate of egg parasitism. Eggs were assigned to the following categories: successfully hatched (presence of a large hole and empty shell), unhatched eggs (intact egg shell), hatched failure (egg shell open as for successfully hatched but with a dead larva inside), successful parasitism by O. pityocampae (egg shell with a small hole not in the centre and with an irregular edge), and successful parasitism by B. servadeii (egg shell with a small hole in the centre and with a regular edge).
Statistical analysis
Analyses were done using a linear mixed model to account for the effects of stand type and distance of the egg batch from the needle bud, on the dependent variables (log transformed number of unhatched eggs, and percentage of eggs parasitized by O. pityocampae and by B. servadeii). Interaction among factors was tested to detect if egg mortality was related to distance of the egg batch from the needle bud in native and planted stands. Trees within sites were used as a random effect. All statistical tests were made using the package “nlme” ([16]) in R ver. 3.2.0.
Results
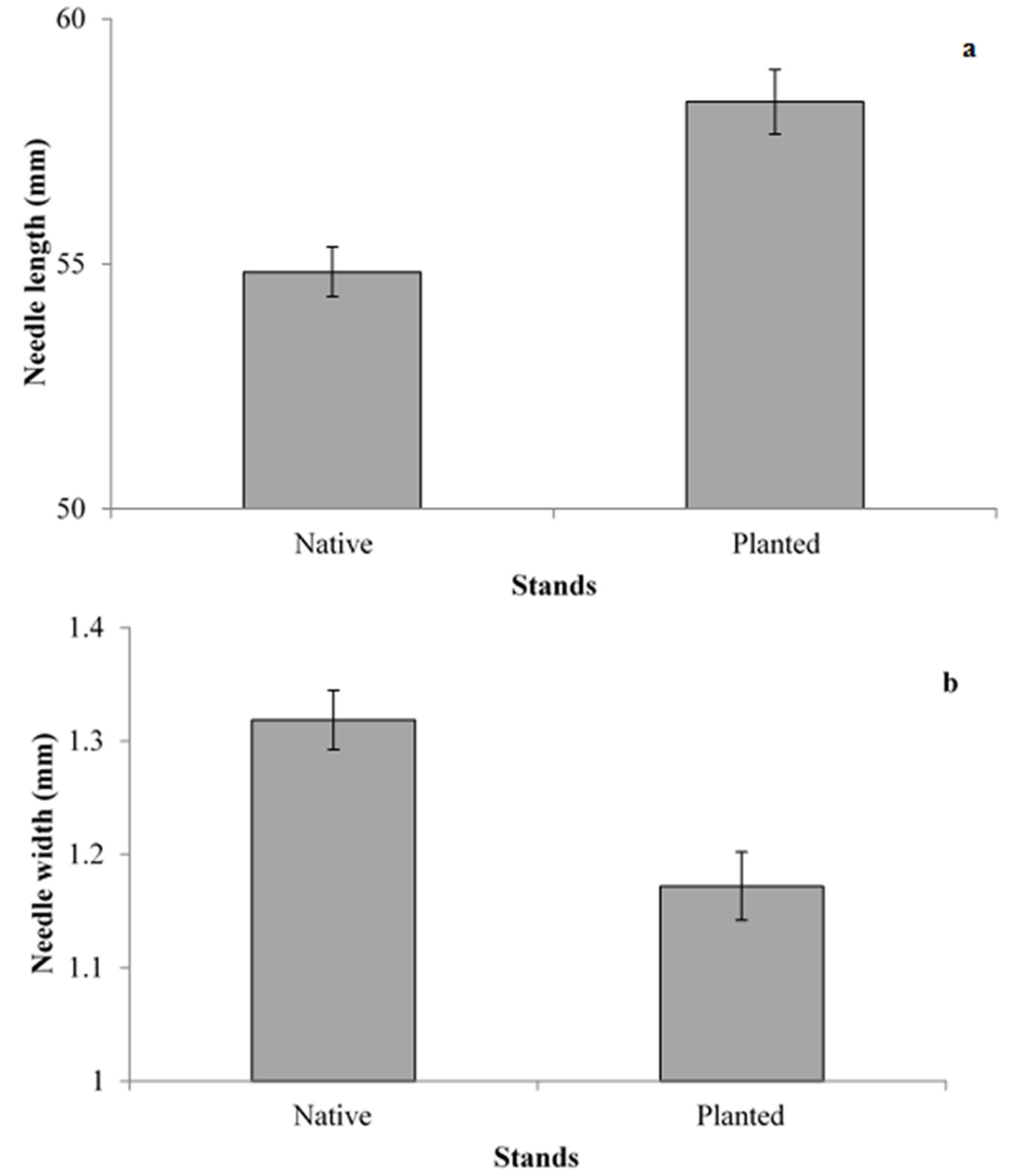
