Fungal community of necrotic and healthy galls in chestnut trees colonized by Dryocosmus kuriphilus (Hymenoptera, Cynipidae)
iForest - Biogeosciences and Forestry, Volume 12, Issue 4, Pages 411-417 (2019)
doi: https://doi.org/10.3832/ifor3014-012
Published: Aug 13, 2019 - Copyright © 2019 SISEF
Research Articles
Abstract
Dryocosmus kuriphilus is a non-native pest that has recently spread through Europe with a special incidence along the Mediterranean Basin. The presence of this exotic wasp (originally from Asia) threatens stands and orchards of sweet chestnut (Castanea sativa Mill.) as it reduces tree growth and consequently fruit production. In this study the living mycobiota in leaves, healthy and necrotic galls collected from two sites in Cantabria (Northern Spain) was investigated. A total of twenty-two fungal taxa based on morphological and molecular traits were determined. In addition, we calculated fungal diversity and identified the dominant taxa among members of the mycobiota. Seven log-linear models were used to analyse whether fungal abundance varied between sites, types of plant material or fungal taxa. Our findings highlight the complex interactions between plant hosts, insect and the endophytic community, and are of potential interest in relation to the biological control of this important pest.
Keywords
ACGW, Biological Control, Castanea sativa, Endophytic Fungi, Entomopathogens, Fungal Community, Necrotic Gall
Introduction
The Asian chestnut gall wasp (ACGW), Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae), a gall-inducing insect native of China, was first introduced to Japan, then North America and most recently to Europe (2002). Currently, ACGW has been spread to fourteen European countries including Spain. In this country the pest was first recorded in Catalonia, in 2012 ([38]), and then in Cantabria ([5]). This invasive insect is now present in nine Autonomic Communities and fourteen provinces in the country. The female emerges over a relatively short period in early summer and immediately lays eggs inside the chestnut buds (Castanea sativa Mill.) that will develop during the following spring ([9], [22], [30]). The ACGW forms galls on leaves and shoots, thus halting growth and causing abortion of flowers and thereby reducing chestnut yield. The ACGW is the most important pest affecting Castanea species worldwide, with loss of chestnuts reaching 80% in cases of severe infestations ([9], [30]).
Several attempts have been made to control this forest pest worldwide. In this regard, the parasitoid Torymus sinensis Kamijo (Hymenoptera, Torymidae), a natural enemy of the ACGW, has been shown to be an effective control agent in the medium or long term ([33]). However, the use of non-native species may have negative impacts on the native parasitoid community such as the risk of hybridization, hyperparasitism or displacement of native species. The search for novel biocontrol agents to manage the infestations of ACGW is therefore of great interest.
Alternative perspectives are offered by fungi, since endophytes sensu lato (i.e., fungi that inhabit plant tissues regardless their lifestyle and the kind of association established with the host) have been successfully used as biological control agents (BCAs - [2]). Endophytes can be isolated from the same ecosystem in which they will be used as biological controllers, and thus no impact on the environment is expected. Comparing to the chemical control, another advantage is that organisms do not generally become resistant to the endophytes ([24]). Moreover, endophytes can provide other benefits to the plants. For instance, many studies have demonstrated that the presence of specific groups of endophytes can promote high growth rates in plants ([4]), resistance to drought stress, defence against herbivores ([8]) as well as resistance against biotic and abiotic stresses ([36]).
Prospero & Forster ([32]) and Meyer et al. ([27]) highlighted the relationship between the ACGW and the fungal community, by noting the link between the D. kuriphilus galls and increased infection by chestnut blight disease (Chryphonectria parasitica [Murrill] Barr). Unfortunately, the ecological impacts of invasive organisms are often analysed separately, thereby overlooking possible interactions and combined effects, which may considerably hinder the determination of actual ecological consequences ([27]). Interactions between pathogenic organisms are specific to the tripartite insect-fungus-tree system ([15]). The importance of fungi in the ecological balance of chestnut micoflora highlights the need of more scientific evidences about the species that colonize this host or their diversity.
The overall objectives of the present study were (i) to detect and identify endophytes colonizing C. sativa trees attacked by D. kuriphilus in two sites located in Northern Spain; (ii) to assess the diversity of the endophytic community; and (iii) to verify the predominant taxa among the fungi colonizing the galls for biological control purposes.
Material and methods
Sample collection and fungal culture
Site 1
Scattered chestnut trees surrounded by pine plantations of Monterey pine (Pinus radiata D. Don) were sampled between June and August 2015, in Vejorís (Cantabria, Northern Spain - Tab. 1). Necrotic galls (i.e., current season galls showing symptoms of degrading activity by fungi) were randomly collected every fortnight from up to ten trees depending on the abundance of necrotic galls. Healthy leaves were also randomly collected monthly in order to identify fungal taxa from asymptomatic tissue. Trees selected for gall collection showed neither symptoms of chestnut blight nor other relevant biotic damages. The galls and leaves were placed in labelled plastic bags and then held at -20 °C until processing. The plant material was surface sterilized by immersion in 70% v/v ethanol for one minute, then in 2% v/v sodium hypochlorite for one minute, and finally in double distilled water for one minute. Each gall and leaf was then cut into four sections (galls) or nine 1-cm2 pieces (leaves) and plated on PDA (3.90% w/v potato-dextrose-agar - Scharlab S.L., Spain) amended with streptomycin (PDAs, 0.60 g L-1 - Sigma Aldrich Química S.L., Spain) to reduce bacterial contamination of the plates. The cultures were incubated for five days at room temperature (i.e., 21 ± 2 °C). Incipient fungal colonies were then subcultured in new PDAs plates and incubated under the aforementioned conditions for one week.
Tab. 1 - Description of sampled sites. (*): Climatic data of Santiurde de Toranzo, the closest location with climatic records; (**): summer values correspond to June-September period. Source of data: CLIMATE-DATA.ORG (⇒ https://es.climate-data.org/).
| Characteristics | Site 1 (Vejoris*) |
Site 2 (Zamudio) |
|---|---|---|
| ETRS89 | 43º 11′ 46.65″ N 03º 53′ 29.01″ W |
43º 17′ 55.76″ N 02º 51′ 04.66″ W |
| Height (m a.s.l.) | 503 | 140 |
| Annual rainfall (mm) | 933 | 1194 |
| Summer rainfall (mm)** | 237 | 293 |
| Mean temp. (°C) | 13.8 | 14.0 |
| Maximal temp. (°C) | 23.2 | 23.4 |
| Minimal temp. (°C) | 5.5 | 6.3 |
| Summer mean temp. (°C)** | 18.6 | 18.9 |
| Summer maximal temp. (°C)** | 23.2 | 23.4 |
| Summer minimal temp. (°C)** | 13.9 | 14.5 |
Site 2
Necrotic galls were collected every fortnight between June and September 2016, in a chestnut plantation located in Zamudio (Basque Country, Northern Spain - Tab. 1) following the tree selection criteria mentioned for Site 1. The sampling site is surrounded by monospecific stands of eucalyptus (Eucalyptus globulus Labill.), pines (P. radiata and Pinus pinaster Aiton) and common oak (Quercus robur L.). At the same time, galls with no signs of necrosis (green galls) were also harvested from the sampled trees and stored at -20 °C. When the availability of completely green galls decreased (i.e., between late July and September), galls with only ≤ 1 cm of necrosis around emergence holes were considered green material. Surface sterilization and culturing were performed following the protocol described for Site 1.
Fungal identification
The emerging colonies were characterized using ten colonial variables (growth rate, shape, above/reverse colour, elevation, texture, type of mycelium, edge, density and zonality), as described by Lacap et al. ([18]), and were consequently grouped in homogeneous operational taxonomic units (OTUs). A representative number of isolates per OTU were subcultured in cellophane-membrane-covered PDA plates for DNA extraction.
Total nucleic acids were extracted from each fungal sample as described by Vainio et al. ([40]). Each OTU was identified by amplification of two rDNA molecular markers: ITS by using the primer pair ITS1F (5’-CTTGGTCATTTAGAGGAAGTAA-3’)/ITS4 (5’-TCCTCCGCTTATTGATATGC-3’), and LSU, by using the primer pair LR0R (5-ACCCGCTGAACTTAAGC-3)/LR16 (5’-TTCCACCCAAACACTCG-3’) as described by Botella & Diez ([7]). The polymerase chain reaction (PCR) was performed with Kapa Taq® PCR Kit (Kapa Biosystems, Wilmington, MA, USA) using a specific amplification program for each molecular marker. For ITS the protocol consisted on 10 min of denaturation at 95 °C; 13 cycles of 35 s at 95 °C, 55 s at 55 °C and 45 s at 72 °C; 13 cycles of 35 s at 95 °C, 55 s at 55 °C and 2 min at 72 °C; 9 cycles of 35 s at 95 °C, 55 s at 55 °C and 3 min at 72 °C; the last extension step consisted on 7 min at 72 °C. The protocol for LSU consisted on 3 min at 94 °C; 36 cycles of 30 s at 94 °C, 30 s at 54 °C, 1 min at 72 °C; and a last extension step of 10 min at 72 °C. The resulting PCR products were run in 1.60% w/v agarose gel stained with 0.004% (v/v) 10.000× GelRedTM (Biotium, USA). DNA fragments were visualized under UV light, and the size of each amplicon was estimated by comparison against a 100 bp ladder (Nippon Genetics Europe, Dueren, Germany).
Amplicons were sent to STAB Vida (⇒ http://www.stabvida.com/) for DNA purification and sequencing. Sequences were trimmed using Geneious Pro 6.0.6 (⇒ https://www.geneious.com/) and compared against sequences deposited in GenBank (NCBI - ⇒ http://blast.ncbi.nlm.nih.gov/Blast.cgi) by using BLASTn and the Fungi database (taxid: 4751). Fungal taxa were assigned to genus or species level when they showed a homology equal or higher than 99% in at least one molecular marker. The sequences representing each OTU were submitted to GenBank (see Tab. S1 in Supplementary Material).
Study of biological diversity
The observed taxonomical richness (S) was calculated for each sampling site and type of material (leaves/healthy galls/necrotic galls). In addition, the fungal diversity and evenness were also calculated according to Shannon (H and J respectively) and Simpson (D and E respectively) indices ([44]).
Sample-based rarefaction curves were computed using EstimateS v9.10 software (⇒ http://viceroy.eeb.uconn.edu/estimates/), as described by Muñoz-Adalia et al. ([29]). The similarity between mycobiota and plant material from different sites was evaluated using the Sorensen (I) and Jaccard (Y) indices ([44]). Taxonomic dominance was also calculated using Camargo’s index. According to this method, a single taxon is considered dominant if pj > 1/S, where pj is the number of isolates of taxon j divided by the total number of fungal isolates. In addition, the functional group (i.e., guild) of each sampled taxon was investigated using the FUNGuild database (⇒ http://www.stbates.org/guilds/app.php). When information of a single species was not available in the database, data referred to fungal genus was used for guild analysis.
Statistical analysis
The variation in fungal community composition was investigated by computing log-linear models in the R environment (⇒ https://www.r-project.org/). The absolute abundance of each taxon (i.e., number of colonies recorded for each taxon) was considered the response variable of each model. The sampling site, type of plant material (green or necrotic), fungal taxa and their corresponding interactions were selected as explanatory variables. Only models that revealed significant effects of variables were investigated further. The most parsimonious model according to Akaike’s Information Criteria (AIC) was selected as best model by the “AICcmodavg” package for R ([26]). This model was then compared with the other computed models by using the χ2 test in the same programming environment.
Results
Identification of fungal taxa
A total of 35 necrotic galls from Site 1 were fragmented and cultured as previously outlined. As a result, 23 galls provided 148 fungal colonies and the others only produced bacterial masses being recorded as negative samples. In parallel, 12 healthy leaves from Site 1 were cultured after being cut in 108 fragments, which provided 101 fungal colonies. Twenty-four green galls from Site 2 were cultured in 96 pieces, of which 45 fragments produced fungal colonies. In the case of necrotic galls from Site 2, 24 fragmented galls yielded 26 isolates. In summary, 320 fungal colonies were identified and morphologically grouped into 22 OTUs. Molecular identification of the OTUs by ITS and LSU markers resulted in 19 genera (Tab. 2 and Tab. S1 in Supplementary material).
Tab. 2 - Abundance of each taxon of sampled mycobiota. Dominant taxa according to Camargo’s index are indicated by an asterisk (*). FUNGuild categories: A (Animal pathogen); Ds (Dung saprotroph); En (Endophyte); Ep (Epiphyte); F (Fungal parasite); L (Lichen parasite); P (Plant pathogen); Ss (Soil saprotroph); U (Unidentified saprotroph); W (Wood saprotroph).
| Fungal taxa | Site 1 | Site 2 | Assigned guild (FUNGuild) | ||||
|---|---|---|---|---|---|---|---|
| Green leave |
Necrotic gall |
Total | Green gall |
Necrotic gall | Total | ||
| Arthrinium arundinis* | *24 (23.76%) | 1 (0.46%) | 25 (7.86%) | 0 | 0 | 0 | Ds/P |
| Aureobasidium pullulans | 0 | 0 | 0 | 3 (6.67%) | 0 | 3 (4.23%) | A/En/Ep/P |
| Bionectria byssicola | 0 | 0 | 0 | 0 | 1 (3.85%) | 1 (1.41%) | F/U |
| Cladosporium cladosporioides | 0 | 0 | 0 | 2 (4.44%) | 0 | 2 (2.82%) | En/Ep/P |
| Colletotrichum acutatum* | 2 (1.98%) | *28 (12.90%) | *30 (9.43%) | *8 (17.78%) | *4 (15.38%) | *12 (16.90%) | En/P |
| Daldinia concentrica | 0 | 0 | 0 | 1 (2.22%) | 0 | 1 (1.41%) | W |
| Epicoccum nigrum | 0 | 5 (2.30%) | 5 (1.57%) | 1 (2.22%) | 1 (3.85%) | 2 (2.82%) | En/L/P |
| Fusarium sp.* | *20 (19.80%) | 20 (9.22%) | *40 (12.58%) | 0 | 0 | 0 | A/En/L/P/Ss/W |
| Fusarium lateritium | 0 | 0 | 0 | 1 (2.22%) | 0 | 1 (1.41%) | En/P |
| Gnomoniopsis castanea* | *27 (26.73%) | *103 (52.07%) | *130 (44.03%) | *17 (37.78%) | *7 (26.92%) | *24 (33.80%) | En |
| Nigrospora oryzae | 0 | 0 | 0 | 2 (4.44%) | 0 | 2 (2.82%) | En/P |
| Paraphaeosphaeria neglecta | 0 | 0 | 0 | 1 (2.22%) | 1 (3.85%) | 2 (2.82%) | U |
| Penicillium glabrum | 0 | 0 | 0 | 1 (2.22%) | 1 (3.85%) | 2 (2.82%) | P |
| Penicillium spinulosum | 0 | 1 (0.46%) | 1 (0.31%) | 0 | 0 | 0 | A |
| Pestalotiopsis sp.* | 4 (3.96%) | 3 (1.38%) | 7 (2.20%) | 3 (6.67%) | 2 (7.69%) | *5 (7.04%) | P |
| Phoma herbarum | 0 | 0 | 0 | 1 (2.22%) | 0 | 1 (1.41%) | P |
| Rhizomucor variabilis | 0 | 0 | 0 | 0 | 1 (3.85%) | 1 (1.41%) | U |
| Sirococcus castaneae* | 0 | 0 | 0 | 3 (6.67%) | *5 (19.23%) | *8 (11.27%) | P |
| Sydowia polyspora* | 0 | 0 | 0 | 1 (2.22%) | *3 (11.54%) | 4 (5.63%) | P |
| Trichoderma atroviride morphotype 1 | 2 (1.98%) | 0 | 2 (0.63%) | 0 | 0 | 0 | En |
| Trichoderma atroviride morphotype 2* | 9 (8.91%) | *45 (20.74%) | *54 (16.98%) | 0 | 0 | 0 | En |
| Mucor sp. like | 0 | 1 (0.46%) | 1 (0.31%) | 0 | 0 | 0 | U |
| Unknown001* | *13 (12.87%) | 0 | 13 (4.09%) | 0 | 0 | 0 | - |
| Total positive samples | 101 | 217 | 318 | 45 | 26 | 71 | - |
Biological diversity
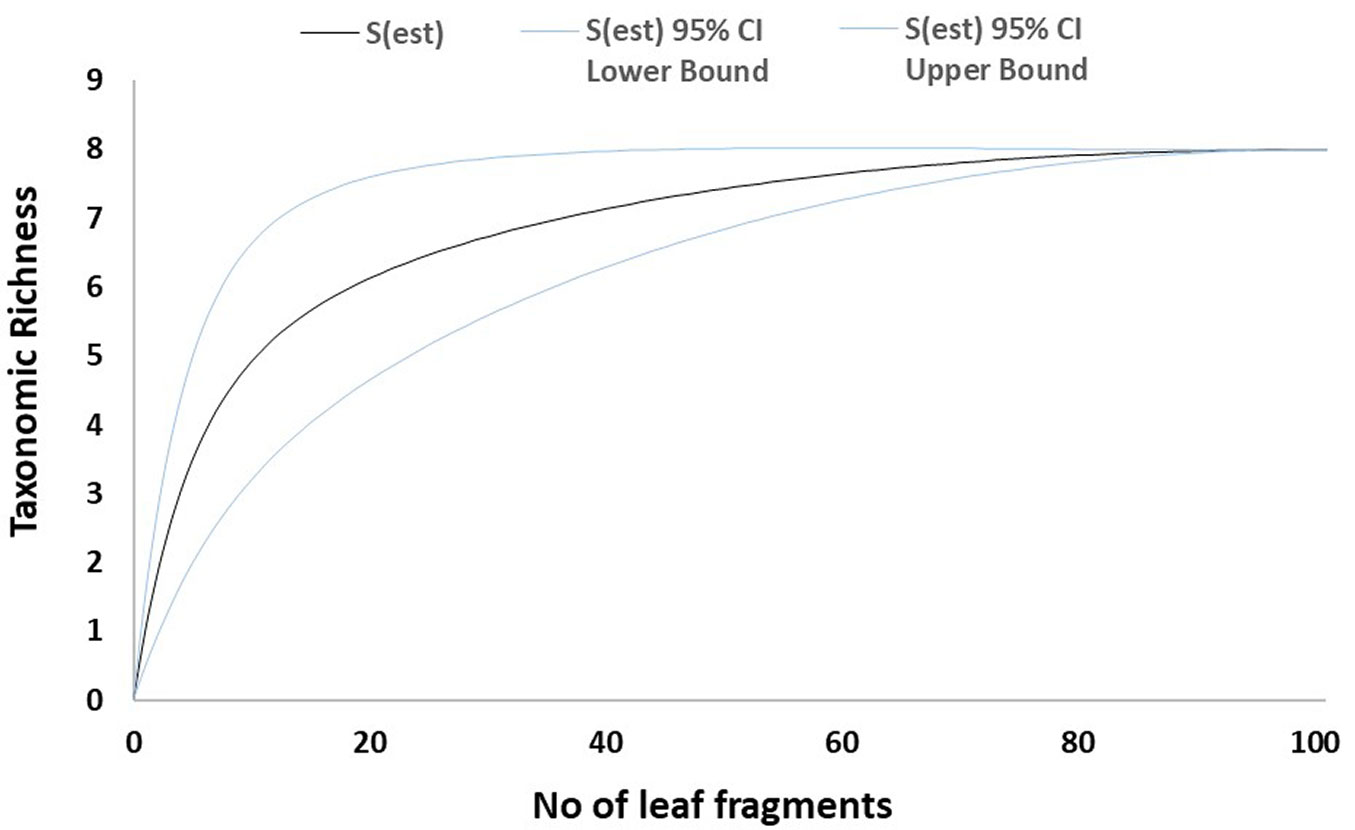
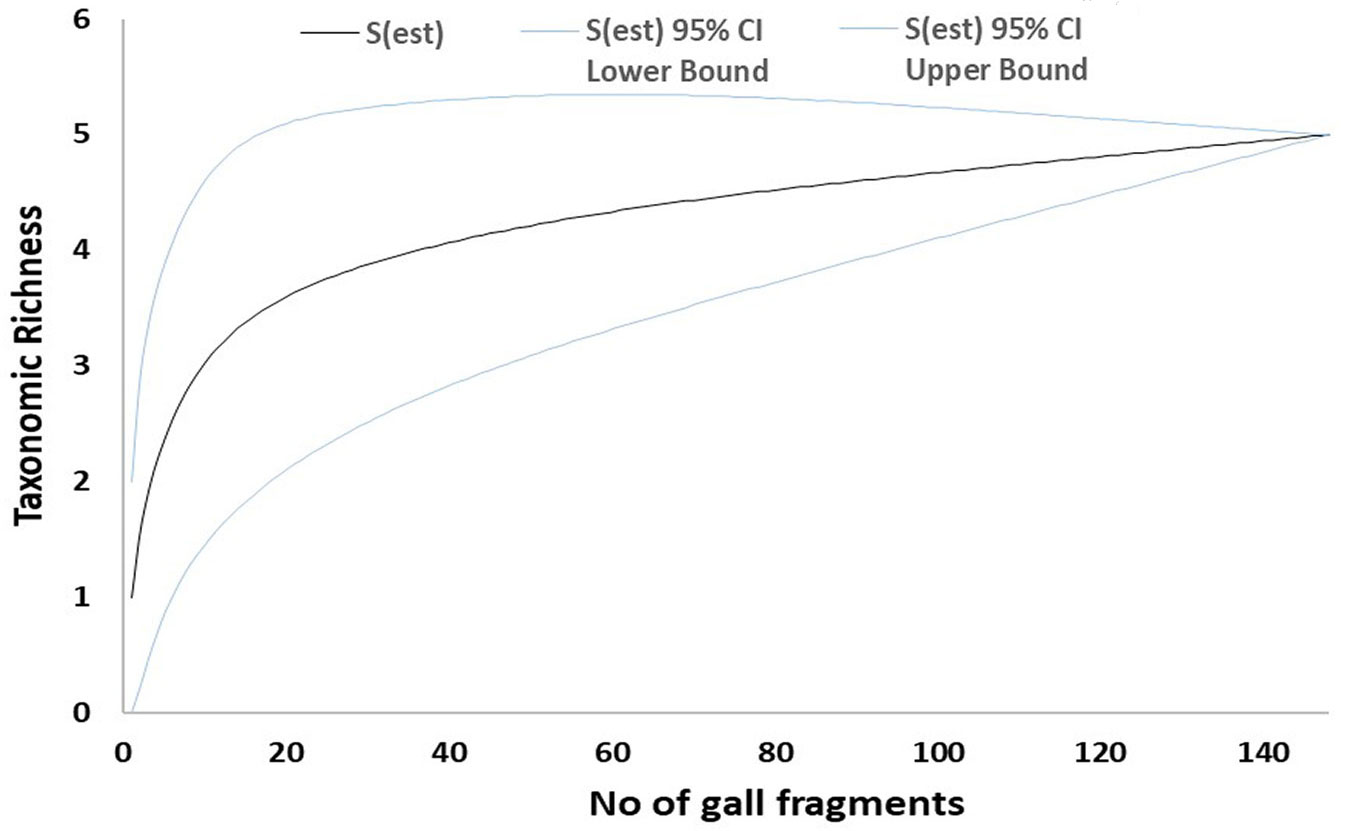
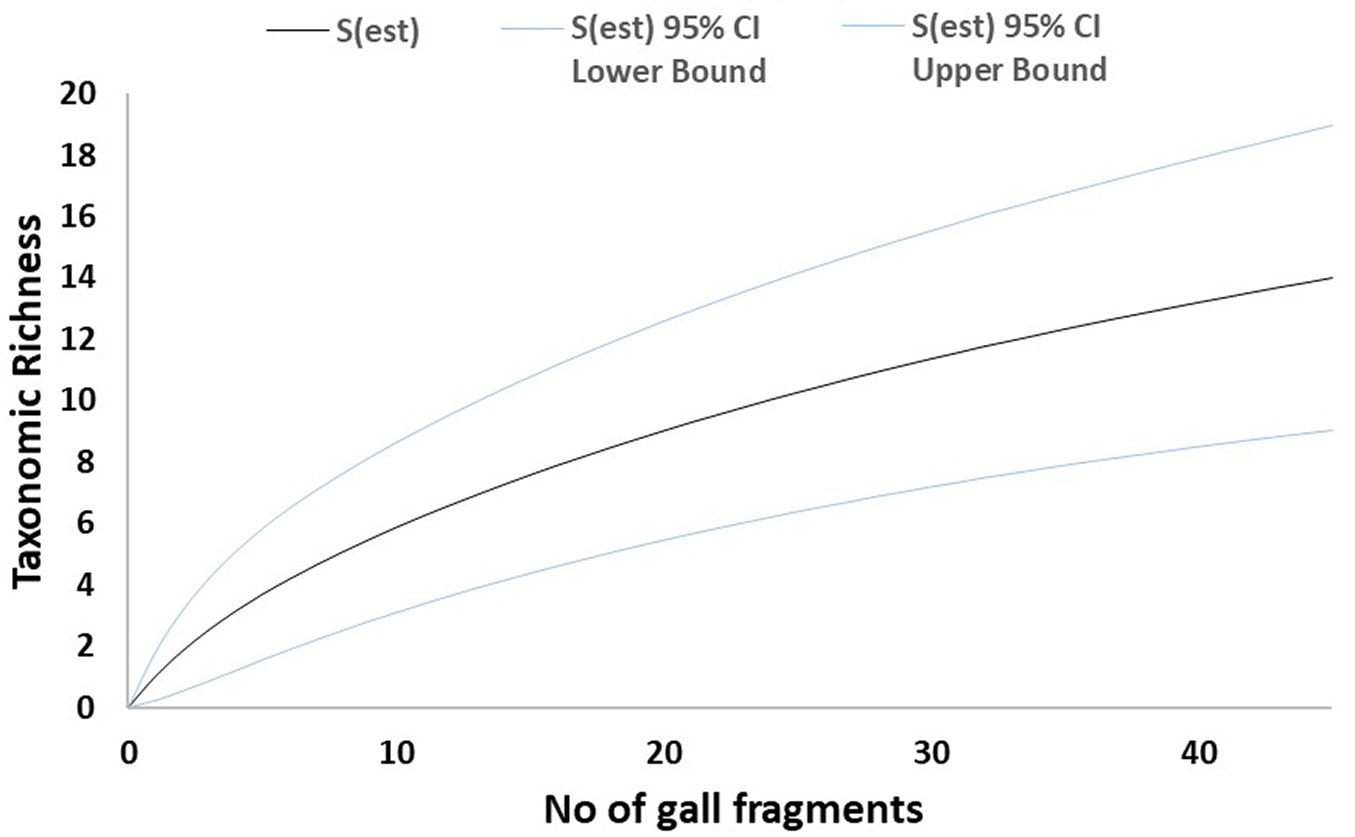
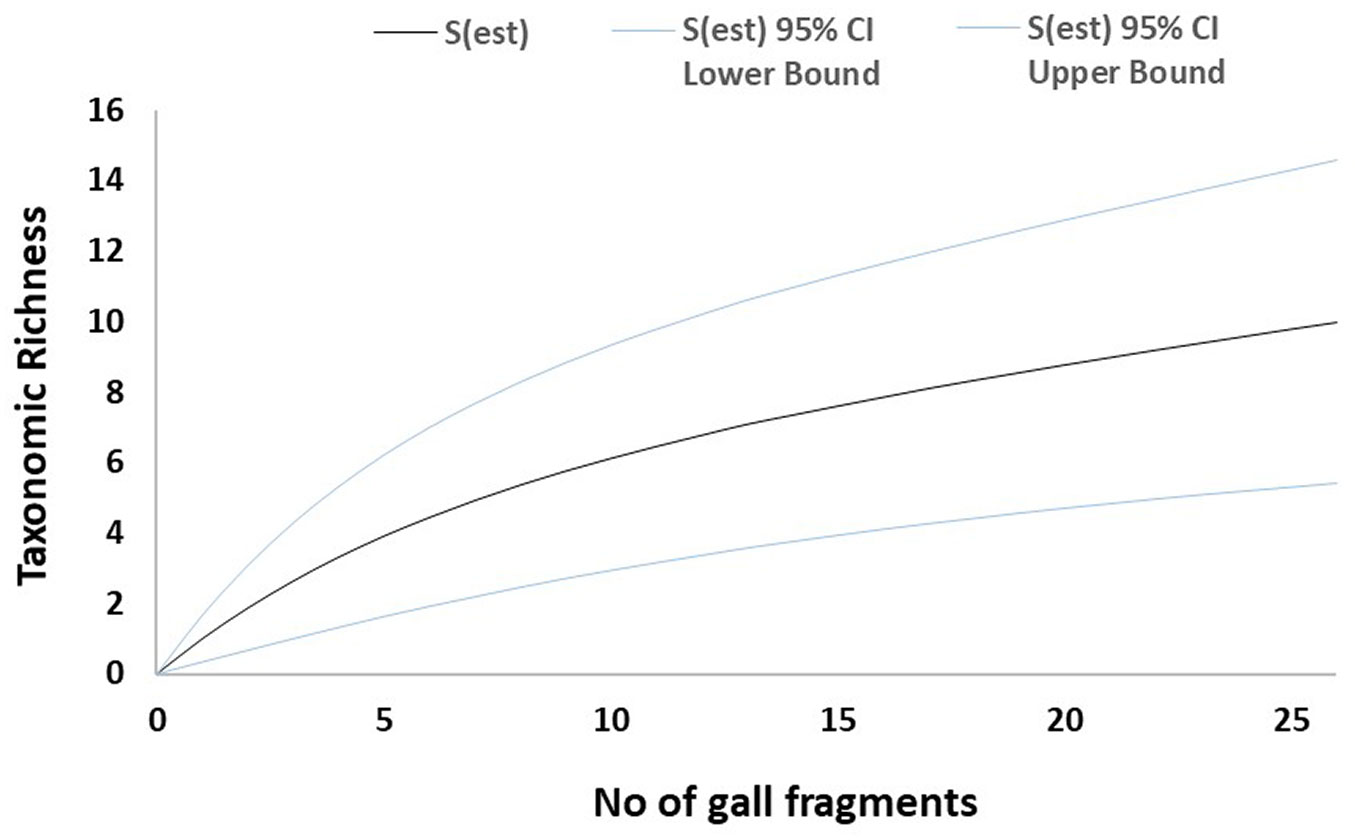
The sampled-based rarefaction curves approached an asymptote for both healthy leaves and necrotic galls in Site 1. By contrast, the curve for Site 2 was not asymptotic (Fig. 1, Fig. 2, Fig. 3, Fig. 4). Asymptotic curves indicate that sampling effort is sufficient for adequate characterization of the studied community. The analysis of variation of absolute abundance provided a total of seven log-linear models. The most parsimonious model (i.e., Model 1 - Tab. 3) included the three factors considered (fungal taxa, sampling site and type of plant material) but no interactions between them as explanatory variables. In addition, this model was also significantly different from the other models computed in all χ2 test pairwise comparisons (p < 0.01).
Fig. 1 - Mao-Tau estimator for healthy leaves from Site 1. Taxonomic accumulation curves for fungal taxa isolated from green leaves from Site 1 (Vejorís). Taxonomic richness computed using Mao-Tau estimator with 95% confidence intervals.
Fig. 2 - Mao-Tau estimator for necrotic galls from Site 1. Taxonomic accumulation curves for fungal taxa isolated from necrotic galls from Site 1 (Vejorís). Taxonomic richness computed using Mao-Tau estimator with 95% confidence intervals.
Fig. 3 - Mao-Tau estimator for green galls from Site 2. Taxonomic accumulation curves for fungal taxa isolated from green galls from Site 2 (Zamudio). Taxonomic richness computed using Mao-Tau estimator with 95% confidence intervals.
Fig. 4 - Mao-Tau estimator for necrotic galls from Site 2. Taxonomic accumulation curves for fungal taxa isolated from necrotic galls from Site 2 (Zamudio). Taxonomic richness computed using Mao-Tau estimator with 95% confidence intervals.
Tab. 3 - Log-linear model selection of absolute fungal abundance as response variable. (×): interaction of variables; (K): number of parameters; (AICc): corrected Akaike information criterion; (ΔAIC): difference of AIC between each model and the model with the lowest AIC; (wi): Akaike weights.
| Model | Model terms | Log Likelihood |
K | AICc | ΔAICc | wi |
|---|---|---|---|---|---|---|
| 1 | Site + Material + Taxa | -227.13 | 25 | 523.95 | 0.00 | 1 |
| 2 | Material + Taxa | -311.91 | 24 | 689.73 | 165.78 | 0 |
| 3 | Taxa | -324.13 | 23 | 710.50 | 186.55 | 0 |
| 4 | Site × Material | -660.98 | 4 | 1330.42 | 806.47 | 0 |
| 5 | Site + Material | -672.99 | 3 | 1352.25 | 828.30 | 0 |
| 6 | Site | -685.21 | 2 | 1374.55 | 850.60 | 0 |
| 7 | Material | -757.77 | 2 | 1519.68 | 995.73 | 0 |
The most frequently isolated fungus was Gnomoniopsis castanea Tamietti (synonym: Gnomoniopsis smithogilvyi L.A. Shuttlew., E.C.Y. Liew & D.I. Guest), which dominated the whole community according to Camargo’s index (Sites 1 and 2). The dominant taxa in Site 1 were G. castanea, Trichoderma atroviride P. Karst morphotype 2, Fusarium sp. and Colletotrichum acutatum J.H. Simmonds, whereas G. castanea, C. acutatum, Sirococcus castaneae (Prill. & Delacr.) J.B. Mey., Senn- Irlet & T.N. Sieber and Pestalotiopsis sp. were the dominant species in Site 2 (Tab. 2). On the other hand, the similarity between studied sites was rather low (I = 0.30, Y =0.17).
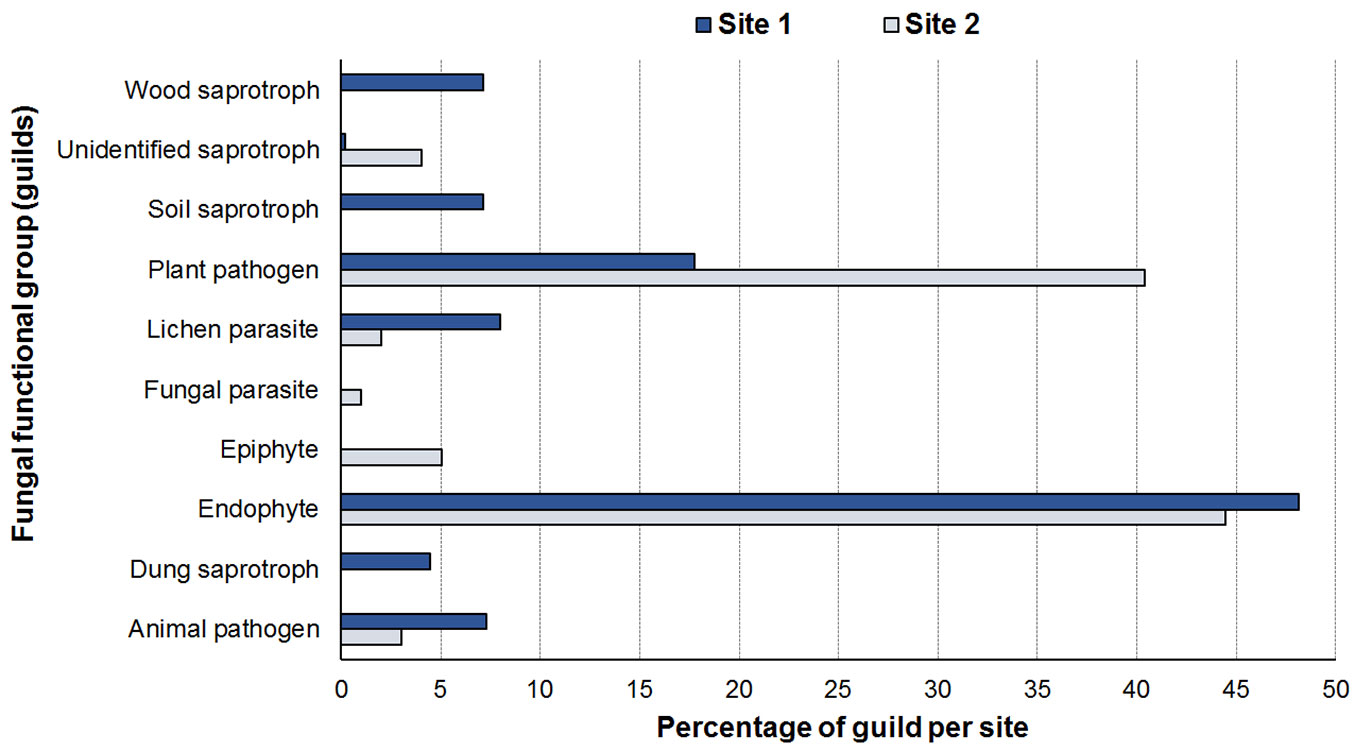
Analysis of the guilds revealed 10 functional groups in sampled mycobiota (Tab. 2). The endophytes and plant pathogens were the most representative groups, with abundances of 48.13% and 17.75% respectively in Site 1 and 44.43% and 40.40% respectively in Site 2 (Fig. 5).
Fig. 5 - Relative abundance of each functional group (guild). Source of data: FUNGuild database (⇒ http://www.stbates.org/guilds/app.php).
Discussion
In this study we described the mycobiota associated with chestnuts infested by ACGW in two different sites in Northern Spain. The observed fungal diversity was moderate to high, relative to endophytic communities affecting other broadleaf species ([25]). More specifically, the Shannon diversity was lower than reported for Quercus species and Eurya japonica Hort. ex K. Koch in the aforementioned study. By contrast, taxonomic evenness was similar to those reported in that study due to the high dominance of a few taxa. Similarly, endophytic community found in twigs of Pinus sylvestris L. by Sanz-Ros et al. ([34]) were also more diverse than in the present study. There were also slight differences in Shannon index values obtained in the present study and for mycobiota isolated from needles of Picea abies (L.) H. Karst. ([17]). Therefore, we believe that the diversity of the mycobiota sampled can be considered moderate. Nevertheless, communities from different host species and tissues should be compared carefully.
Regarding the sampling effort and observed taxonomic richness, the results reported here suggest that the amount of plant material cultured may be enough to characterize the fungal community in Vejorís (Cantabria) for which sample-based rarefaction curves tended to plateau (Fig. 1, Fig. 2). By contrast, the curves for Zamudio (País Vasco) did not tend to be asymptotic, suggesting that more taxa may have been recorded if more plant material had been cultured (Fig. 3, Fig. 4). The statistical analysis showed high variability in fungal abundance for sampling site and plant material. The significant effect of site may be related to climate conditions as the summers are slightly drier in Vejorís (Site 1) and the sampling area is located at a higher altitude (by ~360 m a.s.l.) than in Zamudio (Site 2 - Tab. 1). The sites also differed in habitat structure (i.e., scattered chestnut trees surrounded by Pinus spp. stands in Vejorís in contrast to monospecific chestnut plantation in Zamudio). These variations in host distribution may also explain the observed differences between communities as forest fragmentation is an important factor in the establishment of the mycobiota ([25]). In addition, the host genotype is another source of variation in endophytic communities that should not be overlooked ([3]). The statistical analysis manifested that the type of plant material modulated the abundance of fungal taxa (Tab. 3). Our findings are consistent with those of Lawson et al. ([19]), who reported that the composition of the endophytic community varied greatly depending on the tissue sampled (leaf vs. aphid gall vs. petiole) in poplars (Populus spp.). Regarding these data, we consider that the aforementioned environmental conditions (specially the type of plant material) probably act as selective factors modulating the composition of the mycobiota sampled ([37]). This phenomenon may explain the low similarity between sites shown by the Sorensen and Jaccard indices (both ≤ 0.30). Here, we did not evaluate the effect of time in fungal communities since we sampled each mycobiota for a single season. Hence, future studies are required to elucidate changes in mycobiota composition in longer temporal series (e.g., from incipient infestation of ACGW until the stabilization of its populations).
The guild analysis confirmed that endophytes were the most abundant functional group of fungi inhabiting leaves and galls of C. sativa. Several taxa such as Aureobasidium pullulans (de Bary) G. Arnaud, Epicoccum nigrum Link, Fusarium spp., Phoma herbarum Westend., S. castaneae, Penicillium spp. and Trichoderma spp. have a well-documented role as endophytes of different hosts, including chestnuts ([6], [7], [19], [34]). Surprisingly, plant pathogens were another major group regarding relative abundance, rather than plant saprotrophs as we expected. The abundance of opportunistic pathogens may be explained by an efficient use of gall-derived nutrients (mainly starch) as suggested for endophytes in other pathosystems ([14]). One of the dominant species was G. castanea, which has been reported to be a causal agent of canker in C. sativa ([31]). The genus is thought to be closely associated with the spread of D. kuriphilus ([35]). In addition, Magro et al. ([21]) reported blight symptoms in twigs and intense necrosis in the 90% of galls inoculated with an undescribed member of this fungal genus. These results showed the probable participation of this genus as a latent pathogen that utilizes nutrients made available during gall formation. Gaffuri et al. ([11]) observed the rapid colonization of galls by C. acutatum but did not observe natural necrosis due to this fungus in uninoculated galls. In contrast, this fungus was frequently found in naturally necrotized specimens. The presence of this species highlights the ecological plasticity of this fungus and its potential role as an opportunistic pathogen. The ecological complexity of the sampled mycobiota increases further on considering interactions between species (e.g., fungus-fungus; tree-fungus). In this regard, some community species such as Arthrinium arundinis (Corda) Dyko & B. Sutton, P. herbarum, Sydowia polyspora (Bref. & Tavel) E. Müll. and S. castaneae show multiple different types of behaviour, acting as saprotrophs, endophytes or pathogens depending on the host ([42], [28]). In addition, the presence of fungi with antagonistic activity can also modify the abundance of pathogenic taxa. Specifically, T. atroviride has a well-known anti-fungal effect ([16]) and its dominance in Site 1 could affect the abundance of other taxa. All these considerations deserve further study to elucidate the ecological role of each species during infestation by this invasive pest.
Entomopathogenic fungi comprise an interesting group among the sampled mycobiota. For example, C. acutatum was able to infect larvae of D. kuriphilus causing mortality ([13]). In an earlier study, Marcelino et al. ([23]) reported the entomopathogenic effect of a single variety of this fungus against Fiorinia externa Ferris (Hemiptera, Diaspididae), which acted as an endophyte or caused minor damage in plant hosts. These results support the possible use of this cosmopolitan epiphyte/endophyte as a biocontrol agent. Nevertheless, it also acts as a post-harvest pathogen by colonizing healthy fruits ([12]) thus hindering its use as biological control agent. Pestalotiopsis sp. has also proved to be a successful entomopathogen for Hemiberlesia pitysophila Takagi (Hemiptera, Diaspididae - [20]), producing average mortality rates of up to 67%. The authors considered that the Pestalotiopsis sp. strains used did not damage the host, even though this genus is considered a conifer pathogen. Our findings suggest that the Fusarium spp. were not acting as entomopathogens as they were abundantly represented in Site 1, but not in Site 2 where they were not present in necrotic galls. These findings differ from those reported by Addario & Turchetti ([1]), who showed promising results using the Fusarium incarnatum-equiseti species complex in necrotic galls. Tosi et al. ([39]) also isolated Fusarium proliferatum (Matsush.) Nirenberg from galls and the fungus was often seen covering the insects’ bodies. Cladosporium cladoporioides also showed entomopathogenic potential for controlling the mite Tetranychus urticae Koch (Acari, Tetranychidae), in which it caused mortality > 50%, indicating the species as a candidate biocontrol agent worthy of future study ([10]). In this regard, it is important to note that any candidate species could also affect native or exotic parasitoids of ACGW, playing a plausible counterproductive role that should not be neglected. Despite the previously mentioned pathogenic role of Gnomoniopsis sp. ([43]), G. castanea proved to be a successful entomopathogenic fungi against D. kuriphilus in a recent study performed in Italy ([41]), in which mortality reached > 60%, and was particularly high in the adult wasps. Nevertheless, the authors mentioned that the failure of adults to emerge may be related to the dry state of gall tissues. This would support the possible interaction of other fungi that consume nutrients in the gall as secondary actors in the aetiology and possible control of the chestnut gall wasp.
Conclusions
The mycobiota associated with C. sativa infested by D. kuriphilus in two different sites in Northern Spain was moderately diverse and dominated by a few taxa, including the chestnut pathogen G. castanea.
The taxonomic composition of studied communities varied among locations and type of plant tissue. Together these results suggest an intense effect of environmental and temporal factors that should be considered for a complete characterization of fungal community associated with ACGW.
Fungal endophytes and plant pathogens were the best represented guilds among the identified taxa. Several taxa with entomopathogenic potential were detected in the sampled mycobiota deserving further study.
Acknowledgements
The authors thank Milagros de Vallejo and Juan Blanco (Gobierno de Cantabria, Spain) for their help in carrying out the study. The authors also thank J. Asdrúbal Flores-Pacheco (Bluefields Indian and Caribbean University, Nicaragua), Juan Carlos Vinagrero and Beatriz Fernández-Duque (University of Valladolid, Spain), for their participation in the fieldwork. The authors are also grateful to three anonymous reviewers for their helpful comments on an earlier version of the article.
Author contributions
EJM-A, JD and MF conceived and designed the experiment; EJM-A and DR performed the experiment; EJM-A, DR and MC analysed the data; EJM-A, MC, JD and MF wrote the paper and edited the manuscript.
References
Gscholar
CrossRef | Gscholar
Gscholar
Supplementary Material
Authors’ Info
Authors’ Affiliation
Forest Sciences Center of Catalonia (CTFC), Carretera St. Llorenç de Morunys, km.2, 25280 Solsona (Spain)
María Casado
Mercedes Fernández
Department of Agroforestry Sciences, University of Valladolid. Avenida de Madrid 44, 34071 Palencia (Spain)
Department of Vegetal Production and Forest Resources, University of Valladolid. Avenida de Madrid 44, 34071 Palencia (Spain)
Mercedes Fernández
Sustainable Forest Management Research Institute, University of Valladolid - INIA, Avenida de Madrid 44, 34071 Palencia (Spain)
Corresponding author
Paper Info
Citation
Muñoz-Adalia EJ, Rodríguez D, Casado M, Diez J, Fernández M (2019). Fungal community of necrotic and healthy galls in chestnut trees colonized by Dryocosmus kuriphilus (Hymenoptera, Cynipidae). iForest 12: 411-417. - doi: 10.3832/ifor3014-012
Academic Editor
Alberto Santini
Paper history
Received: Dec 01, 2018
Accepted: Jun 06, 2019
First online: Aug 13, 2019
Publication Date: Aug 31, 2019
Publication Time: 2.27 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2019
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 45303
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 37282
Abstract Page Views: 4058
PDF Downloads: 3056
Citation/Reference Downloads: 0
XML Downloads: 907
Web Metrics
Days since publication: 2392
Overall contacts: 45303
Avg. contacts per week: 132.58
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2019): 12
Average cites per year: 1.71
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
The complexity of mycobiota associated with chestnut galls induced by Dryocosmus kuriphilus in Galicia (Northwestern Spain)
vol. 17, pp. 378-385 (online: 14 December 2024)
Research Articles
Gnomoniopsis castaneae associated with Dryocosmus kuriphilus galls in chestnut stands in Sardinia (Italy)
vol. 10, pp. 440-445 (online: 24 March 2017)
Research Articles
Case study of a new method for the classification and analysis of Dryocosmus kuriphilus Yasumatsu damage to young chestnut sprouts
vol. 5, pp. 50-59 (online: 10 April 2012)
Short Communications
Local spread of an exotic invader: using remote sensing and spatial analysis to document proliferation of the invasive Asian chestnut gall wasp
vol. 5, pp. 255-261 (online: 24 October 2012)
Research Articles
Brown rot on nuts of Castanea sativa Mill: an emerging disease and its causal agent
vol. 6, pp. 294-301 (online: 16 July 2013)
Research Articles
Investigations on yellowing of chestnut crowns in Trentino (Alps, Northern Italy)
vol. 13, pp. 466-472 (online: 07 October 2020)
Review Papers
Soil fungal communities across land use types
vol. 13, pp. 548-558 (online: 23 November 2020)
Research Articles
Effects of drought and nutrient deficiency on grafts originating from sound and shaken sweet chestnut trees (Castanea sativa Mill.)
vol. 9, pp. 109-114 (online: 19 July 2015)
Research Articles
Concordance between vascular plant and macrofungal community composition in broadleaf deciduous forests in central Italy
vol. 8, pp. 279-286 (online: 22 August 2014)
Research Articles
Potential spread of forest soil-borne fungi through earthworm consumption and casting
vol. 8, pp. 295-301 (online: 26 August 2014)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword