Stomatal morphometry of Andean species and their relationship with spatial variation
iForest - Biogeosciences and Forestry, Volume 18, Issue 6, Pages 327-334 (2025)
doi: https://doi.org/10.3832/ifor4689-018
Published: Nov 03, 2025 - Copyright © 2025 SISEF
Research Articles
Abstract
Stomata play a fundamental role in maintaining plant homeostasis by regulating the balance between water loss and CO2 absorption required for photosynthesis. Analysis of stomatal traits across environmental gradients provides insight into how plant species are uniquely adapted to their environment and their potential response to environmental change. In the Andes, there is limited information on how stomatal traits vary spatially and with climatic conditions. This study aimed to characterize the morphometry of Andean woody species and corroborate the relationship of stomata characteristics with leaf traits in response to environmental variation. We measured the following traits on each species: stomatal size (SS), stomatal width (SW), stomatal density (SD), stomatal relative area (SRA), leaf thickness (LT), leaf area (LA), and specific leaf area (SLA). These traits were then analyzed to determine their relationships with mean annual temperature (MAT), mean annual precipitation (MAP), mean solar radiation (MSR), latitude, and longitude at the sampling sites. Our analyses showed that stomatal traits largely vary among species. Some species (e.g., Morella pubescens) exhibit high plasticity, suggesting greater resilience to unfavorable climatic conditions. However, the relationships with MAP, MSR, and MAT varied, suggesting that these species employ different mechanisms to avoid water stress and optimize water use. Moreover, we observed relationships between stomatal traits, particularly SS and SW, with latitude and longitude. Similarly, we identified significant correlations between leaf and stomatal traits. Our results suggest that the functional traits of stomata in the individual evaluated from these species respond to their geographic origin and, therefore, to the climatic conditions of their habitat.
Keywords
Stomatal Density, Stomatal Size, Stomatal Conductance, Plasticity, Functional Traits, Andean Woody Species
Introduction
Climate change is altering plant communities along with the associated processes and functions of ecosystems ([38]). Investigating plant functional traits, particularly the relationship between trait variation and climatic factors, can yield valuable insights into the potential impacts of these changes on critical ecosystem functions, such as the regulation of carbon and water cycle ([49]). Even the functional traits of vegetation in mountain forests are already being affected by the unusual temperature increase ([15]). Leaf traits are particularly significant in this context ([47]), as stomata are essential for maintaining plant homeostasis through gas exchange and regulating water loss and CO2 uptake ([24]). This implies that it supports ecological processes vital to the balanced functioning of ecosystems ([38]).
Previous studies have indicated species-specific variation in stomatal morphometric characteristics, such as stomatal length (SL), width (SW), size (SS), and density (SD) ([2], [11]). These traits often reflect the prevalent environmental conditions of the habitats where plants have evolved ([9]). Stomatal traits may also evolve at different rates across species, with some showing relatively rapid changes and others exhibiting little to no change over millions of years ([42]). Nevertheless, traits and environmental patterns can be observed at the individual level ([30]), despite the considerable variation existing at the species level. This suggests that the strongest relationships are likely to emerge from analyses conducted at the individual level ([1]). Consequently, an improved understanding of how stomatal traits evolve in response to environmental variation is essential for predicting plant responses to climate change.
Research on forest plant species has demonstrated that stomatal traits can be highly plastic ([18]). For example, some studies indicate that SD is directly proportional to light intensity, including blue wavelengths ([23]), and inversely proportional to CO2 levels in the environment ([48]). Similarly, water stress has been shown to influence SL and SS in some species ([29]), whereas stomatal relative area (SRA) is associated with solar radiation, latitude, and longitude ([27]). Some studies showed that temperature can influence stomatal traits (e.g., SL and SD), whereas others did not ([41]). Plants can also adjust stomatal size and density according to their spatial position within the habitat ([14]), resulting in substantial variation in stomatal traits among forest species ([5]). Overall, these findings make it challenging to conclusively relate patterns of stomatal density and their distribution in the leaf to environmental variables ([34]).
Likewise, stomata can also be related to the functional traits of the leaves themselves, such as leaf area and specific leaf area. Indeed, previous research reported that stomatal size and density are correlated with leaf area ([37]). Stomata also show a relationship with leaf thickness, as most stomata are located on the underside of leaves, where gas exchange mainly occurs ([39]). Additionally, there is a relationship of stomata characteristics with other traits such as plant height and petiole size (not studied here), that allow the plant to receive solar radiation, thereby increasing SD, SL, and therefore, SS ([20]).
Previous research on stomata has been conducted in Alnus ([12]) and Hedyosmum spp. ([22]), but not in native Andean species. However, these studies have been primarily descriptive and have not attempted to relate climatic or spatial variables to functional traits. The Andes host an array of diverse ecosystems and habitats, including forests under different climatic conditions. Understanding how stomata interact with the climate in these habitats will help us better understand plant adaptations and responses to these varying environmental conditions. This research aims to (i) characterize the morphometry of nine native Andean woody species throughout a wide altitudinal gradient, (ii) confirm stomatal and leaf traits correlation in response to environmental variation. We hypothesize that stomatal traits show environmental plasticity, and leaf traits, including stomatal traits, respond to environmental changes. The results of this study will improve our understanding of how these species may respond to a rapidly changing climate.
Materials and methods
Study site
The study was carried out from January to March 2019 in four forest sites: Llaviucu, Zhurucay, Angas, and Miguir, located on the two slopes of the western Andes cordillera in the province of Azuay, southern Ecuador (Fig. 1). The study sites are distributed along an altitudinal gradient ranging from 2800 to 3500 meters a.s.l.
Species collection
Samples of nine native species were collected on two slopes of the western cordillera in Azuay. The nine species selected are ecologically important to the Andean forest and are distributed across the four study sites. Five of these species were trees (a total of 100 individuals), and four were shrubs (a total of 80 individuals - Tab. 1). The individuals were randomly selected, with a minimum distance of 100 m between individuals of the same species. Leaf samples were collected from branches exposed to solar radiation. For each individual, a branch with leaves was cut, placed in a moistened plastic bag ([33]), and taken to the Laboratory of Seeds and Forest Ecology at the University of Cuenca for processing.
Tab. 1 - Type of the nine studied species, number of individuals, and number of images analyzed for each species.
| Code | Species | Individuals | Images | Type |
|---|---|---|---|---|
| Aa | Alnus acuminata Kunth | 20 | 100 | Tree |
| Bs | Brugmansia sanguinea (Ruiz & Pav.) D. Don | 20 | 100 | Shrub |
| Em | Escallonia myrtilloides L. f | 20 | 100 | Tree |
| Hl | Hedyosmum luteynii Todzia. | 20 | 100 | Tree |
| Mp | Morella pubescens Benth. | 20 | 100 | Shrub |
| Mr | Myrcianthes rhopaloides (Kunth) McVaugh | 20 | 100 | Tree |
| Og | Oreocallis grandiflora (Lam.) R.Br. | 20 | 100 | Shrub |
| Vs | Vallea stipularis L.f | 20 | 100 | Shrub |
| Wf | Weinmannia fagaroides Kunth | 20 | 100 | Tree |
Quantification of stomatal functional traits
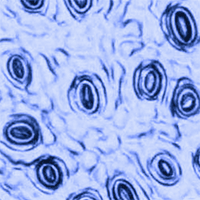
Stomatal data were obtained by applying transparent nail polish or cyanoacrylate glue on the underside of each leaf. Once the nail polish was dry, the epidermis layer was removed and placed on a slide marked with sample information ([40]). For each species, 100 images (one per leaf, totaling 900 images - Fig. 2) were processed using a microscope (Olympus™, ⇒ https://www.evidentscientific.com/) at 100× magnification, equipped with a camera (Lumenera™ - ⇒ https://www.lumenera.com/) and image capture software (Infinity Analyze v. 7). Stomatal length, width, and size were measured following Cai et al. ([3]). To measure stomatal density (SD), the number of stomata was counted and extrapolated to 1 mm2. For each image, the SL and SW of five randomly selected stomata were measured and averaged using ImageJ (⇒ https://imagej.net/ij/ - [31]).
Fig. 2 - Stomata of the nine species selected. (a): Alnus acuminata; (b): Brugmansia sanguinea; (c): Escallonia myrtilloides; (d): Hedyosmum luteynii; (e): Morella pubescens; (f): Myrcianthes rhopaloides; (g): Oreocallis grandiflora; (h): Vallea stipularis; (i:) Weinmannia fagaroides.
The stomatal relative area (SRA) was also quantified. The SRA represents the anatomical restriction on the maximum gas exchange capacity of the leaf, which determines the maximum stomatal conductance. The SRA was calculated as follows ([37] - eqn. 1):
Leaf functional traits
Leaf thickness (LT) was measured with a digital caliper ([26]). Leaf area (LA) was obtained by scanning the leaf, and the area was measured in ImageJ ([46]). For specific leaf area (SLA), the sample was placed in an oven for 72 hours at 60 °C, weighed, and the dry weight divided by LA ([33]).
Climate and spatial data
Mean annual temperature (MAT), mean annual precipitation (MAP), and mean daily solar radiation (MSR) were extracted from TIF images from WoldClim (⇒ https://www/⇒ https://www.worldclim.org/). The data covers the years 1970 to 2000, and the images have a pixel size of approximately 1 km2 ([4]). Latitude and longitude were obtained in situ using a GPS device.
Statistical analysis
To describe the stomatal traits of woody species, we used metrics of central tendency and dispersion. To confirm the effects of climate, spatial variables, and leaf traits individually on SS, SD, SRA, and SW, mixed linear models (LMMs) were fitted, with species/forest as a nested random effect, and marginal R2 was evaluated ([25]). Also, we assessed the interaction of climate variables and leaf traits separately on stomatal functional traits using LMM ([25]). Scatter plots were constructed to represent each stomatal relationship with climate factors. Statistical analyses and climate data extraction were performed in R ([35]) using the lme4 library ([45]).
Results
The species exhibiting the highest mean stomatal size (SS) was H. luteynii (474.8 ± 197.9 µm2), which also had the maximum stomatal size (926.2 µm2). In contrast, the species showing the lower SS were V. stipularis and M. pubescens (76.3 ± 51.7 and 105.2 ± 79.4 µm2, respectively). The above three species also recorded the largest and smallest stomatal lengths (SL: 34.4 ± 6.9, 14.3 ± 3.7, and 12.4 ± 4.6 µm, respectively) and widths (SW: 16.9 ± 4.0, 6.3 ± 3.9, and 9.4 ± 4.2 µm, respectively), and a coefficient of variation (CV) ranging from 0.2 to 0.3. Likewise, H. luteynii and O. grandiflora had the lowest mean stomatal density (SD: 132.7 ± 28.9 and 237.4 ± 37.2 mm2, respectively). In addition, V. stipularis, B. sanguinea, and O. grandiflora exhibited the lowest stomatal relative area (SRA: 3.3%, 4% and 4.4%, respectively). Conversely, M. pubescens showed a stomatal density (SD) of 652.2 per mm2 and a CV of 0.4, making it the species with the highest values for this trait. Finally, E. myrtilloides had the largest SRA (7.7 %), while M. pubescens showed the largest CV (0.6 - Tab. 2).
Tab. 2 - Measures of central dispersion of the four stomatal traits for each species. (STD): standard deviation; (CV): coefficient of variation. For species labels, see Tab. 1.
| Species | Stomatal Size SS (µm2) |
Stomatal Density SD (mm2) |
Stomatal relative area - SRA (%) | Stomatal Length SL (µm) |
Stomatal Width SW (µm) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ±STD |
CV | Min -Max |
Mean ±STD |
CV | Min -Max |
Mean ±STD |
CV | Min -Max |
Mean ±STD |
CV | Min -Max |
Mean ±STD |
CV | Min -Max |
|
| Aa | 187.3±101 | 0.5 | 44.7-366.9 | 380.5±112.4 | 0.3 | 201.5-622 | 6.1±1.7 | 0.3 | 3.7-10.2 | 22.2±4.5 | 0.2 | 13.4-28.8 | 10.4±4.4 | 0.4 | 2.5-16.7 |
| Bs | 155.1±87.6 | 0.6 | 2.6-273.9 | 278.3±68.4 | 0.2 | 171.4-407.8 | 4±1.7 | 0.4 | 1.7-8.7 | 22.8±6.9 | 0.3 | 10.5-34.4 | 8.1±4.2 | 0.5 | 0.3-14.4 |
| Em | 202±69.4 | 0.3 | 65.9-297.7 | 457.3±102.2 | 0.2 | 312.3-708 | 7.7±2.3 | 0.3 | 4.4-13.6 | 22.5±3 | 0.1 | 17.5-28.6 | 11.5±3.9 | 0.3 | 4.7-16.5 |
| Hl | 474.8±197.9 | 0.4 | 159.8-926.2 | 132.7±28.9 | 0.2 | 75.5-196.7 | 6.2±1.8 | 0.3 | 3.6-9.7 | 34.4±6.9 | 0.2 | 21.9-47.6 | 16.9±4 | 0.2 | 9.3-24.8 |
| Mp | 105.2±79.4 | 0.8 | 14.9-256.2 | 652.2±275.6 | 0.4 | 280-1122.3 | 6.8±4.2 | 0.6 | 2.3-16 | 12.4±4.6 | 0.4 | 6.3-20.5 | 9.4±4.2 | 0.4 | 3-17.1 |
| Mr | 116.9±80.3 | 0.7 | 18.3-268.4 | 647.6±111.9 | 0.2 | 420.6-827.8 | 6.6±2.5 | 0.4 | 3.6-12.6 | 15.5±5.2 | 0.3 | 5.9-22.6 | 8.6±4.4 | 0.5 | 2.9-16.4 |
| Og | 207.1±86.7 | 0.4 | 49.7-334 | 237.4±37.2 | 0.2 | 167.4-308 | 4.4±1.7 | 0.4 | 2.1-8.4 | 24±4 | 0.2 | 17.2-30.1 | 10.8±3.8 | 0.3 | 2.8-15.3 |
| Vs | 76.3±51.7 | 0.7 | 3.4-156.6 | 561.4±170.2 | 0.3 | 346.4-975.8 | 3.3±1.1 | 0.3 | 1.7-5.6 | 14.3±3.7 | 0.3 | 5.1-18.1 | 6.3±3.9 | 0.6 | 0.4-11.3 |
| Wf | 131.9±84.9 | 0.6 | 12-251.5 | 540±113.5 | 0.2 | 331.1-735.3 | 6±2.1 | 0.4 | 2.8-10.5 | 16.7±6 | 0.4 | 5.7-23.5 | 9.1±4.5 | 0.5 | 2.7-14.7 |
Climatic variables
Linear regression analysis revealed that most pairwise relationships between stomatal functional traits and climatic variables were significant, though weak and positive, except for mean annual precipitation (MAP). However, the distribution of climatic data was unequal across the range analyzed, with a limited number of records with precipitation > 900 mm (Fig. 3c, Fig. 3f, Fig. 3i). The temperature trend for all stomatal traits was negative, with a marginal R2 < 0.10, with exception of SL (R2 = 0.16, p-value < 0.001 - Fig. 3i). As for SRA and SW, it was not statistically significant (Fig. 3h, Fig. 3j). The MSR showed a positive relationship (p-value < 0.05) with SS (Fig. 3a), SD (Fig. 3b), and SW (Fig. 3d), with a cluster of data points around 14.000 KJ m-2 day-1. Similar results were obtained for the relationship between climatic variables and leaf traits (Fig. S1 in Supplementary material).
Leaf functional traits
All stomatal traits demonstrated dependence on leaf area (LA); specifically, the regression of this trait with SD showed an R2 of 0.04 (Fig. 4b), while SW had an R2 of 0.13 (Fig. 4e). Also, a significant relationship between SLA and SD, SRA, and SL was found, however, the variance explained (R2) values were >0.04 (Fig. 4f-Fig. 4j). On the contrary, Fig. 4k to Fig. 4o indicates that LT is independent of stomatal traits. Additionally, Fig. 4shows that most data lie on the left side of the scatter plots of leaf traits versus stomatal traits.
Spatial variation and patterns
We found significant associations of spatial variables with four stomatal traits (Fig. 5). All relationships between stomatal characteristics and latitude were negative, whereas those with longitude were positive. LMMs revealed a dependence of SS (Fig. 5a), SD (Fig. 5b), and SW (Fig. 5e) on latitude; this last model had an R² of 0.15. A similar trend was observed for the longitude, which revealed a significant association with SL. Likewise, the best fit (R2 = 0.33) was achieved with SW (Fig. 5j). Furthermore, the unique stomatal trait showing no significant relationship with geographic coordinates of the sampling sites was the stomatal relative area (SRA - Fig. 5c, Fig. 5h).
Climatic variables and leaf traits models
The analysis of climatic variable interactions indicated that stomatal density (SD) responded to all three climatic variables analyzed (p-value < 0.05), whereas SL and SW showed an exclusive dependence on MAT (mean annual temperature) and MAP (mean annual precipitation). In leaf trait models, most stomata variables interact with specific leaf area (SLA) and, to a lesser extent, with leaf area (LA) and thickness (LT). In addition, in the performed models, the random effect for species/forest was found to be statistically significant. On the contrary, SRA showed no association with climatic variables, whereas SD did not relate to any predictor. However, the variance explained (R2) in each case was relatively low (2% to 18%), while the ICC ranged from 0.1 to 0.8; the latter value suggests that our results are due to the interaction between species/forest (Tab. 3).
Tab. 3 - LMM performed for each stomatal trait with climatic and leaf variables. (df): Degrees of freedom; (STD): standard deviation; (ICC): intraclass correlation coefficient; (MAT): mean annual temperature; (MAP): mean annual precipitation; (SLA): specific leaf area; (LA): leaf area; (LT): leaf thiskness; (***): p<0.001; (**): p<0.01; (*): p<0.05; (NS): not significant.
| Group | Trait | Predictors | df | F | Var exp (%) | Random Effect | ICC | STD | σ2 |
|---|---|---|---|---|---|---|---|---|---|
| Environmental | SS | MAT × log(MAP) NS | 1 | 0.01 | 11 | Species/Forest | 0.82 | 56.95 | 4169.80 |
| Residuals | - | 66.07 | - | ||||||
| SD | MAT × log(MAP) × log(MSR)* | 1 | 0.98 | 12 | Species/Forest | 0.87 | 99.75 | 6230.50 | |
| Residuals | - | 78.93 | - | ||||||
| SRA | MAT × log(MAP) NS | 1 | 0.20 | 4 | Species/Forest | 0.28 | 1.41 | 3.48 | |
| Residuals | - | 1.86 | - | ||||||
| SL | MAT × log(MAP)** | 1 | 4.45 | 17 | Species/Forest | 0.10 | 2.51 | 11.05 | |
| Residuals | - | 3.32 | - | ||||||
| SW | MAT × log(MAP)* | 1 | 4.31 | 2 | Species/Forest | 0.66 | 3.99 | 3.45 | |
| Residuals | - | 1.86 | - | ||||||
| Leaf traits | SS | log(SLA) × LT NS | 1 | 0.36 | 2 | Species/Forest | 0.85 | 122.10 | 7403.30 |
| Residuals | - | 86.04 | - | ||||||
| SD | LA × log(SLA) × LT** | 1 | 3.52 | 12 | Species/Forest | 0.83 | 98.91 | 3807.60 | |
| Residuals | - | 61.71 | - | ||||||
| SRA | LA × log(SLA) × LT** | 1 | 5.15 | 14 | Species/Forest | 0.47 | 1.36 | 3.43 | |
| Residuals | - | 1.85 | - | ||||||
| SL | LA × log(SLA)** | 1 | 3.37 | 11 | Species/Forest | 0.86 | 5.08 | 10.17 | |
| Residuals | - | 3.19 | - | ||||||
| SW | LA × LT** | 1 | 7.38 | 8 | Species/Forest | 0.85 | 4.27 | 3.37 | |
| Residuals | - | 1.84 | - |
Discussion
Examining the coefficients of variation for each stomatal trait revealed relative differences in plasticity among the nine species analyzed in our study ([32]). Our results indicate that M. pubescens has the greatest plasticity of four out of five stomatal traits, while V. stipularis was the most plastic in terms of stomatal width. Species with larger stomata tend to exhibit reduced stomatal density, thereby facilitating more efficient regulation of water and gas exchange ([17]). This trend was evident in the tree species H. luteynii. Species with reduced size of stomata tend to increase their density, as is the case with M. pubescens (Tab. 2). This suggests that this species may be better adapted to the climatic conditions of its habitat and potentially more resilient to climate change impacts.
The stronger relationships observed in this study were between stomatal traits and leaf functional traits (LT) and MAP. In contrast, the relationships with the other climatic and spatial variables appeared to be less robust (Fig. 3, Fig. 4, Fig. 5). Leaf area (LA) was related to all stomatal traits, likely due to the fact that a larger leaf area could host larger stomata. However, this leads to a reduced stomatal density or width, and these patterns were found in our study ([24], [31]). This indicates that correlation exists between stomata, leaf traits, and mesophyll architecture for the proper functioning of this process ([6]).
In addition, as higher stomata density leads to lower length (SL) or size (SS) of stomata, they will close more quickly, thereby avoiding unnecessary water loss ([8]). Indeed, a smaller SL can facilitate rapid stomatal opening and closing through altering cell turgor ([50]). Besides, lower LA and SLA allow the plant to mitigate water stress in dry environmental conditions ([44]). Our findings suggest that stomatal length is associated with a decrease in their density on the leaf, likely preventing unnecessary water loss ([25]). In addition, coordination among stomatal traits was verified; for example, SL and SS tend to reduce while SD increases (Fig. S2 in Supplementary material).
Stomatal density was negatively related to specific leaf area (SLA, Fig. 4e), indicating that as SLA increases, stomatal density tends to decrease. This aligns with previous studies ([31]), possibly due to the balance between the leaf area and its mass (SLA), which is related to the number of guard cells ([16]). Although the relation between leaf thickness and stomatal variables was found to be statistically insignificant, our study revealed a negative trend with stomatal density. This is because leaf gas exchange is regulated by the architecture of the mesophyll, stomata density, and characteristics of the stomata, which together determine the maximum gas flux ([24], [36]).
According to the LMM results (Tab. 3), climatic variables at the sampling site, such as precipitation (MAP) and temperature (MAT), influence the functional traits of individuals. Earlier studies have demonstrated that these factors play a significant role in determining stomatal morphometry ([10]). Our findings suggest that the stomata may respond to different climate conditions. For example, MAP was positively related to stomatal density, size, length, and width, as well as to stomatal relative area, consistent with prior research ([37]). This supports the hypothesis that plants develop mechanisms for optimal water use, such as the efficient opening of stomata to minimize water loss ([2]), while also maintaining photosynthetic rates or adjusting stomatal conductance to cool leaves ([23]). But this may also result in unnecessary water loss ([28]).
In this study, all stomatal variables were negatively, though weakly, related to mean annual temperature. Previous research reported the same trend ([37]). Higher temperatures allow the leaf to modify stomatal length, width, and size ([19]), thereby increasing water-use efficiency and CO2 absorption ([21]). Solar radiation influences stomatal transpiration, increasing leaf and mesophyll temperatures, thereby altering plant respiration and photosynthesis ([23]). Additionally, leaf exposure to wavelengths at which photosynthesis occurs may modify stomatal size and density ([41]).
Our findings indicate that the individuals of the collected species have adapted their leaf (Fig. S1 in Supplementary material) and stomatal traits to the local climate (Fig. 3) in order to maintain the proper plant functionality. For example, a reduced stomatal density and an increased leaf area can contribute to keeping the leaves cool and refreshed ([23], [41]).
Our results also indicate that stomatal size and density, and stomatal relative area are associated with longitude or latitude (Fig. 5), consistent with previous research ([7], [10]). Other studies have analyzed geographic patterns in stomatal traits across large gradients in longitude and latitude, such as in China, reporting similar results. Likewise, stomatal functional traits, such as stomatal density, increase with increasing latitude and longitude ([43], [10]). In our study, the geographic gradients are relatively short, suggesting that this relationship may be robust even at shorter geographic distances.
The length, width, and size of stomata decrease as the sampling sites are closer to the western Andes cordillera. This trend is linked to the influence of moisture from the Pacific and Amazon regions ([13]). This moisture influences precipitation levels: the western slopes, influenced by the Amazon, experience higher precipitation, while the eastern slopes, affected by the Pacific, receive less. In our study, two forests are located on the eastern slope of the western Andes Cordillera and two on the western slope, where we observed higher values for stomatal traits.
Conclusions
The stomatal functional traits of nine shrub and tree species showed varying levels of adaptation to their local environments. Species such as M. pubescens and V. stipularis, which exhibit high plasticity, may better adapt to climate change. In contrast, E. myrtilloides may be negatively affected by climate change due to its lower plasticity in stomatal traits. Other species, such as H. luteynii, which had the highest stomatal length and size but the lowest stomatal density, may exhibit variable responses to climate change.
Generally, the variation in stomata characteristics responds to climatic variables, especially precipitation. Leaf functional traits are correlated with stomatal traits, especially LA and SLA. This means that the plant adjusts its functional traits to save and optimize resources. In addition, our findings showed that plants exposed to higher temperatures have reduced stomatal length, width, and size, likely as a mechanism to reduce water loss.
We observed a significant relationship between stomata characteristics and geographic coordinates (latitude and longitude) of the sampling sites. While these relationships have been observed across wide ranges of latitude and longitude, our study indicates that such relationships also hold across relatively short geographic gradients. Overall, these results suggest that native Andean species exhibit varying degrees of stomatal trait plasticity and possibly varying abilities to cope with climate change. Future research on additional Andean woody species is needed to shed light on how these critically important plant communities are likely to respond to current and future climate changes.
List of abbreviations
SL: stomata length; SW: stomata width; SS: stomata size; SD: stomata density; LA: leaf area; SLA: specific leaf area; SRA: stomatal relative area; LT: leaf thickness; MAT: mean annual temperature; MAP: mean annual precipitation; MSR: mean solar radiation; LMM: linear mixed model; Aa: Alnus acuminata; Bs: Brugmansia sanguinea; Em: Escallonia myrtilloides; Hl: Hedyosmum luteynii; Mp: Morella pubescens; Mr: Myrcianthes rhopaloides; Og: Oreocallis grandiflora; Vs: Vallea stipularis; Wf: Weinmannia fagaroides; ICC: Intraclass correlation coefficient; σ2: variance.
Acknowledgements
The authors thank the Laboratory of Seeds and Forest Ecology at the University of Cuenca, Ecuador.
References
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Faculty of Agricultural Sciences, Av. 12 de Octubre, University of Cuenca (Ecuador)
Department of Natural Resources and the Environment, 420 Kendall Hall, University of New Hampshire, NH (USA)
Corresponding author
Paper Info
Citation
Macancela-Herrera A, Smith R (2025). Stomatal morphometry of Andean species and their relationship with spatial variation. iForest 18: 327-334. - doi: 10.3832/ifor4689-018
Academic Editor
Michele Carbognani
Paper history
Received: Jul 16, 2024
Accepted: Jun 10, 2025
First online: Nov 03, 2025
Publication Date: Dec 31, 2025
Publication Time: 4.87 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2025
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 1621
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 447
Abstract Page Views: 656
PDF Downloads: 449
Citation/Reference Downloads: 0
XML Downloads: 69
Web Metrics
Days since publication: 94
Overall contacts: 1621
Avg. contacts per week: 120.71
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
(No citations were found up to date. Please come back later)
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Drought tolerance in cork oak is associated with low leaf stomatal and hydraulic conductances
vol. 11, pp. 728-733 (online: 06 November 2018)
Research Articles
A comparison between stomatal ozone uptake and AOT40 of deciduous trees in Japan
vol. 4, pp. 128-135 (online: 01 June 2011)
Research Articles
Adaptive variation in physiological traits of beech provenances in Central Europe
vol. 11, pp. 24-31 (online: 09 January 2018)
Research Articles
Role of photosynthesis and stomatal conductance on the long-term rising of intrinsic water use efficiency in dominant trees in three old-growth forests in Bosnia-Herzegovina and Montenegro
vol. 14, pp. 53-60 (online: 28 January 2021)
Research Articles
Testing a dual isotope model to track carbon and water gas exchanges in a Mediterranean forest
vol. 2, pp. 59-66 (online: 18 March 2009)
Research Articles
Light acclimation of leaf gas exchange in two Tunisian cork oak populations from contrasting environmental conditions
vol. 8, pp. 700-706 (online: 08 January 2015)
Research Articles
Gas exchange characteristics of the hybrid Azadirachta indica × Melia azedarach
vol. 8, pp. 431-437 (online: 17 December 2014)
Research Articles
Prediction of ozone effects on net ecosystem production of Norway spruce forest
vol. 11, pp. 743-750 (online: 15 November 2018)
Research Articles
Developing stand transpiration model relating canopy conductance to stand sapwood area in a Korean pine plantation
vol. 14, pp. 186-194 (online: 14 April 2021)
Research Articles
Towards a functional phytosociology: the functional ecology of woody diagnostic species and their vegetation classes in Northern Italy
vol. 14, pp. 522-530 (online: 22 November 2021)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword