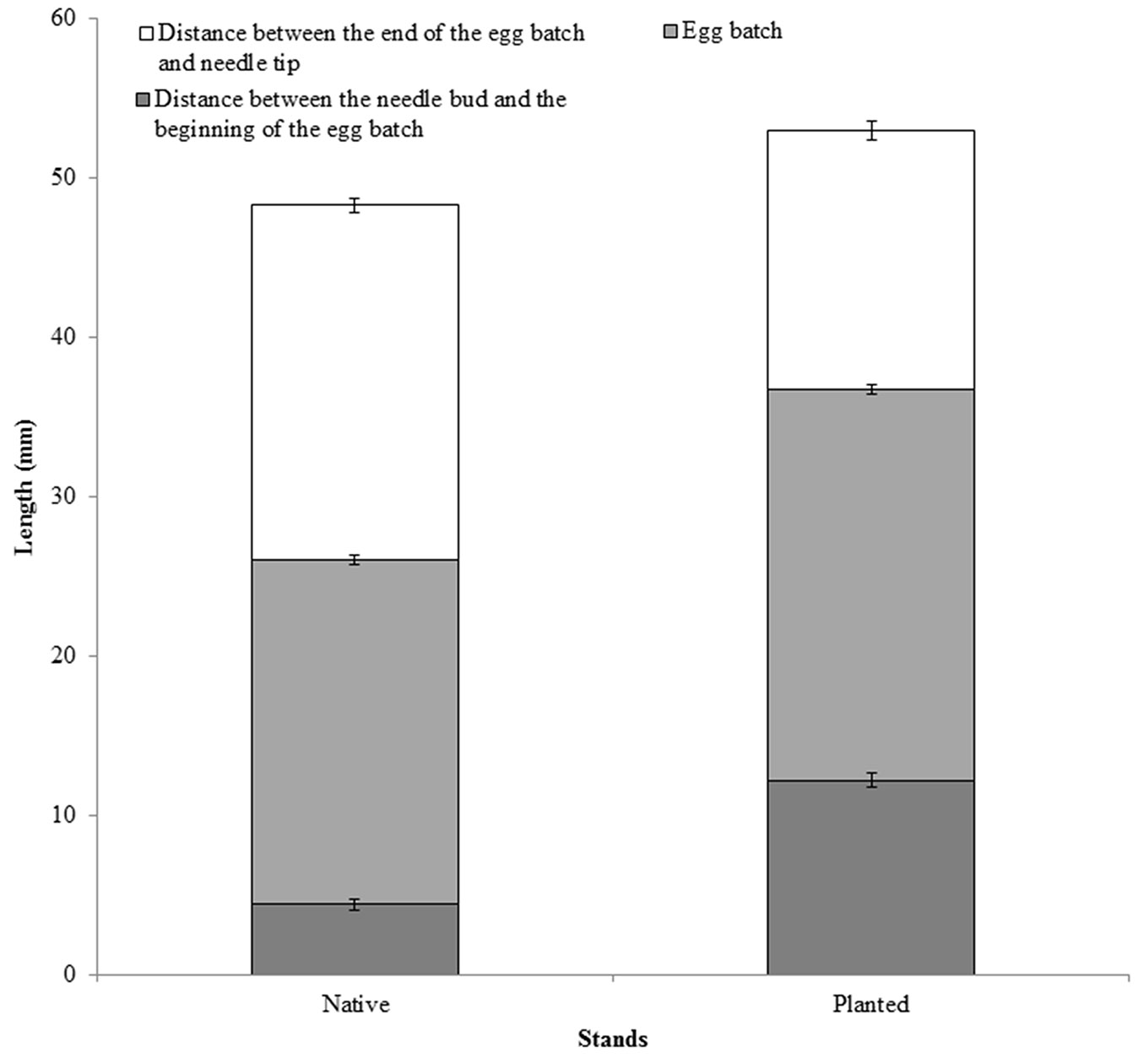
Two years of sampling did not result in significant differences between the types of stand for any of the variables considered, and for this reason the data were pooled. Needle pairs without egg batches were slightly longer and heavier in planted than in native stands (Fig. 1a), although differences were marginally significant (p = 0.08). Thickness of the needles, measured as needle width, differed significantly between the stands (F1, 172 = 13.7, p < 0.01 - Fig. 1b). Distance between the needle bud and the beginning of the egg batch was not significantly different between native and planted stands, even though the egg batches tend to be closer to needle buds in native stands (F1, 165 = 1.14, p = 0.397 - Fig. 2). As needles from planted stands were slightly longer and thinner than those of the native stands, the section of the needles carrying eggs was longer in planted than in native stands (F1, 499 = 33.38, p < 0.01 - Fig. 2), while the average number of eggs per egg batch was similar between planted and native stands (Tab. 2).
Fig. 1 - Length (a) and width (b) of needles (± SE) from trees growing in native and planted stands.
Fig. 2 - Distance between needle bud and the beginning of the egg batch, length of the section carrying the eggs (= length of the egg batch), and distance between egg batch and the needle tip in native and planted stands (± SE).
Tab. 2 - Characteristics of the egg batches laid on trees of native and planted stands (average ± SE).
| Parameters | Stands | |
|---|---|---|
| Native | Planted | |
| Number of egg batches | 104 | 138 |
| Number of eggs | 134.6 ± 3.5 | 135.3 ± 2.8 |
| Successfully hatched eggs | 87.0 ± 5.3 | 107.2 ± 2.7 |
| Hatched eggs with dead larvae | 15.6 ± 2.2 | 3.5 ± 0.5 |
| Unhatched eggs | 41.7 ± 3.7 | 13.1 ± 0.6 |
| Eggs parasitized by O. pityocampae | 3.3 ± 0.4 | 2.1 ± 0.07 |
| Eggs parasitized by B. servadeii | 7.1 ± 0.6 | 7.0 ± 0.5 |
| % of O. pityocampae | 2.4 | 1.5 |
| % of B. servadeii | 5.3 | 5.2 |
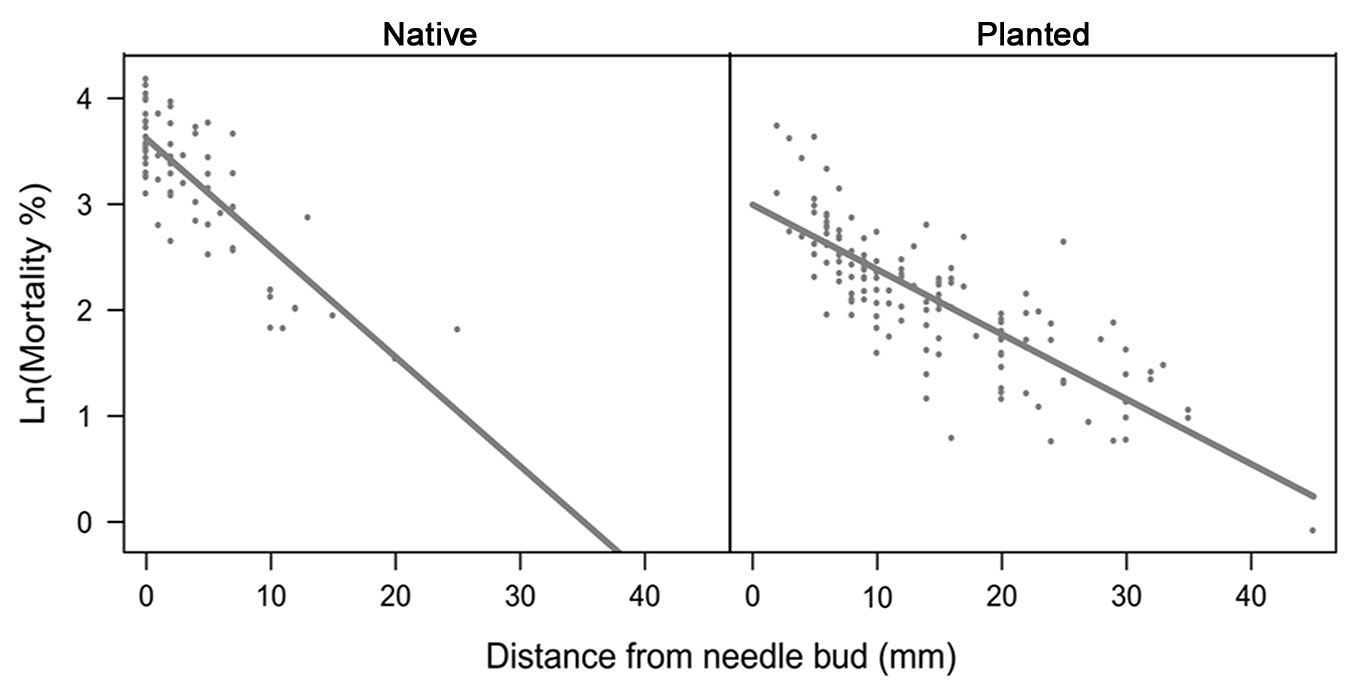
Overall egg mortality (including all factors shown in Tab. 2) decreased significantly with the increase in distance of the egg batch from the needle bud, for both native and planted stands (F1, 165 = 251.64, p < 0.01), indicating that egg mortality was higher when eggs were laid closer to the needle bud. Interestingly, slope of the regression was significantly higher in native than in planted stands (F1, 165 = 13.25, p < 0.01 - Fig. 3), suggesting a stronger role of mortality factors in native stands.
Fig. 3 - Relationships between the number of unhatched eggs and distance between needle bud and the beginning of the egg batch in native and planted stands. Dots represent the individual egg batches.
Number of eggs successfully parasitized by O. pityocampae (overall 1.9%) and by B. servadeii (overall 5.2%) did not differ significantly between the two stands, as well as the distance of egg batch from the needle bud (Tab. 2).
Discussion
At the very southern edge of the range of both the host plant (P. halepensis) and its main defoliating insect (T. pityocampa), extreme conditions are imposing a drastic reduction in P. halepensis needle size and T. pityocampa fecundity, as observed by Mirchev et al. ([11]) and Zamoum et al. ([23]). This observation is not surprising considering the low amount of precipitation (less than 400 mm, semi-arid zone) and high temperatures during summer. Interestingly, we have found that there were morphological differences in pine needles between native and planted stands which affected egg mortality rates and oviposition patterns of the pine processionary moth.
Egg mortality of T. pityocampa was lower when eggs were laid on pine needles far from the needle bud in both native and planted stands; effects were more pronounced in the latter, likely because the needles were slightly longer and thinner than in native stands. As number of eggs laid by female moths did not vary between the types of stands, the observed differences are likely to be explained by the needle size. According to Demolin ([4]), the ideal diameter of the needle pair for suitable oviposition varies from 1.6 to 2 mm; while in our case, the diameter of the needle pair did not exceed 1.4 mm. Female fecundity, however, is proportionally lower at the southern edge of the range (130-140 eggs per female - this study and [23]) compared to the core range (200-240 eggs per female - [4], [15]). As female moths respond to small variations in needle diameter and inspect pine needles from the base ([4]), it is likely that they found optimal conditions for ovipositing at a distance from the base on longer needles. Our needles were thinner but the number of eggs per female were similar. Therefore, T. pityocampa eggs were laid over a longer distance compared to the general oviposition behaviour of this species. Based on the same principle, Pimentel et al. ([15]) also found that needle morphology affects the length of egg batches.
Position of the eggs on needles had some effects on the performance of pine processionary moth. Mortality was generally higher in egg batches laid closer to the base of the needles, irrespective of the type of stand. Stronger effects of mortality observed in native stands is possibly due to its shorter and thicker needles, which lead to the egg batch being more compact and closer to the needle bud. There were no significant effects of parasitism on mortality rates of T. pityocampa in both stands. A likely explanation for this observed mortality is the abiotic conditions that may have impaired embryonic development and egg hatching. Milani ([10]) observed that the scales laid by the female moth to cover the eggs may help to capture solar radiation and significantly increase the temperature of the eggs, compared to egg batches in the shade or egg batches without scale cover. Based on these observations, position of the eggs on the needles could be a factor affecting the amount of solar radiation it receives and thus result in different temperatures. Additionally, shape of the egg batch would also influence the way solar radiation affects the temperature of eggs. Therefore, both position and shape of the egg batch would influence the cooling effect associated with ventilation of the pine shoot. Temperature is known to affect embryonic development in the pine processionary moth, and differences are already detected among populations ([20]). Consequently, oviposition pattern may have affected the egg survival in this population at the southern edge of the range under rather extreme conditions. Measuring temperatures experienced by the eggs in native and planted stands are required to establish the causes of difference in mortality rates.
In conclusion, we suggest that egg mortality is associated with distance of the egg batch from the needle bud. Egg mortality from egg parasitoids O. pityocampae and B. servadeii did not vary with distance of the egg batches from the needle bud; therefore, other mortality factors have to be invoked. Among these, abiotic factors associated with thermoregulation should be considered, because of the extreme conditions these eggs are exposed to. Specific measures of egg thermal niche should be taken in the field and laboratory to understand the observed pattern and to use the data for predictive models on egg mortality. This will be important for the survival of pine forests in this region, and it is essential for combating desertification and providing local communities with essential services.
Acknowledgements
The authors thank Lorenzo Marini for helping with the statistical analysis and University of Padova, DAFNAE for hosting the first author scientific visit. Special thanks to Ahmad A.D. Alshashani for his advice and suggestions, to Mustafa Avci for his comments, to anonymous reviewers who helped to improve the manuscript, and to Mizuki Uemura for language check and editing.
References
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Department DAFNAE-Entomology, University of Padova, Agripolis, I-35020 Legnaro, PD (Italy)
Corresponding author
Paper Info
Citation
Hezil S, Chakali G, Battisti A (2018). Plant phenotype affects oviposition behaviour of pine processionary moth and egg survival at the southern edge of its range. iForest 11: 572-576. - doi: 10.3832/ifor2675-011
Academic Editor
Massimo Faccoli
Paper history
Received: Nov 03, 2017
Accepted: Jul 03, 2018
First online: Sep 01, 2018
Publication Date: Oct 31, 2018
Publication Time: 2.00 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 44750
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 37644
Abstract Page Views: 3034
PDF Downloads: 3073
Citation/Reference Downloads: 4
XML Downloads: 995
Web Metrics
Days since publication: 2720
Overall contacts: 44750
Avg. contacts per week: 115.17
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2018): 4
Average cites per year: 0.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
The impact of post-defoliation foliage of Pinus halepensis Mill. on the larval performance of Thaumetopoea pityocampa and its relationship with the tree-induced defense
vol. 18, pp. 186-193 (online: 01 July 2025)
Research Articles
Abundance and impact of egg parasitoids on the pine processionary moth (Thaumetopoea pityocampa) in Bulgaria
vol. 14, pp. 456-464 (online: 02 October 2021)
Research Articles
Effects of defoliation by the pine processionary moth Thaumetopoea pityocampa on biomass growth of young stands of Pinus pinaster in northern Portugal
vol. 3, pp. 159-162 (online: 15 November 2010)
Research Articles
Performances of an expanding insect under elevated CO2 and snow cover in the Alps
vol. 1, pp. 126-131 (online: 27 August 2008)
Research Articles
The physicomechanical and thermal properties of Algerian Aleppo pine (Pinus halepensis) wood as a component of sandwich panels
vol. 15, pp. 106-111 (online: 21 March 2022)
Research Articles
Wildfires in Algeria: problems and challenges
vol. 8, pp. 818-826 (online: 25 March 2015)
Research Articles
Controlled-release fertilizers combined with Pseudomonas fluorescens rhizobacteria inoculum improve growth in Pinus halepensis seedlings
vol. 8, pp. 12-18 (online: 12 May 2014)
Research Articles
Analysis of forest fires causes and their motivations in northern Algeria: the Delphi method
vol. 6, pp. 247-254 (online: 13 June 2013)
Research Articles
Seasonal variations in monoterpene profiles and ecophysiological traits in Mediterranean pine species of group “halepensis”
vol. 1, pp. 65-74 (online: 28 February 2008)
Research Articles
Pinus halepensis Mill. in the Mediterranean region: a review of ecological significance, growth patterns, and soil interactions
vol. 18, pp. 30-37 (online: 15 February 2025)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword