Conservation of Betula oycoviensis, an endangered rare taxon, using vegetative propagation methods
iForest - Biogeosciences and Forestry, Volume 13, Issue 2, Pages 107-113 (2020)
doi: https://doi.org/10.3832/ifor3243-013
Published: Mar 23, 2020 - Copyright © 2020 SISEF
Research Articles
Abstract
Ojcow birch (Betula oycoviensis Besser) is a rare Central European tree taxon, micro-populations of which are found in only several localities. With a view to maintaining the B. oycoviensis gene pool, this study tested the species’ potential for micropropagation, grafting, and propagation by cuttings. Plant material for vegetative propagation was collected from ten genotypes in the Czech Republic. In vitro culture was established from axillary buds surfaces sterilized with 0.1% HgCl2 and cultivated on woody plant (WP) medium supplemented with 1 mg l-1 6-benzylaminopurine (BAP). Two genotypes of the species were successfully multiplied by in vitro propagation using WP medium supplemented with 0-2 mg l-1 BAP. The BAP concentration of 1 mg l-1 proved to be optimal, yielding 2.5 new shoots per explant in genotype 516 and 3.5 shoots per explant in genotype 545. The shoots were rooted on half-strength Murashige and Skoog (MS) medium supplemented with various concentrations of α-naphthylacetic acid (NAA) and indole-3-butyric acid (IBA). The highest rooting percentages (72.5% and 77.5% for genotypes 516 and 545, respectively) were achieved on the medium with the combination of both auxins at concentrations of 0.3 mg l-1. The rooted plants were transferred ex vitro in substrate composed of sand, peat, and perlite (1:1:1) and acclimated in the greenhouse. After 4 weeks, more than 90% of plants survived. Grafting was carried out in spring using Betula pendula as rootstock. The efficiency of this technique ranged from 0% to 50% across genotypes, and 4 out of 10 genotypes were successfully propagated by grafting. The cuttings were treated with commercial root stimulators Stimulax I and Stimulator AS-1, planted in a mixture of peat and sand (1:1) in the greenhouse, and watered regularly. This technique resulted in 0% rooting, however, and no cutting survived until the end of the vegetation period. The results of this study show that protocols for in vitro propagation and grafting can be employed for effective mass propagation of B. oycoviensis, although these processes show genotype-dependent responses.
Keywords
Introduction
Betula oycoviensis Besser is a rare and heretofore insufficiently researched taxon in the Czech Republic ([12], [4]), its occurrence being reported from only a few sites (Pladias, database of Czech flora and vegetation - ⇒ https://pladias.cz/en/). The only confirmed location of B. oycoviensis in the country is in a nature reserve near Volyne u Výsluní in the Ore Mountains (western Czech Republic). Native distribution of Ojcow birch has been also reported for Poland, Ukraine, Russia, Denmark, Romania, and southern Sweden ([22], [23], [44], [45], [5]).
In the nature reserve near Volyne u Výsluní, only a few dozen individuals exist. The natural regeneration of this population is slow, and many of the trees suffer from poor health conditions. Only a small percentage of individuals from the birch offspring bear the phenotype of the taxon, which is considered a hybrid species ([25], [24]). Although the taxonomic status of B. oycoviensis is not clear yet ([26]), it is crucial to maintain its extant populations both in the Czech Republic and elsewhere. This is important both in terms of maintaining the genetic diversity of this birch and to resolve its taxonomic status within our key taxonomic literature ([25], [24], [20]). B. oycoviensis is morphologically distinguishable from silver birch (Betula pendula), although a number of phenotypic transitions can be usually found in natural populations. In such cases, efforts should be undertaken to integrate ex situ conservation approaches and maintain endangered populations ([35]).
Micropropagation, especially via meristem culture, is an in vitro technology suitable for maintaining endangered birch genotypes through the rapid proliferation of clone planting material. In vitro methods are advantageous as opposed to conventional cuttings, especially in old plants where the formation of new shoots and roots can be more easily controlled and stimulated by the influence of substances added to the nutrient medium ([8], [43]). Micropropagation protocols have developed in recent decades for a large number of farm and ornamental tree species. However, each plant material needs to be tested on various culture media with the additions of plant growth regulators to optimize the growth of each individual. In this study, we used protocols formerly optimized for silver birch (B. pendula) as a starting point for B. oycoviensis propagation ([17], [41], [40], [47]).
Propagation by cuttings proved difficult in birches ([14]), though partially successful results have been previously reported for several birch species. Pellett & Alpert ([33]) achieved a 60% success rate with rooted cuttings of Betula papyrifera. Marczynski & Joustra ([30]) studied the influence of length and light intensity on the survival and rooting of Betula utilis cuttings, achieving more than 90% success in rooting. In most cases, 2-3 years old trees grown from summer or winter cuttings have a chance to survive ([46], [14], [30]); however, this was not the case of our populations of B. oycoviensis.
Grafting is a propagation method demanding advanced manual skills. Tolerability of grafts and rootstocks to inosculation may vary substantially within the genus, and potential incompatibility has been reported among numerous combinations of rootstocks and grafts across different species, genera, and families ([7]). Andrews & Marquez ([2]) reported potential factors that may contribute to rootstock and graft incompatibility, such as cellular recognition, wound response, growth regulators, and toxins. Moreover, inosculation failure may be caused by anatomical inconsistency, poor processing, environmental conditions, and diseases ([13]). Václav ([46]) stated that the most commonly used combination in grafting birches is a union of an early sprouted rootstock and a winter graft of 2-year-old wood, which typically inosculates within 2-3 weeks.
In this study, we tested the viability of the three vegetative propagation methods described above (in vitro organogenesis, grafting, and cutting) in order to obtain viable individuals from mature B. oycoviensis tree branches.
Material and methods
Plant material
As the initial plant material, branches 1-2 years old and bearing buds were used. The branches were collected from ten trees 40-50 years old, numbered 34, 511, 516, 518, 520, 521, 540, 545, 533, and 552. Sampling was carried out in early spring (March) 2018 from the locality near Volyne village, in the vicinity of the town of Výsluní, Chomutov District, Czech Republic. All the collected material was stored in black polyethylene bags at 3-5 °C before its processing (grafting, cutting, in vitro culture). Experiments on B. oycoviensis propagation via cuttings and grafting were established the second day after branches were collected. Five days after the branches collection, the plant material was used for in vitro propagation. For grafting B. oycoviensis, containerized B. pendula seedlings 2-3 years-old were used as rootstock.
In vitro culture
Branchlets of B. oycoviensis were divided into one-nodal segments 1-2 cm long. The scales were removed from buds and the explants were washed in 200 ml distilled water with addition of 1-2 drops of Tween 20® (Sigma-Aldrich, St. Louis, MO, USA) for 10 min. The buds were then sterilized in 0.1% HgCl2 for 6 min, rinsed three times in sterile distilled water, and placed in 230 ml baby food culture jars containing 30 ml of woody plant (WP) medium ([28]) solidified with 8 g l-1 Danish® agar (Carl Roth, Karlsruhe, Germany), containing 100 mg l-1 myo-inositol, and supplemented with 1 mg l-1 6-benzylaminopurine (BAP). After adjustment of pH to 5.7, the medium was sterilized in an autoclave at 121 °C and 118 kPa for 30 min. The explants were cultivated under 16/8 h light/dark photoperiod (photosynthetic photon flux density 35 ± 2 μmol m-2 s-1 cool white fluorescent light), at temperature 24 ± 1 / 20 ± 1 °C (light/dark).
From each genotype, 20 explants were used after surface sterilization in two replications. The newly sprouted shoots were regularly subcultured every 2-3 weeks on the same medium until sufficient plant material was obtained. Dry, brown and contaminated explants were discarded during the in vitro cultivation.
In vitro multiplication and rooting
In vitro propagation experiment was carried out on segments of about 1.5 cm in length with two buds. The explants were cultivated on WP medium supplemented with 0.5, 1, and 2 mg l-1 BAP. As a control, plants grown on the medium without growth regulators were used. The in vitro cultures were kept under the cultivation conditions described above. After 8 weeks of cultivation, the shoot length, number of newly developed shoots, and approximate length of new shoots were evaluated. The intensity of callus development was also recorded.
The shoots developed in vitro were rooted on a half-strength MS medium ([32]) with addition of 0.3 or 0.5 mg l-1 indole-3-butyric acid (IBA), 0.3 or 0.5 mg l-1 α-naphthylacetic acid (NAA), or their combination (0.3 mg l-1 IBA + 0.3 mg l-1 NAA). Culture medium without auxins was used as a control. The number of roots per shoot, length of roots, and rooting percentage were evaluated after 4 weeks of cultivation.
Ex vitro transfer
Well-rooted shoots were removed from the cultivation jars and the roots were washed with water to remove residues of the culture medium. The plants were transferred into sterile substrate (sand:peat:perlite, 1:1:1) within plastic pots (7×7×8 cm), watered, and then treated with 1% Previcur Energy® (propamocarb 530 g l-1, fosetyl-Al 310 g l-1 - Bayer Garden, Leverkusen, Germany). The plants were cultivated in a greenhouse under a photoperiod of 16/8 h (day/night) and temperature 24 ± 2 / 18 ± 2 °C (day/night). They were acclimated by gradually decreasing air humidity from 95% to 60%. One month after the ex vitro transfer, the plants were transferred to partially shaded open growing plots. During the ex vitro cultivation, the plants were fertilized using 0.1% Kristalon Start (Agro CZ; N-P-K = 19-6-20 + 3% Mg + 7.5% S + microelements B, Mo, Fe, Cu, Mn, Zn) once every 2 weeks during vegetation growth.
Cuttings
Winter (hardwood) cuttings 10-12 cm in length were treated with Stimulax® I powder root stimulator (IAA, 3-indoleacetic acid min. 0.06%, NAA min. 0.06%, IBA min. 0.05% - Hü-Ben, Cerčany, Czech Republic) and Stimulator AS-1 (nicotinic acid min. 1%, NAA min. 1% - Nohel Garden, Dobríš, Czech Republic). The basal parts of the cuttings were dipped into the rooting stimulator and planted in substrate composed of peat and sand (1:1). Cuttings not treated with the stimulator were used as control. The cuttings were cultivated in the greenhouse and watered to keep a constant substrate moisture. To prevent fungal infection of the cultures, they were treated with 1% Previcur Energy® every 2 weeks.
Grafting
Grafting of B. oycoviensis was performed on B. pendula rootstocks at the beginning of March 2018. The rootstock was cut to about 30-40 cm in length and the winter scions (10-15 cm long) with no shoots were placed on the rootstock at two heights on the side (side-veener graft) or on the top (splice graftor top bark grafting forthicker rootstock). The graft point was tied and fastened by rubber tape and coated with Tervanol F® balsam (turpentine, oil, 1% thiabendazole, limonene - Dr. Stähler, Schopf GmbH, Bitterfeld-Wolfen, Germany). The plants were left in a transparent plastic foil tunnel until the end of May 2018, then moved to an open growing area. During vegetation, the plants were watered as needed. If newly sprouted shoots (epicormic branches) appeared on the rootstock, these were removed.
Experimental design and data analysis
For both in vitro multiplication and rooting experiments, 20 explants in two replications were used per treatment and tested genotype. The experiment was set up in a completely randomized design. Percentage success of shoots initiation, length increment of the initial shoots, number of newly created shoots per explant, length of the newly grown shoots, callus formation, number of roots per explant, length of roots, and percentage of rooting were monitored at regular intervals.
For cuttings, 20 explants in two replications were used per treatment and tested genotype. The rooting percentage was evaluated after 6 months.
In grafting, for each genotype, 12 rootstocks were used in two replications. The success of graft acceptance was monitored by genotype and location of the graft.
The significance of differences among means was assessed using Kruskal-Wallis test (as the assumptions of the ANOVA were not satisfied in all cases). Subsequent multiple comparison tests ([42]) were conducted for identifying statistically significant differences between particular variants.
Binomial testing ([1]) was used for evaluating the differences between variants where binomial parameters were recorded (i.e., initiation of the culture [yes/no], successful rooting [yes/no], ex-vitro transfer [successful/unsuccessful], success of grafting [successful/unsuccessful]).
All statistical analyses were conducted using the R software ([39]). Significance level (α) was set at 0.05 for all analyses. Plots were created using the R package “ggplot2” ([50]).
Results and discussion
In vitro culture
Our study aimed to compare the success of vegetative propagation in B. oycoviensis using different methods (in vitro cultures - especially via meristem cultures -, grafting and cuttings). Particular attention was given to the successful initiation of in vitro culture and propagation of B. oycoviensis. Our study showed the efficacy of the surface sterilization of explants using 0.1% HgCl2, which prevented the contamination of cultures and their browning. Most authors do not report data about the sterilization success in their studies, but according to Raghu et al. ([36]) and Badoni & Chauhan ([3]) 30% loss of explants is caused by contamination.
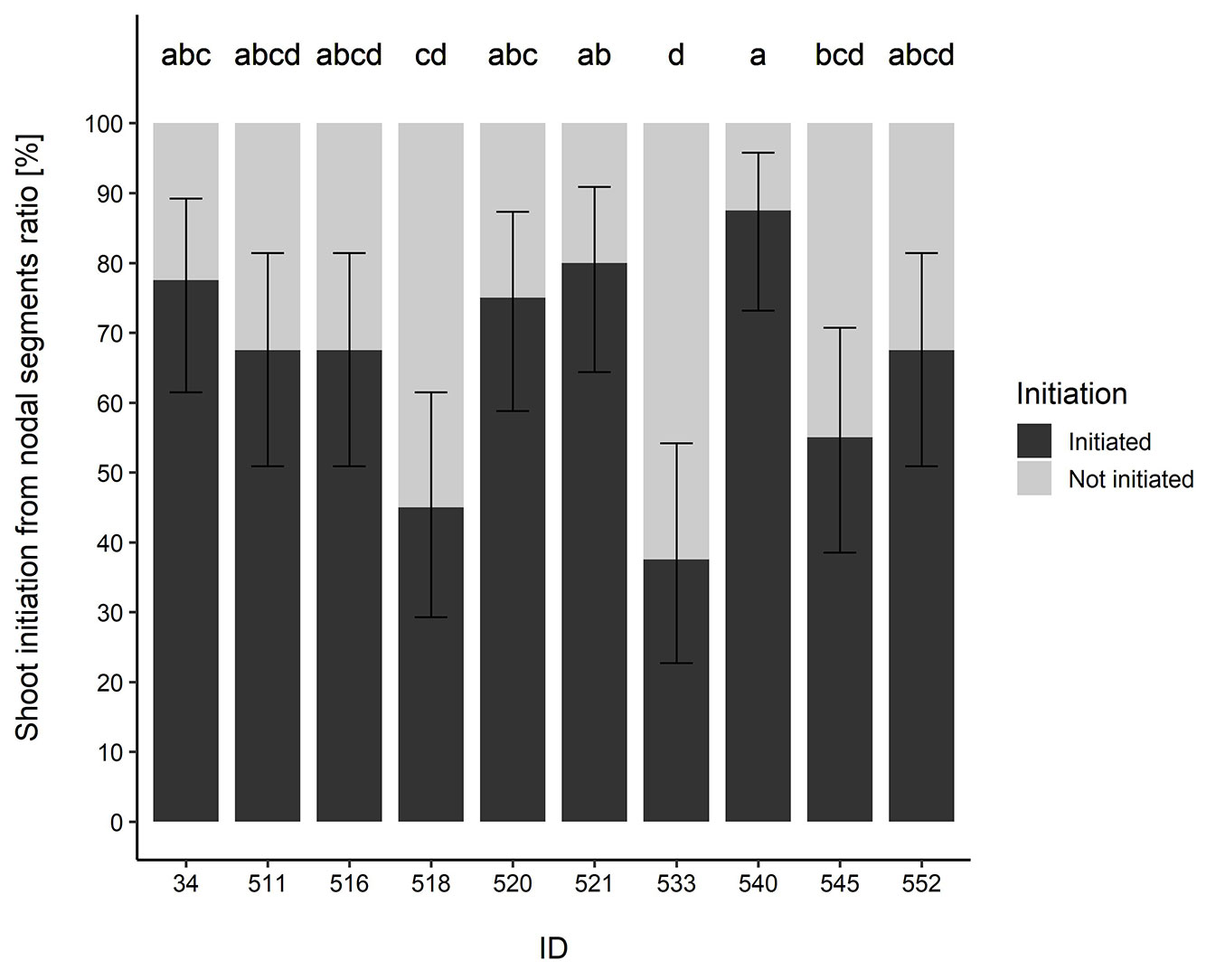
The buds started to sprout after 2-3 weeks of cultivation on WP medium supplemented with 1 mg l-1 BAP. Two weeks later, 37.5-87.5% of the explants of particular genotypes had germinated (Fig. 1). This corresponds to former results reported in the literature ([17], [40], [41]), where lower concentrations of 0.5-2.0 mg l-1 BAP without addition or auxin or with addition of auxin NAA, IBA, or IAA at concentrations 0.0-0.2 mg l-1 appeared to be suitable concentrations of cytokinins (BAP) for B. pendula culture. The highest sprouting efficiency was recorded in genotype 540, the lowest in genotype 533 (Fig. 1). During the initial phases of cultivation, explants of some genotypes tended to turn brown and, due to generally slow growth, they did not produce new and viable shoots. Despite regular subculture of the explants onto the fresh medium, all explants of genotypes 518 and 533 died after 2 months, followed by genotypes 34, 520, 521, 540, and 552 three months later. Only for genotypes 516 and 545, the in vitro cultures were successfully maintained and the plants regularly multiplied (Fig. 2a). Therefore, only these two genotypes were used for the multiplication experiment using different concentrations of BAP. Ryynänen & Ryynänen ([40]) had succeeded to grow 2 out of 5 plants to mature trees after micropropagation in curly birch (Betula pendula var. carelica). Contrastingly, Máchová et al. ([29])successfully transferred all (7) individuals taken from the wild to in vitro culture. In our case, only 2 out of 10 tested trees were successfully grown to a mature stage. This demonstrates one of the potential pitfalls of the in vitro culture, as taxon and clone specificity are manifested in micropropagation ([49]).
Fig. 1 - Sprouting percentage of nodal segments (n=40) belonging to 10 Betula oycoviensis genotypes, 28 days after their initial cultivation on WP medium supplemented with 1 mg l-1 BAP. Means followed by the same letter within columns do not differ significantly (p>0.05) after Kruskal-Wallis test. Error bars depict 95% confidence interval.
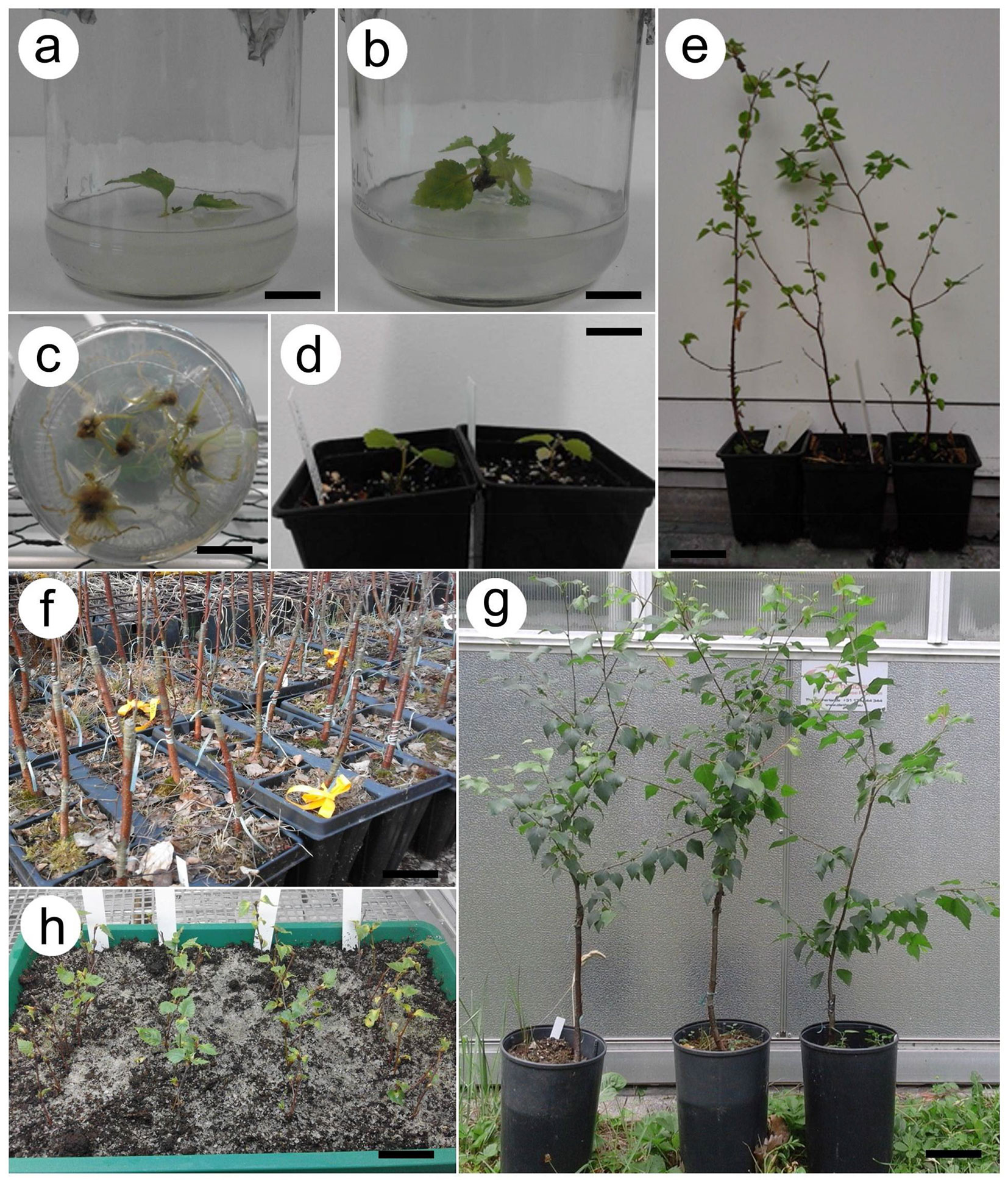
Fig. 2 - (a) Betula oycoviensis initial explant on WP medium with 1 mg l-1 BAP (scale bar = 1.2 cm). (b) B. oycoviensis explant after 8 weeks on WPM medium with 1 mg l-1 BAP (scale bar = 1.1 cm). (c) Rooting explant on ½ MS with 0.3 mg l-1 IBA and 0.3 mg l-1 NAA (bar = 1.2 cm). (d) Rooted B. oycoviensis plants transplanted into substrate after 4 weeks of acclimatization (scale bar = 1.5 cm). (e) Growing B. oycoviensis 8 months old from the in vitro culture (genotype 545 - scale bar = 6 cm). (f) Rootstocks with scions of B. oycoviensis in side (side-veneer graft) and top (splice graft or bark grafting) positions (scale bar = 4 cm). (g) B. oycoviensis plants 1 year after bark grafting (scale bar = 6.4 cm). (h). Cuttings of B. oycoviensis 8 weeks after culture initiation (scale bar = 4 cm).
Ditmar ([9]) used various cultivation media for growing curly birch; the best results were achieved on WP medium, as also confirmed by Chalupa ([16]), Wynne & McDonald ([51]), Häggman et al. ([15]), Businge et al. ([6]), and Girgžde & Samsone ([11]). Cultivation on MS medium has not been so successful ([9], [18]). On the contrary, Rathwell et al. ([37]) reported more promising results in Betula lenta cultures on MS and Driver and Kuniyuki Walnut medium (DKW - [10]) as compared to WP medium. In our case, the shoots were vigorous and green on the control WP medium without BAP supply, but they grew less intensively, reaching just 2 and 4 mm after 4 and 8 weeks, respectively (Tab. 1). Almost no new shoots developed (0.2 and 0.15 shoots per explant, respectively) in genotypes 516 and 545. Callus on the base part of the explants did not develop (Tab. 1).
Tab. 1 - Effect of benzylaminopurine (BAP) concentrations on new shoot development within 8 weeks of in vitro cultivation of Betula oycoviensis explants (n=40). Means followed by the same letter within columns do not differ significantly (P>0.05) after Kruskal-Wallis test. Data were recorded after 8 weeks of culture. (-): no callus; (*): little callus; (**): moderate callus; (***): large callus.
| Nutrient media | BAP Conc. (mg l-1) |
Genotype | Length increment of initial shoots (mm) |
Number of new shoots / explant |
Length of new shoots (mm) |
Callus |
|---|---|---|---|---|---|---|
| (mean ± SD) | (mean ± SD) | (mean ± SD) | ||||
| WP | 0 | 516 | 4.60 ± 2.93 bc | 0.20 ± 0.40 bc | 1.05 ± 2.18 c | - |
| 545 | 4.10 ± 1.99 c | 0.15 ± 0.36 c | 0.90 ± 2.30 c | - | ||
| WP | 0.5 | 516 | 9.80 ± 3.75 ab | 1.10 ± 0.62 ab | 6.68 ± 3.21 ab | * |
| 545 | 14.45 ± 2.56 ad | 1.75 ± 0.69 ad | 7.83 ± 2.80 b | * | ||
| WP | 1 | 516 | 12.15 ± 5.58 a | 2.50 ± 1.02 a | 6.90 ± 2.28 ab | ** |
| 545 | 18.80 ± 3.25 d | 3.50 ± 1.12 d | 7.50 ± 2.81 b | ** | ||
| WP | 2 | 516 | 10.30 ± 3.70 a | 2.20 ± 0.68 a | 5.39 ± 1.51 a | *** |
| 545 | 11.10 ± 2.55 a | 2.10 ± 0.83 a | 5.59 ± 1.86 a | *** |
The plants on WP medium supplemented with 0.5 mg l-1 BAP started to produce new and healthy shoots (1.1 and 1.75 shoots per explant in genotypes 516 and 545, respectively) during 8 experimental weeks while showing no signs of necrosis. The callus started to develop on the plant base. A similar tendency was recorded in plants on culture medium with 1 mg l-1 BAP (2.5 and 3.5 shoots per explant in genotypes 516 and 545, respectively). However, plants subjected to this treatment yielded the highest number of new shoots, as compared with other BAP concentration (Tab. 1, Fig. 2b). The newly produced shoots were green, viable, and without morphological abnormalities. Similarly, Ditmar ([9]) used WP medium supplemented with 0.5 mg l-1 BAP to produce 2.9 shoots per explant of curly birch.
The callus development was more intensive when BAP concentration was increased beyond 1 mg l-1. Increasing BAP concentration too far beyond that level, however, reduced new shoot development and led to necrosis. Thus, the highest BAP concentration tested (2 mg l-1) led to the production of fewer shoots, which after 3-4 weeks of cultivation, had brown shoot tips and leaves that also turned brown. This treatment brought the most intensive production of callus seen in the whole experiment. Mirabbasi & Hosseinpour ([31]) report a similar experience with Ulmus glabra, wherein increasing cytokinin concentrations also increased the tendency for callus formation and necroses. When they used no plant growth regulators, neither necroses nor callus were recorded.
The shoots that had developed within the multiplication experiment did not root, and thus they were transferred onto the ½ MS media containing auxins (IBA, NAA). On culture medium without plant growth regulators, approximately 17.5% and 22.5% of shoots rooted for genotype 516 and genotype 545, respectively. Prolonged cultivation of plants on this medium, however, caused browning and death of the plants.
Although the rooting percentage and number of roots per explant increased with auxin concentration, the roots showed poor growth in length (Tab. 2, Fig. 3). Addition of IBA into the culture medium led to formation of callus on the basal part of explants. This phenomenon was not observed in plants cultivated on media with added NAA, but shoot tips of plants cultivated longer on the medium with higher NAA concentration (0.5 mg l-1 NAA) turned brown and started to wither.
Tab. 2 - Effect of indole-3-butyric acid (IBA) and α-naphthylacetic acid (NAA) concentrations on new root development within 4 weeks of in vitro cultivation of Betula oycoviensis explants (n=40). Means followed by the same letter within columns do not differ significantly (P>0.05) after Kruskal-Wallis test. Data were recorded after 4 weeks of culture.
| Nutrient medium | Concentration (mg l-1) |
Genotype | Number of roots by explant |
Root lengths (cm) |
|
|---|---|---|---|---|---|
| IBA | NAA | (mean ± SD) | (mean ± SD) | ||
| ½ MS | 0 | 0 | 516 | 0.20 ± 0.40 a | 1.12 ± 0.48 a |
| 545 | 0.25 ± 0.43 ab | 1.42 ± 0.56 ab | |||
| ½ MS | 0.3 | 0 | 516 | 0.50 ± 0.92 ab | 0.95 ± 0.45 ab |
| 545 | 0.80 ± 1.17 ab | 1.16 ± 0.44 abc | |||
| ½ MS | 0.5 | 0 | 516 | 1.75 ± 1.99 abc | 1.09 ± 0.46 bc |
| 545 | 2.55 ± 2.46 abcd | 1.27 ± 0.60 c | |||
| ½ MS | 0 | 0.3 | 516 | 2.60 ± 2.40 abcd | 0.93 ± 0.34 bc |
| 545 | 2.70 ± 2.32 abcd | 1.05 ± 0.41 c | |||
| ½ MS | 0 | 0.5 | 516 | 2.85 ± 2.55 abcd | 0.98 ± 0.39 c |
| 545 | 3.65 ± 2.59 bcd | 1.03 ± 0.40 c | |||
| ½ MS | 0.3 | 0.3 | 516 | 4.55 ± 3.12 cd | 0.81 ± 0.27 bc |
| 545 | 5.70 ± 3.05 d | 0.79 ± 0.25 bc | |||
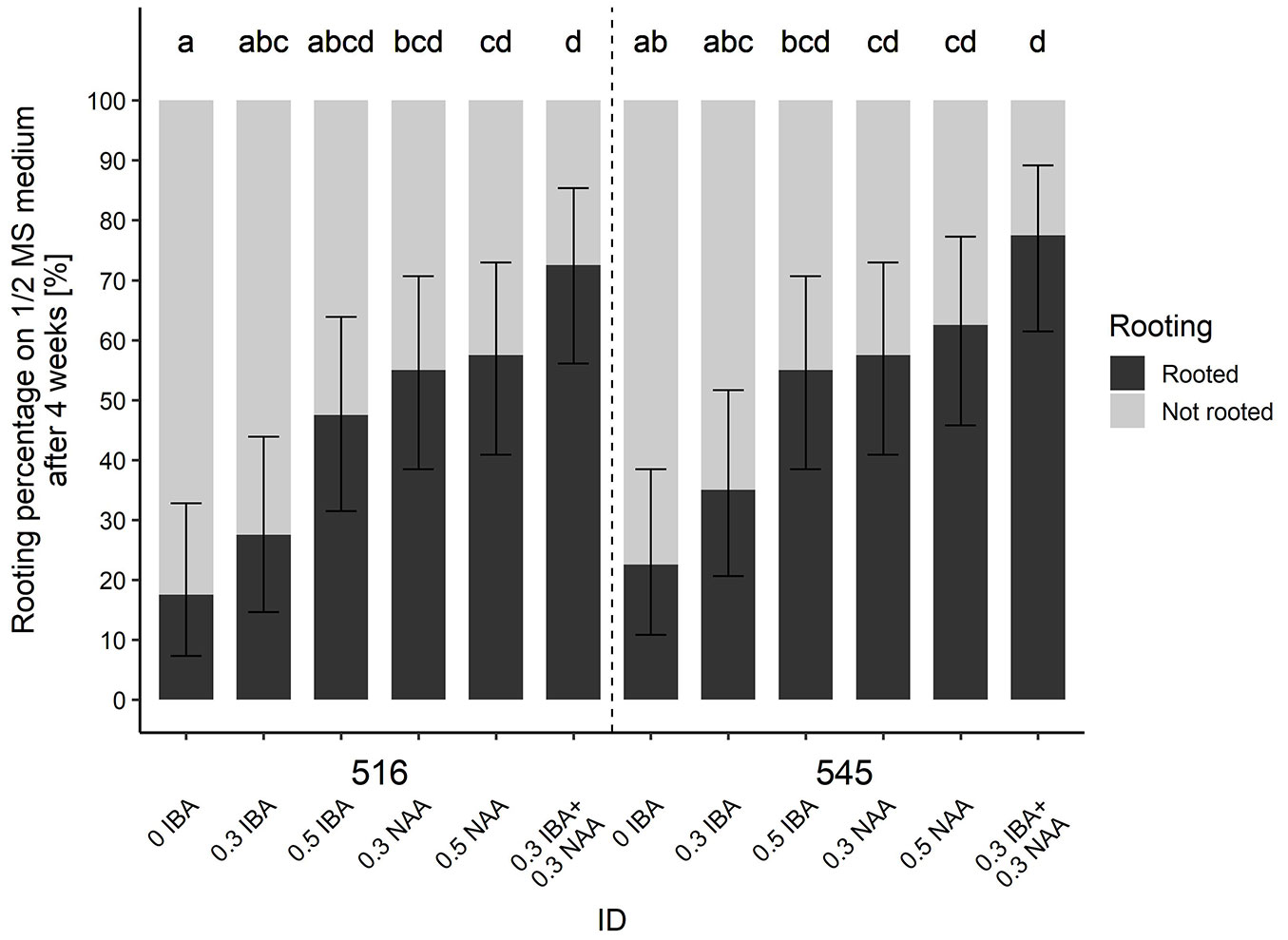
Fig. 3 - Effect of auxins on rooting of Betula oycoviensis shoots (n=40) belonging to genotypes 516 and 545 after 28 days of their cultivation on half-strength MS medium. Means followed by the same letter within columns do not differ significantly (P>0.05) after Kruskal-Wallis test. Error bars depict 95% confidence interval.
The most efficient rooting medium proved to be half-strength MS medium with a combination of IBA and NAA, both at concentration 0.3 mg l-1 (Fig. 2c). On this medium, we observed the highest rooting percentage for genotypes 516 and 545 (72.5% and 77.5%, respectively - Fig. 3) and the highest number of roots per explant (4.5 and 5.7, respectively - Tab. 2). Wynne & McDonald ([51]) reported 60-100% Betula pendula rooting percentage in relation at IBA concentration of 0-0.3 mg l-1. Máchová et al. ([29]) reported 100% rooting efficiency in Betula nana grown on WP medium with 0.5 mg l-1 IBA. Successful rooting results of Betula pubescens are also reported by Kauppi et al. ([21]) and by Wakita et al. ([48]) in Betula platyphylla on MS medium with IBA auxin (0.5 mg l-1) and NAA (0.02 mg l-1).
Rooted shoots were transferred ex vitro and acclimated in the greenhouse. Plants rooted on all types of media were included into this experiment. Eight weeks after ex vitro transfer (Fig. 2d), at least 90% of plants were still surviving (100% of plants in the case of all rooting media, 90% of plants from the control medium - Fig. 2e). Similarly, Máchová et al. ([29]) reported 98% success in ex vitro transfer in the case of B. nana. Rathwell et al. ([37]) reported that acclimatization of B. lenta was poor with only 36.8% success, even though the in vitro rooting efficiency had been 80% on ½ MS and ½ DKW medium with 4.1 mg l-1 IBA. In our experiment, no differences in plant survival were observed among plants from various rooting media. All plants kept growing, new leaves developed, and no abnormalities were observed.
Cuttings
Hardwood cuttings of B. oycoviensis treated with root stimulators were transferred to the greenhouse (Fig. 2h) as described above. The cuttings were evaluated at the end of the vegetative season in 2018. No roots developed on any of the cuttings and no callus development on the basal part of the cuttings was observed (regardless they were treated with root stimulators or not). Hartmann & Kester ([14]) had stated that birch cuttings are difficult to root due to low species-specific rooting potential. Similarly, Václav ([46]) reported that in curly birch winter cutting growth was not very successful. Winter cuttings of this species usually take longer to root (even as long as 2 years) than do summer cuttings from trees over 3 years of age. Marczynski & Joustra ([30]) used Rhizopon® AA powder containing 2% IBA for rooting and achieved 96-99% successfully rooted cuttings of B. utilis, 77-85% of which survived until the following year. Unlike our study, the aforementioned experiments used young plant material for vegetative propagation. Nevertheless, cuttings collected from older trees are generally regarded as difficult to root ([13], [34]).
Grafting
A partial success with variable results were observed for B. oycoviensis grafted onto silver birch rootstock (Fig. 2f). The best results were achieved with genotype 34. At the end of the vegetation season, 50% of the grafted trees from that genotype were assessed as successfully growing. The success rate was 33% in genotypes 520 and 545, and 8% in genotype 521. The grafts on rootstocks of the mentioned trees grew without abnormalities during the growing season and created a new assimilation apparatus and new shoots (Fig. 2g). No inosculated graft was found at the end of the 2018 vegetation season on the other grafted trees. The position of the graft (side vs. top) did not significantly affect the success of the inosculation, as well as the position of the scion (side vs. top). In fact, an identical 33% success rate was achieved for scions growing in (i) both side and top position, (ii) only in the side position, or (iii) only in the top position.
Jermakov ([19]) used curly birch scions grafted onto wild birch rootstocks 4-5 years old in open areas, achieving the highest success rate of 60% with the best clone and 26% success on average. The same author further reported that grafting in the greenhouse had an average success rate of 52%, and several clones showed as much as 100% success in inosculation and subsequent growth. Ljubavskaja ([27]) summarized her numerous experiments with curly birch grafting and concluded the best grafting method to be the side-veneer in B. pubescens and possibly in B. verrucosa. The diameter of the graft should be > 4 mm and length 4-5 cm, bearing 3-4 buds. The root material (rootstock) should be 4-5 years old with a stem diameter of 3-5 cm, and the best period for transplantation is in February. Václav ([46]) used 2-year-old B. pendula rootstocks grafted in January and 93% of the curly birch grafts successfully inosculated. Ranney & Whitman ([38]) investigated the possibility of vegetatively propagating B. platyphylla var. japonicavia its scion grafted onto various birch rootstocks (B. nigra, B. papyrifera, B. szechuanica, and B. pendula). They obtained the best results using B. nigra as rootstock, whereby 100% of the grafts survived for 2 years after grafting. That was in contrast with B. pendula, upon which only 80% of the grafts survived. The poorest results were achieved with B. szechuanica, where only 30% of the grafts survived. Choice of a suitable grafting rootstock is therefore of great importance.
Conclusion
Our results on the vegetative propagation of B. oycoviensisvia organogenesis and grafting suggest a real possibility for successfully addressing the need to maintain the B. oycoviensis gene pool. Consideration should be given to conducting further research in the field of cuttings (especially in relation to summer cuttings) and for further improving the growth of explants in vitro. Selected results of this study can point to further directions in investigating the reproduction of birches and other genera by vegetative propagation, through in vitro cultures, grafting, and cutting.
List of abbreviations
The following abbreviations have been used throughout the text:
- BAP: 6-benzylaminopurine;
- IAA: 3-indoleacetic acid;
- DKW: Driver & Kuniyuki (1984) medium;
- IBA: indole-3-butyric acid;
- MS: Murashige & Skoog (1962) medium;
- NAA: α-naphthylacetic acid;
- WP: Lloyd & McCown (1980) medium.
Authors’ Contributions
JV and IK conceived and designed the experiments; MB and RL collected the samples and evaluated the different traits on the trees. RL did the statistical analysis of the data. JV, IK and IV wrote the manuscript with the assistance of the other authors.
Acknowledgements
This work was supported by the TA CR agency (Project No. TACR TH03030339). Technical facilities for experiments were provided by the in vitro laboratory of the Faculty of Forestry and Wood Sciences and nursery facilities by the Department of Silviculture in Truba, Kostelec nad Cernými lesy (Czech Republic). We thank English Editorial Services and Environmental English Ltd. for proofreading of the manuscript.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Ivan Kuneš 0000-0002-1875-384X
Rostislav Linda 0000-0002-9602-7915
Martin Baláš
Czech University of Life Sciences Prague, Faculty of Forestry and Wood Sciences, Kamýcká 1176, Praha 6 Suchdol (Czech Republic)
Czech University of Life Sciences, Faculty of Tropical AgriSciences, Kamýcká 129, Praha 6 Suchdol (Czech Republic)
Corresponding author
Paper Info
Citation
Vítámvás J, Kuneš I, Viehmannová I, Linda R, Baláš M (2020). Conservation of Betula oycoviensis, an endangered rare taxon, using vegetative propagation methods. iForest 13: 107-113. - doi: 10.3832/ifor3243-013
Academic Editor
Werther Guidi Nissim
Paper history
Received: Sep 18, 2019
Accepted: Jan 21, 2020
First online: Mar 23, 2020
Publication Date: Apr 30, 2020
Publication Time: 2.07 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2020
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 43790
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 36177
Abstract Page Views: 3598
PDF Downloads: 3180
Citation/Reference Downloads: 2
XML Downloads: 833
Web Metrics
Days since publication: 2167
Overall contacts: 43790
Avg. contacts per week: 141.45
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2020): 2
Average cites per year: 0.33
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Technical Reports
De novo adventitious root formations in mini-cuttings of Azadirachta indica in response to different rooting media and auxin treatments
vol. 8, pp. 558-564 (online: 09 December 2014)
Research Articles
Effect of family, crown position, number of winter buds, fresh weight and the length of needle on rooting ability of Pinus thunbergii Parl. cuttings
vol. 9, pp. 370-374 (online: 11 January 2016)
Technical Reports
Effects of different mechanical treatments on Quercus variabilis, Q. wutaishanica and Q. robur acorn germination
vol. 8, pp. 728-734 (online: 05 May 2015)
Research Articles
Use of alternative containers for promoting deep rooting of native forest species used for dryland restoration: the case of Acacia caven
vol. 10, pp. 776-782 (online: 02 September 2017)
Short Communications
Effect of four levels of shade on survival, morphology and chlorophyll fluorescence of Nothofagus alessandrii container-grown seedlings
vol. 8, pp. 638-641 (online: 08 January 2015)
Research Articles
Effects of different nut pretreatments and substrates on germination and seedlings growth of Neocarya macrophylla Sabine in Basse Casamance, Senegal
vol. 17, pp. 346-352 (online: 03 November 2024)
Research Articles
Seedling quality and short-term field performance of three Amazonian forest species as affected by site conditions
vol. 17, pp. 80-89 (online: 21 March 2024)
Research Articles
Growth and physiological acclimation to shade in young plants of Adesmia bijuga Phil., a critically endangered species in central Chile
vol. 14, pp. 307-312 (online: 01 July 2021)
Short Communications
The effects of salicylic acid, oxalic acid and chitosan on damping-off control and growth in Scots pine in a forest nursery
vol. 13, pp. 441-446 (online: 24 September 2020)
Research Articles
Growth, morphology, and biomass allocation of recently planted seedlings of seven European tree species along a light gradient
vol. 13, pp. 261-269 (online: 03 July 2020)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword