Effects of different mechanical treatments on Quercus variabilis, Q. wutaishanica and Q. robur acorn germination
iForest - Biogeosciences and Forestry, Volume 8, Issue 6, Pages 728-734 (2015)
doi: https://doi.org/10.3832/ifor1423-008
Published: May 05, 2015 - Copyright © 2015 SISEF
Technical Reports
Abstract
Delayed and uneven germination of acorns has a negative effect on seedling quality and yield in seedlings. To address this issue, the effects of different mechanical treatments were studied, including a control (CK), removal of cup scar (RS), removal of pericarp (RP), removal of pericarp and 1/2 of the cotyledon (HC) and removal of pericarp and 2/3 cotyledon (TC), on the germination of Quercus variabilis, Q. wutaishanica and Q. robur acorns and pericarp thickness. The results showed that (1) RP and HC treatments significantly decreased root and shoot mean germination time, increased rooting and shooting germination percentage, and improved the root and shoot synchronization and vigor indexes of the three species’ acorns; (2) the acorns from the TC treatment significantly reduced root and shoot mean germination time and significantly induced the root and shoot synchronization index for all three species; and (3) the RS treatment significantly reduced the root and shoot mean germination time of the three species. Therefore, RP and HC treatments can effectively accelerate germination and regular seedling, which are important in the propagation of Q. variabilis, Q. wutaishanica and Q. robur seedlings. Even and quick germination help reduce acorn predation.
Keywords
Pericarp and Cotyledon Excision, Pericarp Thickness, Acorn Germination, Quercus wutaishanica
Introduction
Due to their richness in proteins and carbohydrates, acorns are very attractive to animals as a food source and suffer heavy predation by a number of consumers. It is well documented that resistance and tolerance to biotic and abiotic stresses are alternative defense strategies for plants ([23]). These mechanisms prevent consumption from predators using a number of physical barriers and chemical defenses ([12], [28]): (1) to escape certain pathogens or insects, the time of first and last acorn germinations can differ by up to several weeks ([26]), which leads to asynchronous germination ([9]); (2) under moderate conditions, the biomass remaining in the cotyledon serves as a reserve that can be used for seedling growth and survival, and the remaining biomass is used to defend against such stress factors as frequent shoot destruction, low light and low soil fertility ([7], [5], [14]); (3) chemical defense by plant secondary metabolites, e.g., coumarin, quinine, tannins ([22], [27]). While these mechanisms assist oak acorns during germination, they have a negative effect on seedling quality in a nursery setting, where fast and simultaneous germination is required. If germination time is long, the earlier plants quickly develop leaves that may overshadow neighboring seedlings and restrict access to water ([10]).
Over the past several decades, numerous studies have found that acorns of many oak species exhibit characteristics of low germination percentage ([2]) and delayed and irregular germination in field and laboratory experiments ([26], [10]). Several feasible practice technologies and their associated mechanisms have been published for some oak species popular in Europe, North America and Asia ([26], [25], [29], [11], [10]). Removing pericarp can increase germination percentage, and cutting off parts of the distal end of the cotyledons can induce faster emergence and improve germination percentage ([25], [11], [10]). Our previous work on the acorns of Q. aliena var. acuteserrata has supported these results ([16]). Moreover, previous studies on mechanical scarification have dealt exclusively with acorn germination and seedling emergence ([10]). The consequences of mechanical scarification for roots are poorly understood.
We chose three oak species to test the effects of mechanical treatments on acorn germination and seedling growth. These species were selected because: (1) of the proven resistance of the pericarp of Q. variabilis, restricting water uptake and gas exchange ([24]), and the cutting off of different parts of the cotyledon having provoked different effects on Q. variabilis acorn germination ([11]); (2) several similar studies have been conducted on Q. robur acorns, but the results are inconsistent ([25], [20], [10]); (3) Q. wutaishanica is an important and common species in warm temperate zone deciduous forest in China. The time from root to shoot germination is long, and our previous research indicated that mechanical treatments affect root and shoot emergence of Q. aliena var. acuteserrata ([16]). The aim of this study was to determine the effects of mechanical treatments on delayed and asynchronous germination of three oak species, and whether the effects are the same among the different species. We also analyzed the role that pericarp thickness plays in acorn germination, and assumed that the thinner the pericarp, the more easily the shoot could emerge from the apex.
Materials and methods
Acorn collection and treatment
In mid-September of 2009, acorns of Q. variabilis, Q. wutaishanica and Q. robur were collected from multiple trees growing in the Beijing Botanical Garden of the Chinese Academy of Sciences (116° 20′ E, 39° 56′ N). The mother tree characteristics of Q. variabilis, Q. wutaishanica and Q. robur acorns are presented in Tab. 1.
Tab. 1 - Characteristics of Quercus variabilis, Q. wutaishanica and Q. robur trees from which acorns were collected. (DBH): diameter at breast height.
| Tree Species | DBH (cm) |
Plant height (m) |
Clear bole height (m) |
Crown width EW/NS (m) |
Quantity (kg) |
Acorn maturity time |
|---|---|---|---|---|---|---|
| Q. variabilis | 29 | 12 | 4.5 | 7.5/6.2 | 10 | late August |
| Q. wutaishanica | 33 | 5.7 | 2.2 | 8.0/7.4 | 10 | mid-September |
| Q. robur | 26 | 8.0 | 3.2 | 6.0/5.2 | 10 | mid-September |
After collection, acorns were soaked in water, and all those still floating after 5 min were considered nonviable and removed ([17], [15]). The remaining acorns were air dried and stored in 5-ml (0.127 mm) polyethylene bags, which are permeable to carbon dioxide and oxygen, but largely impermeable to moisture ([2]), under temperature of 3 ± 1 °C.
Prior to storage, 100 acorns were randomly selected so that certain basic acorn morphological characteristics could be measured (Fig. 1). Acorn mass (3.93 ± 1.31 g for Q. variabilis, 2.07 ± 0.47 g for Q. wutaishanica and 2.23 ± 0.46 g for Q. robur) was measured using a 1/1000 electronic balance. Acorn length, package length, diameter, pericarp thickness and diameter of the cup scar were determined by a vernier caliper. The difference in pericarp thickness at the apex, middle, and base of acorns was recorded to determine whether or not pericarp thickness had an effect on acorn germination.
Fig. 1 - Profile of Quercus variabilis, Q. wutaishanica and Q. robur acorn measurement. (L): Length; (PL): Package length; (D): Diameter; (SCD): Diameter of cup scar.
Germination tests
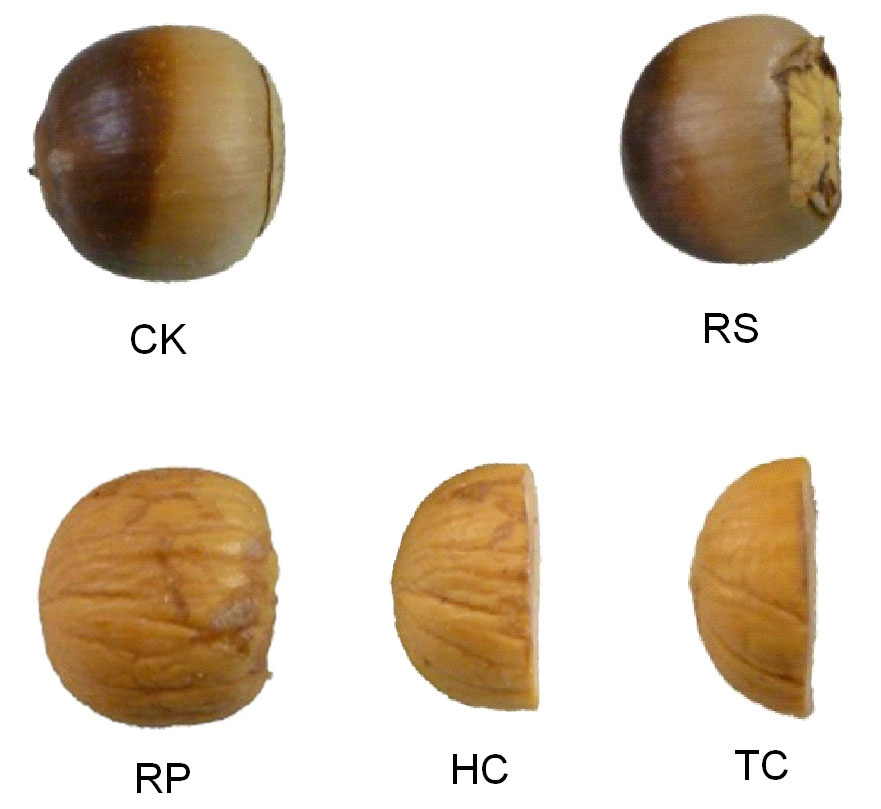
To test the effect of pericarp and cotyledon on root and shoot emergence and establishment, the acorns of each species were randomly assigned to one of the following mechanical scarification treatments or categories (Fig. 2): (1) the control (CK); (2) removal of the cup scar (RS); (3) removal of the pericarp (RP); (4) removal of the pericarp and half of the cotyledon (HC); (5) removal of the pericarp and 2/3 of the cotyledon (TC). On September 19, 2009, 15 acorns from each category were placed onto three pieces of filter paper moistened with distilled water in petri dishes (11.5 cm diameter) at a constant temperature of 25 °C with 8 hours light in an incubator. Each category had three replicates. As acorns germinated, root (the length of radicle being longer than acorn length) and shoot emergence were checked every seven days, and the length of all roots and shoots was measured at the end of the experiment (137 days).
Fig. 2 - The schematic diagram of different mechanical treatments applied to Q. variabilis acorns. (CK): control; (RS): removal of cup scar; (RP): removal of pericarp; (HC): removal of pericarp and half of the cotyledon; (TC): removal of pericarp and 2/3 of the cotyledon.
Germination percentage (GP), vigor index (VI), mean germination time (t - [21]) and the synchronization index (Z - [21]) of roots and shoots were calculated using the following formulae (eqn. 1 to eqn. 4):
In these formulae, ni refers to the number of seeds germinated in time i (not the accumulated number, but the number corresponding to the ith Cn1, 2 observation); T is the total number of tested seeds; S represents the average length of root and shoot on the 137th day; ti refers to the time from the start of the experiment to the ith observation (days); and k is the last germination time.
Statistical analyses
Analysis of variance (ANOVA) was used to compare differences in the final root and shoot emergence rate and length, t, Z, VI and pericarp thickness among different mechanical scarifications. The results were expressed in percent, and data were transformed using arcsin for ANOVA analyses. All statistical tests were performed using the software package SPSS® version 11.5 (IBM, NY, USA) and considered significant at P=0.05.
Results
Effect of different mechanical treatments on acorn emergence
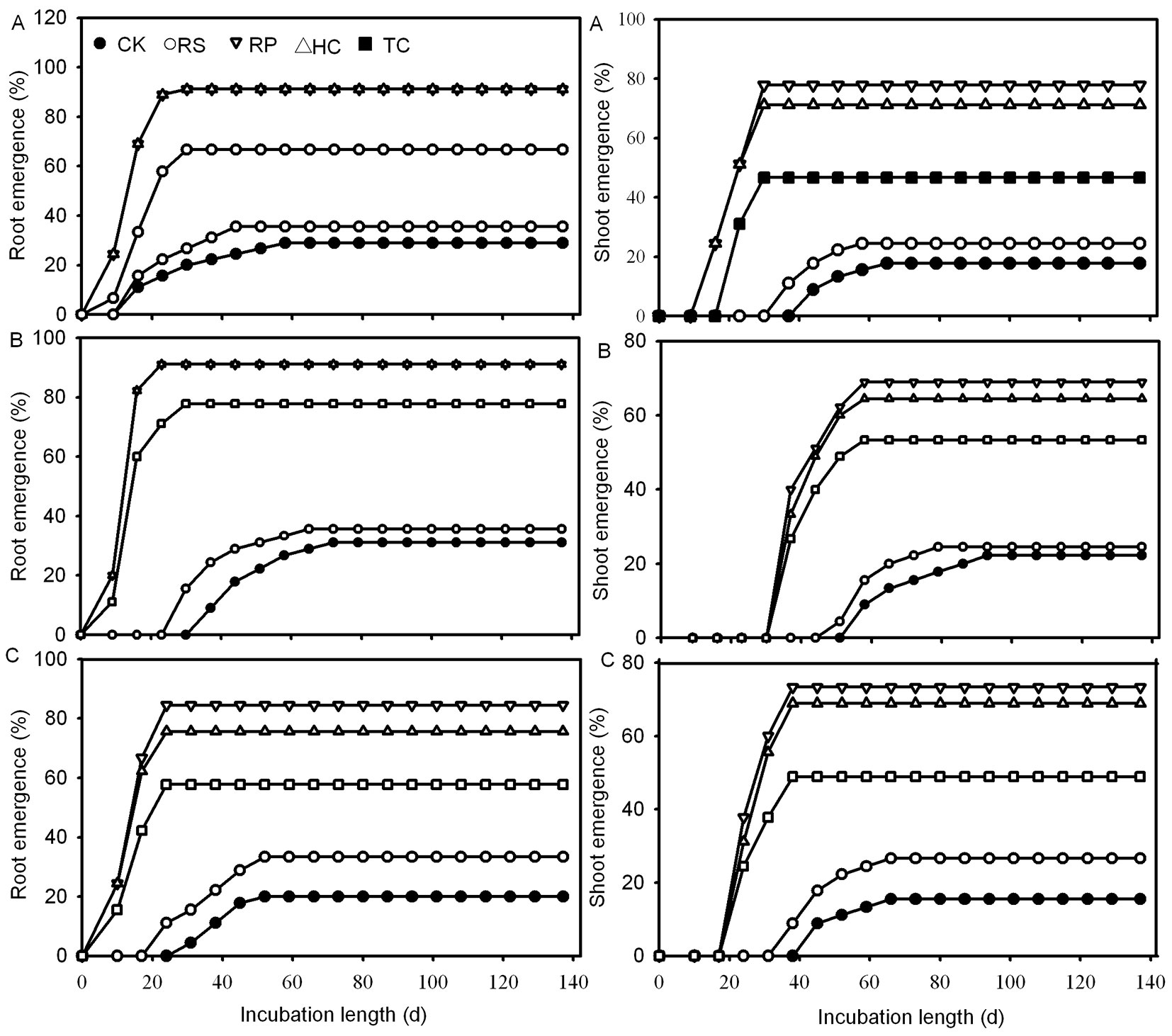
RP, HC and TC treatments all caused a significant increase in the root and shoot final germination rate of Q. variabilis acorns when compared to control acorns (Fig. 3). Acorns of Q. variabilis and Q. robur from the TC treatment had significantly lower root and shoot germination rates than those from the RP and HC treatments, while those of Q. wutaishanica had no significant difference from those from the former treatments. There was no significant difference in root and shoot germination rate between RS and control treatments for all three species.
Fig. 3 - The effects of five mechanical scarification treatments on root and shoot emergence from Q. variabilis (A), Q. wutaishanica (B) and Q. robur (C) acorns. (CK): the control; (RS): removal of cup scar; (RP): removal of pericarp; (HC): removal of pericarp and 1/2 of the distal end of cotyledon; (TC): removal of pericarp and 2/3 of the distal end of cotyledon.
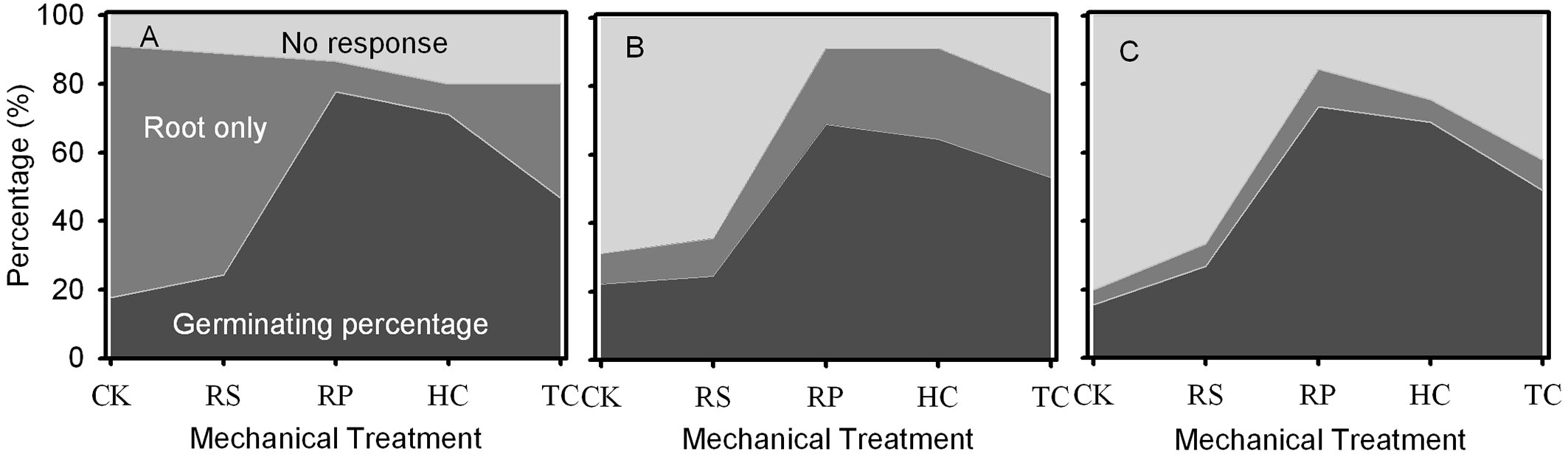
When the germination experiment was completed, some acorns had only roots (Fig. 4). RP, HC and TC treatments significantly reduced the percentage of only roots for Q. variabilis, while decreasing that of Q. wutaishanica, and having no effect on that of Q. robur acorns (Fig. 4).
Fig. 4 - Percent of germinating, rooting and non-germinating acorns at the end of the 137-day study period for Q. variabilis (A), Q. wutaishanica (B) and Q. robur (C) acorns. Using the packing diagram area to represent the ratio of germinated acorns, only roots emerged acorns and non-germinated acorns under different mechanical treatments. (CK): the control; (RS): removal of cup scar; (RP): removal of pericarp; (HC): removal of pericarp and 1/2 of the distal end of cotyledon; (TC): removal of pericarp and 2/3 of the distal end of cotyledon.
Effect of pericarp and cotyledon on root and shoot mean germination time
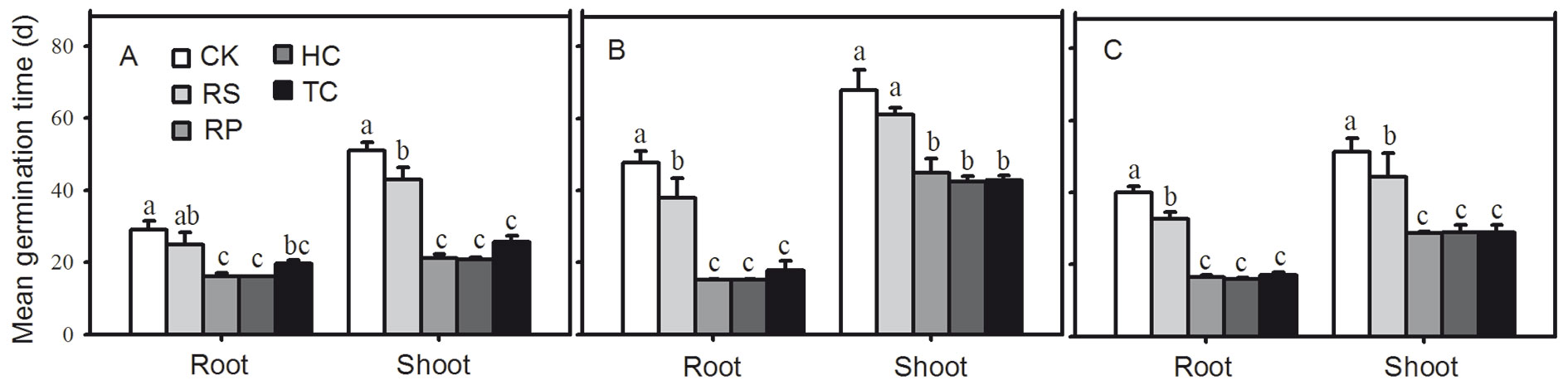
Removing the pericarp and cutting off the distal end of the cotyledon could give rise to faster root and shoot emergence. The first roots of Q. variabilis, Q. wutaishanica and Q. robur acorns from the RP, HC and TC treatments emerged 9, 9 and 10 days after incubating, respectively, which amounted to 7, 28 and 21 days earlier than those of the control treatment (Fig. 3). The first shoots of RP and HC acorns emerged 4, 3 and 3 weeks faster than those of the control treatment, while the first shoots of TC acorns emerged one week later than those of RP and HC treatments (Fig. 3). All RP, HC and TC treatments significantly reduced the root and shoot mean germination time of Q. variabilis, Q. wutaishanica and Q. robur acorns (Fig. 5). The root mean germination time of Q. wutaishanica and Q. robur acorns and the shoot mean germination time of Q. variabilis and Q. robur acorns were significantly decreased by RS compared with those under control condition.
Fig. 5 - Mean germination time of root and shoot from Q. variabilis (A), Q. wutaishanica (B) and Q. robur (C) acorns. Means with the same letter are not significantly different from each other (P>0.05) after the Scheffe’s test. Error bars represent the standard deviation from the mean.
Effect of the pericarp and cotyledon on root and shoot synchronization index
Q. variabilis, Q. wutaishanica and Q. robur acorns from the RP, HC and TC treatments showed a much higher synchronization index than those from the control treatment (Fig. 6). No significant difference was observed between RS and the control treatments for Q. variabilis and Q. wutaishanica acorns.
Fig. 6 - Synchronization index of root and shoot for Q. variabilis (A), Q. wutaishanica (B) and Q. robur (C) acorns. Means with the same letter are not significantly different from each other (P>0.05) after the Scheffe’s test. Error bars represent the standard deviation from the mean.
Effect of the pericarp and cotyledon on root and shoot vigor index and length
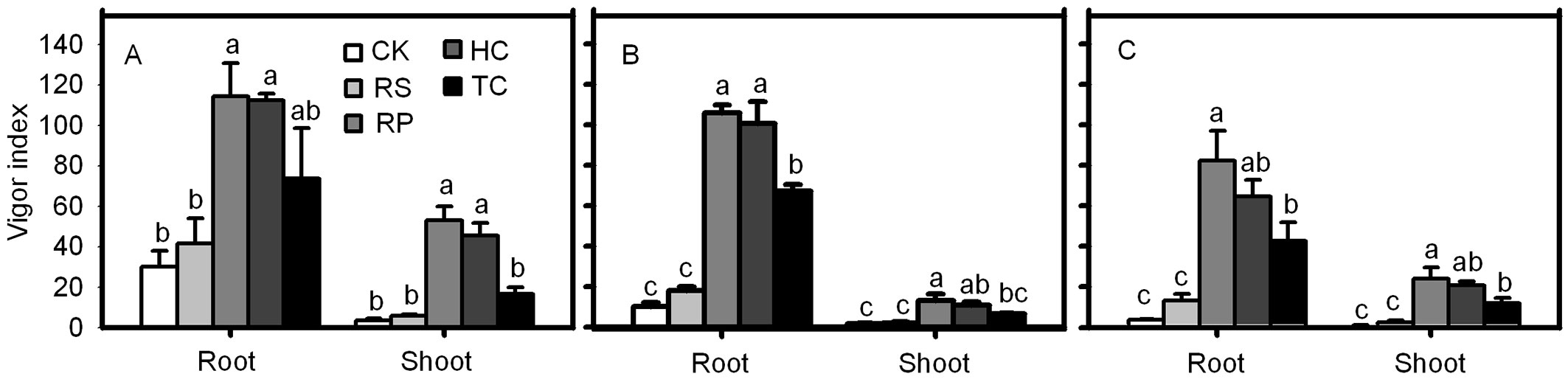
RP and HC treatments significantly improved the root and shoot vigor index of Q. variabilis, Q. wutaishanica and Q. robur acorns (Fig. 7). The root vigor index of Q. variabilis and Q. wutaishanica acorns from the TC treatment was significantly higher than those from the control, but no significant differences were detected among TC, RP and HC treatments. The shoot vigor index of Q. variabilis and the root and shoot vigor index of Q. robur acorns from the TC treatment were much higher than the control, but significantly lower than those from the RP and HC treatments. The root and shoot vigor index of the three species from RS and the control were not significantly different (Fig. 7). The final root emergence of Q. variabilis, Q. wutaishanica and Q. robur acorns from the RS treatment was not significantly different from the control (Fig. 7). A similar result was detected in the final shoot emergence of the three species.
Fig. 7 - Vigor indexes of root and shoot from Q. variabilis (A), Q. wutaishanica (B) and Q. robur (C) acorns. Means with the same letter are not significantly different from each other (P>0.05) after the Scheffe’s test. Error bars represent the standard deviation from the mean.
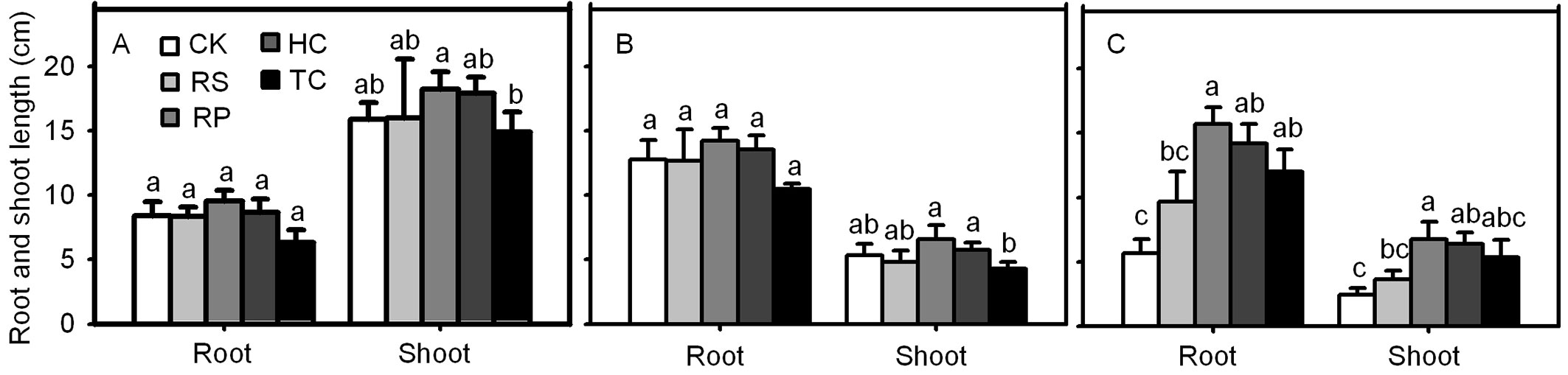
The final root length and shoot height of Q. variabilis and Q. wutaishanica acorns were not significantly different from the roots and shoots from the RP, HC, TC and RS and the control (Fig. 8). However, Q. robur acorns from the RP and HC treatments had significant higher root length and shoot height than the control. The final root length of Q. robur acorns from the TC treatment was significantly longer than those from the control, but the final shoot height showed no significant different between the oaks subjected to the TC treatment and the control treatment.
Fig. 8 - Influence of experimental treatments on mean root length and shoot height (cm) for Q. variabilis (A), Q. wutaishanica (B) and Q. robur (C) acorns at the end of the experiment. Means with the same letter are not significantly different from each other (P>0.05) after the Scheffe’s test. Error bars represent the standard deviation from the mean.
Effect of pericarp thickness on acorn germination
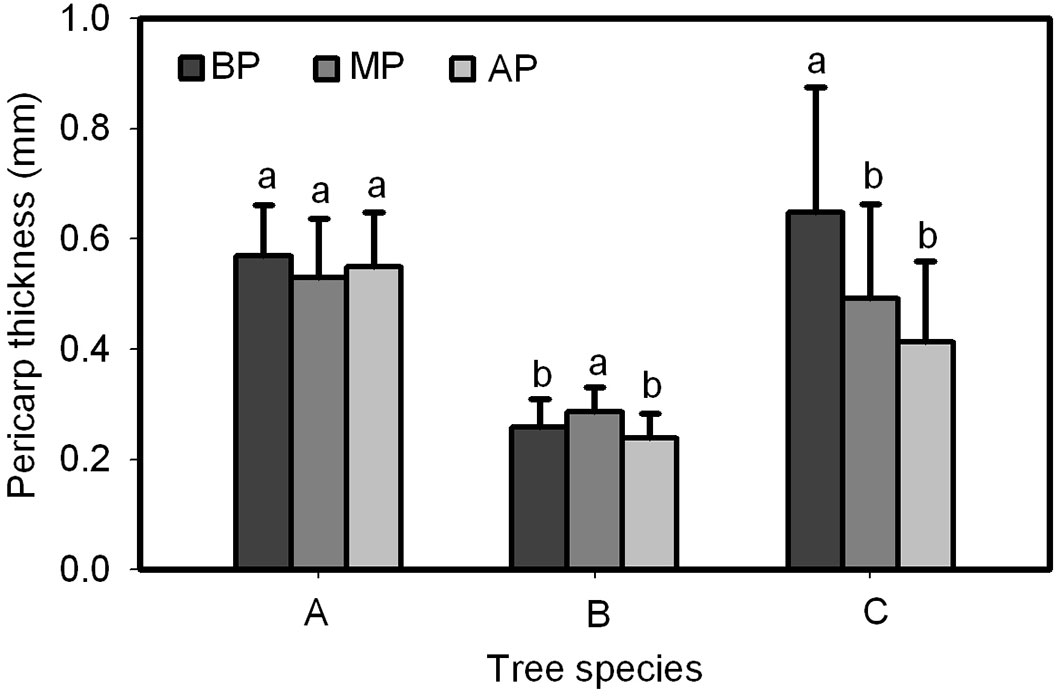
A significant difference was found in pericarp thickness at the base, middle and apex of Q. wutaishanica and Q. robur acorns, and no significant difference was found for the Q. variabilis acorns (Fig. 9). Pericarp thickness of Q. wutaishanica and Q. robur acorns at the base and apex and at the base and medial was significantly lower than that at other sections; however, no difference was observed between the two lower parts. The pericarp thickness of Q. wutaishanica acorns was significantly thinner than that of Q. variabilis and Q. robur acorns.
Fig. 9 - The comparison of pericarp thickness at the apex, middle, and base of Q. variabilis (A), Q. wutai-shanica (B) and Q. robur (C) acorns. Means with the same letter are not significantly different (P>0.05) after the Scheffe’s test. Error bars represent the standard deviation from the mean. (BP): pericarp thickness at the base of acorns; (MP): pericarp thickness at the middle of acorns; (AP): pericarp thickness at the apex of acorns.
Discussion
Effects of different treatments on acorn germination
Mean germination time was used as the index to evaluate the phenomenon of germination speed of oak acorns. It is a measurement of the average length of time required for maximum germination of a seed lot ([21]). RP, HC and TC treatments significantly reduced root and shoot mean germination time for Q. variabilis, Q. wutaishanica and Q. robur acorns, indicating that the three treatments can induce faster root and shoot emergence for the acorns of these species, which is in accordance with results from Giertych & Suszka ([10]). The results are also consistent with the effects of cutting off approximately 1/3 of the distal end of acorns ([25]). The acorns of the three species under the same treatment had similar root and shoot mean germination times. One reason for faster germination results from a more rapid penetration of water into the acorn ([6]). Another explanation is that increased levels of plant growth regulators were provoked, especially of IAA - indole-acetic acid ([6], [19]), which is closely related to water uptake. Moreover, cotyledons of Q. wutaishanica and Q. robur acorns separate rapidly when they absorb water, which improves water access to the embryonic axis ([2]).
The synchronization index can describe acorn emergence patterns, with a higher index representing a more uniform germination. The root and shoot synchronization index of Q. variabilis and Q. wutaishanica acorns from the RP, HC and TC treatments was significantly higher than those from the control, illustrating that the three treatments can promote simultaneous germination. The reason may be that removing pericarp and cutting off the distal end of acorns causes faster root and shoot emergence, and reduces root and shoot germination time, causing the majority of roots and shoots to emerge in a shorter period.
RP, HC and TC treatments significantly increased the root and shoot emergence as well as the final germination rate of Q.variabilis, Q. wutaishanica and Q. robur acorns, a finding similar to those of other studies on water oak acorns (e.g., Q. nigra L. - [3], [2]). ISTA ([13]) also showed that removing the acorn pericarp and cup scar has a positive impact on acorn emergence. The reasons for this positive impact is that removing the pericarp can reduce the resistance of the radicle to pierce the pericarp, which can improve acorn water uptake and gas exchange ([4], [18], [24]). Moreover, Bonner & Vozzo ([2]) have indicated that an acorn is a raw material rich in tannins, which can inhibit radicle emergence. The presence of inhibitory substances has also been confirmed in the pericarps of mature Quercus nigra acorns ([18]) and in the embryo and cotyledon of Quercus aliena var. acuteserrata acorns ([16]). RP, HC and TC treatments had different effects on enhancing the development of roots but not of the shoots of all three species.
No significant difference was found in the root and shoot mean germination time, synchronization index and final germination rate of Q. variabilis, Q. wutaishanica and Q. robur oak acorns between the RS and the control treatments. This finding is consistent with the effect of RS treatment carried out by Liu et al. ([16]), but not with the findings of Rakić et al. ([20]), who reported that removing the cup scar of Q. robur acorns can significantly improve the shoot percentage from 18 to 89%. Although RS treatment can alleviate some of the mechanical strength, the main mechanical resistance of the pericarp is not removed, as the radicle breaks through at the acorn apex, where a powerful internal binding force is present. In addition, germination inhibitors may have impacted our results.
Effects of the different treatments on root and shoot vigor index and length
Vigor index, a comprehensive account of acorn germination and seedling growth, is a suitable index to evaluate seed vigor. Q. variabilis and Q. wutaishanica acorns from RP and HC treatments had a markedly higher root and shoot vigor index than those from the control. However, TC treatment only significantly improved the root vigor index of Q. wutaishanica and Q. robur acorns and the shoot vigor index of Q. robur acorns, indicating that RP and HC treatments have a stronger effect on increasing acorn vigor than the TC treatment. No significant difference was observed in the root length and shoot height of Q. variabilis, Q. wutaishanica and Q. robur acorns among RS, RP, HC, TC and control treatments. These results are supported by previous works by Bonner & Vozzo ([2]) and Andersson & Frost ([1]). Fukumoto & Kajimura ([8]) argue that removing too much of the cotyledon (1/2 and 1/3) has a negative effect on the growth of Q. variabilis. This negative effect may be because, even if nutrients reserved in the cotyledon exceed those used in acorn germination, removing too much of the cotyledon can cause nutrient deficiencies.
Relationship between pericarp thickness and acorn germination
Our hypothesis was that pericarp thickness has a positive effect on acorn germination. However, Q. wutaishanica acorns, having the lowest pericarp thickness, emerged 21 and 6 days later than Q. variabilis and Q. robur acorns, respectively, indicating that thinner pericarp thickness may not lead to faster germination. Pericarp thickness at the middle Q. wutaishanica acorns was the thickest, but for Q. robur, it was thickest at the base of the acorns. However, no significant difference in pericarp thickness was observed between the base, middle and apex of Q. variabilis acorns, which was inconsistent with the findings of Hou et al. ([11]), who reported that pericarp thickness at the base and middle parts was significantly lower than at the apex. Our results indicate that pericarp thickness does not correlate with faster or higher acorn germination rates. Sobrino-Vesperinas & Viviani ([24]) illustrated that microstructures of the pericarp at the cupule and the apex of acorns that can restrain water are different from other parts of the pericarp. Therefore, the acorn pericarp anatomy appears to have a closer relationship with acorn germination than pericarp thickness.
Conclusions
Mechanical treatments before sowing have their advantages. RP and HC treatments significantly decreased root and shoot mean germination time, increased rooting and shooting germination percentage, and improved the root and shoot synchronization and vigor indexes of the three species’ acorns, which effectively accelerated germination and regular seedling. Based on our results, removing the pericarp or cutting off 1/2 of the cotyledon would be optimal for seedling producers.
Acknowledgements
We are very grateful to Lily van Eeden for various useful suggestions and for correcting the language of this paper. Yan Liu and Longyu Hou have contributed equally to this work. Conceived of and designed the experiments: Qingmei Li, Yan Liu, Longyu Hou. Conducted the experiments: Yan Liu, Longyu Hou. Analyzed the data: Yan Liu. Contributed reagents / materials / analysis tools: Qingmei Li. Wrote the paper: Yan Liu. The study was carried out at Research Institute of Forestry, Chinese Academy of Forestry, Beijing (China).
The work has been funded by the General Financial Grant from China Postdoctoral Science Foundation grant 2014M550886, National Basic Research Program of China (973 Program) grant 2010CB951301-6 and the National Key Project of Scientific and Technical Supporting Programs of China under grants 2006BAD09B06 and 2006BAD 03A0308.
References
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Qingmei Li
State Key Laboratory of Tree Genetics and Breeding, Research Institute of Forestry, Chinese Academy of Forestry, Beijing 100091 (China)
Grassland Science Department, Animal Science and Technology, China Agricultural University, Beijing 100193 (China)
Corresponding author
Paper Info
Citation
Liu Y, Hou L, Li Q (2015). Effects of different mechanical treatments on Quercus variabilis, Q. wutaishanica and Q. robur acorn germination. iForest 8: 728-734. - doi: 10.3832/ifor1423-008
Academic Editor
Gianfranco Minotta
Paper history
Received: Aug 06, 2014
Accepted: Jan 23, 2015
First online: May 05, 2015
Publication Date: Dec 01, 2015
Publication Time: 3.40 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 52822
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 43703
Abstract Page Views: 3169
PDF Downloads: 4272
Citation/Reference Downloads: 14
XML Downloads: 1664
Web Metrics
Days since publication: 3926
Overall contacts: 52822
Avg. contacts per week: 94.18
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 3
Average cites per year: 0.27
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Germination and seedling growth of holm oak (Quercus ilex L.): effects of provenance, temperature, and radicle pruning
vol. 7, pp. 103-109 (online: 18 December 2013)
Research Articles
Conservation of Betula oycoviensis, an endangered rare taxon, using vegetative propagation methods
vol. 13, pp. 107-113 (online: 23 March 2020)
Research Articles
Effects of brassinosteroid application on seed germination of Norway spruce, Scots pine, Douglas fir and English oak
vol. 10, pp. 121-127 (online: 02 October 2016)
Research Articles
Influence of mother plant and scarification agents on seed germination rate and vigor in Retama sphaerocarpa L. (Boissier)
vol. 7, pp. 306-312 (online: 08 April 2014)
Research Articles
Effects of different nut pretreatments and substrates on germination and seedlings growth of Neocarya macrophylla Sabine in Basse Casamance, Senegal
vol. 17, pp. 346-352 (online: 03 November 2024)
Research Articles
Fertilisation of Quercus seedlings inoculated with Tuber melanosporum: effects on growth and mycorrhization of two host species and two inoculation methods
vol. 10, pp. 267-272 (online: 13 December 2016)
Research Articles
Using field and nursery treatments to establish Quercus suber seedlings in Mediterranean degraded shrubland
vol. 13, pp. 114-123 (online: 26 March 2020)
Research Articles
The effectiveness of short-term microwave irradiation on the process of seed extraction from Scots pine cones (Pinus sylvestris L.)
vol. 13, pp. 73-79 (online: 13 February 2020)
Technical Reports
De novo adventitious root formations in mini-cuttings of Azadirachta indica in response to different rooting media and auxin treatments
vol. 8, pp. 558-564 (online: 09 December 2014)
Short Communications
Effect of four levels of shade on survival, morphology and chlorophyll fluorescence of Nothofagus alessandrii container-grown seedlings
vol. 8, pp. 638-641 (online: 08 January 2015)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword