Effect of four levels of shade on survival, morphology and chlorophyll fluorescence of Nothofagus alessandrii container-grown seedlings
iForest - Biogeosciences and Forestry, Volume 8, Issue 5, Pages 638-641 (2015)
doi: https://doi.org/10.3832/ifor1321-007
Published: Jan 08, 2015 - Copyright © 2015 SISEF
Short Communications
Abstract
Nothofagus alessandrii (ruil) is a threatened, endemic tree of the Mediterranean zone of Chile. As a result of past anthropogenic activities, its current cover has been reduced to only 314 hectares across several fragmented and degraded areas. Although activities to conserve and recover such forests have been developed, little is known about their propagation and nursery cultivation, since the plant’s quality is one of the most important factors for restoration and reforestation plans, re-vegetation, or forest enrichment. The success of restoration programs in these areas will require improvements in plant production, being important to test the shade effects on seedling survival and growth. This paper reports the results of testing for survival, morphological and chlorophyll fluorescence differences in N. alessandrii seedlings grown for approximately 32 weeks in unshaded conditions and under three different levels of shading (18%, 50%, and 80% shade). Morphological traits (stem height, root collar diameter, specific leaf area, shoot and root biomass, and quality indexes) and survival were measured. Chlorophyll fluorescence was also measured to analyze the shade tolerance of the species. Analysis showed significant differences for most traits as a consequence of the shade level. Seedlings exposed to 18% shade showed the highest total dry biomass, while those exposed to 80% shade showed the highest survival rate (92%). Chlorophyll fluorescence was high in the unshaded conditions and medium in the 18-50% shade. Morphological and chlorophyll fluorescence responses differed greatly among shade levels and corresponded with the degree of shade tolerance of the species.
Keywords
Introduction
Nothofagus alessandrii Espinosa (ruil) is a threatened and endemic tree of the Mediterranean zone of Chile ([21]). At the beginning of the last century, ruil forests have been subjected to logging and burning to clear land for cultivation ([6]), leaving the remnant populations highly fragmented and surrounded by Pinus radiata D. Don plantations ([2]). Current anthropogenic pressures on ruil forests led to consider such species as one of the most threatened trees in Chile. Today, the total area of ruil forests covers only 314 ha, decreasing by 11.8% in last 17 years ([20]).
The restoration of these forest ecosystems is a priority task, and calls for a better understanding of the factors that improve the seedling quality. However, in Chile, local authorities are still in the process of policy formulation, and there is no national recovering policy for the species. Besides, little information is available about the seedling cultivation of N. alessandrii ([18], [19]).
It is recognized that seedlings of this species grow under the protection of adult trees and shaded by the understory. Shading is an important cultural practice for seedling quality and survival, especially for seedlings to be established in Mediterranean climate regions ([23]).
The importance of shading for the development of Nothofagus genus species, including ruil, has been already reported ([17], [18], [19]), and the shade-cloth method is widely used to protect plants from direct radiation ([10]). Shade promotes the development of morphological traits ([18]) and induces physiological responses in ruil nursery seedlings. However, no studies have examined the effects of shade on physiological response of N. alessandrii so far. Chlorophyll fluorescence has been increasingly used to understand both the mechanism of photosynthesis and the factors affecting it. Vidaver et al. ([22]) suggested that chlorophyll fluorescence can rapidly provide useful information about photosynthetic responses to environmental stresses.
The objective of this study was to evaluate the effects of three different shade levels and unshaded conditions on the survival, morphology, and chlorophyll fluorescence of N. alessandrii seedlings cultivated in containers. The study assessed the hypothesis that N. alessandrii seedlings require some degree of protection from direct sunlight in their early stages of development.
Materials and Methods
Plant material
The N.alessandrii seeds were collected in a natural stand near the locality Lo Ramirez, Curepto Commune (35° 10′ S, 72° 06′ W, elevation 385 m a.s.l.), which is located in the province of Talca, Maule Region, Central Chile. Fruits were collected directly from the branches and transported to the nursery at the Catholic University del Maule (Talca, Chile). Seeds were cleaned and weighed according to the ISTA ([8]) standards.
The seeds were then sown in rigid plastic containers of 140 ml (Termomatrices®, Santiago, Chile) at an approximate depth of 0.5 cm. The substrate consisted of a mixture of composted bark of Pinus radiata and perlite (7:3 ratio), which was combined with the slow-release fertilizer Basacote® 9 M (COMPO GmbH & Co. KG, Munster, Germany - 16% N, 8% P2O5, 12% K2O, 12% SO3, 2% MgO, 0.02%B, 0.05% Cu, 4% Fe and 0.06% Mn), at a dose of 3 g L-1 of substrate. During their cultivation and before the shade treatments, seedlings were protected by a plastic mesh sunshade (Raschel®, Yantai Sanhai Industry Ltd., Shandong, China).
Shade treatments
The study used a completely randomized blocks design: 3 blocks, 3 shade treatments and 20 seedlings per treatment. Seedlings were grown either under full sun or under shade-cloth shelters. The shading treatments applied were: (i) unshaded conditions, which received 100% of the photosynthetically active radiation (PAR); (ii) 18% shade (45% PAR); (iii) 50% shade (41% of PAR); and (iv) 80% shade (20% PAR). PAR emissions in each treatment were induced by using the EARS PPM200 portable pulse-modulated fluorometer (EARS, Delft, the Netherlands), in which adaptation to dark is not required for certain measurements. During their germination seedlings were watered daily using micro-sprinklers. After seedlings emergence, the substrate was maintained at field capacity by weighing the pots three times per week and adding amounts of water equal to the loss in weight. Seedlings were grown for approximately 32 weeks.
Morphological measurements
The morphological traits measured were: stem length (L), root collar diameter (D), aboveground biomass (stem + leaves, AB), root biomass (RB), and total biomass (TB). Starting since eight weeks after sowing and until the end of the experiment, all seedlings were measured for L and survival at 2-week intervals. Survival was measured according to a categorical scale (1 = alive, 0 = dead). To determine biomass, nine seedlings per treatment and replication were randomly selected (9 seedlings × 4 treatments × 3 replicates = 108 seedlings). Several indexes were calculated as follows: the slenderness index (SI), the shoot:root index (SRI - [9]) and the Dickson’s index (DI - [5]), according to the following formulas (eqn. 1, eqn. 2, eqn. 3):
Specific leaf area (SLA, cm2 g-1), i.e., the ratio of foliar surface to dry weight, was also estimated. For this purpose, five seedlings per treatment were randomly selected, and three leaves per plant were sampled (5 seedlings × 4 treatments × 3 leaves). Leaves were scanned using a common desk-top scanner (Hewlett-Packard, Cupertino, CA, USA) and the surface was measured by using a digital planimeter (Tamaya, Tokyo, Japan). To determine the foliar dry weight, the samples were oven-dried at 60 °C for 48 h in a convection oven (Memmert, Germany) and then weighed with a precision balance (Boeco, Germany).
Chlorophyll fluorescence measurements
Chlorophyll fluorescence (F) was measured in five seedlings per treatment with an EARS PPM200 portable pulse-modulated fluorometer (5 seedlings × 4 treatments × 3 blocks = 60 seedlings). A portion of branches and terminal shoot of each seedling was placed in the fluorometer sphere and illuminated to 100 mmol m-2 sec-1. All measurements were performed between 09:00 and 15:00 hours.
Experimental design and statistical analyss
Vertical and lateral shade was ensured for every shading treatment, and a three-meter buffer between shade treatments was maintained in order to avoid light or shade overlap. Stem length data measured over time were subjected to the repeated measures analyses procedure, while all other variables were analyzed by the analysis of variance (ANOVA). Pairwise comparisons of survival between groups were done using a Chi-square test. To satisfy model assumptions, data for biomass (i.e., shoots, roots and shoot:root ratio) were log-transformed. The analysis of variance and the comparisons of means were performed using the general linear model (GLM) procedure implemented in the software package SPSS® ver. 18 for Windows®. Differences among mean values were tested by the post-hoc Tukey’s test (α = 0.05).
Results
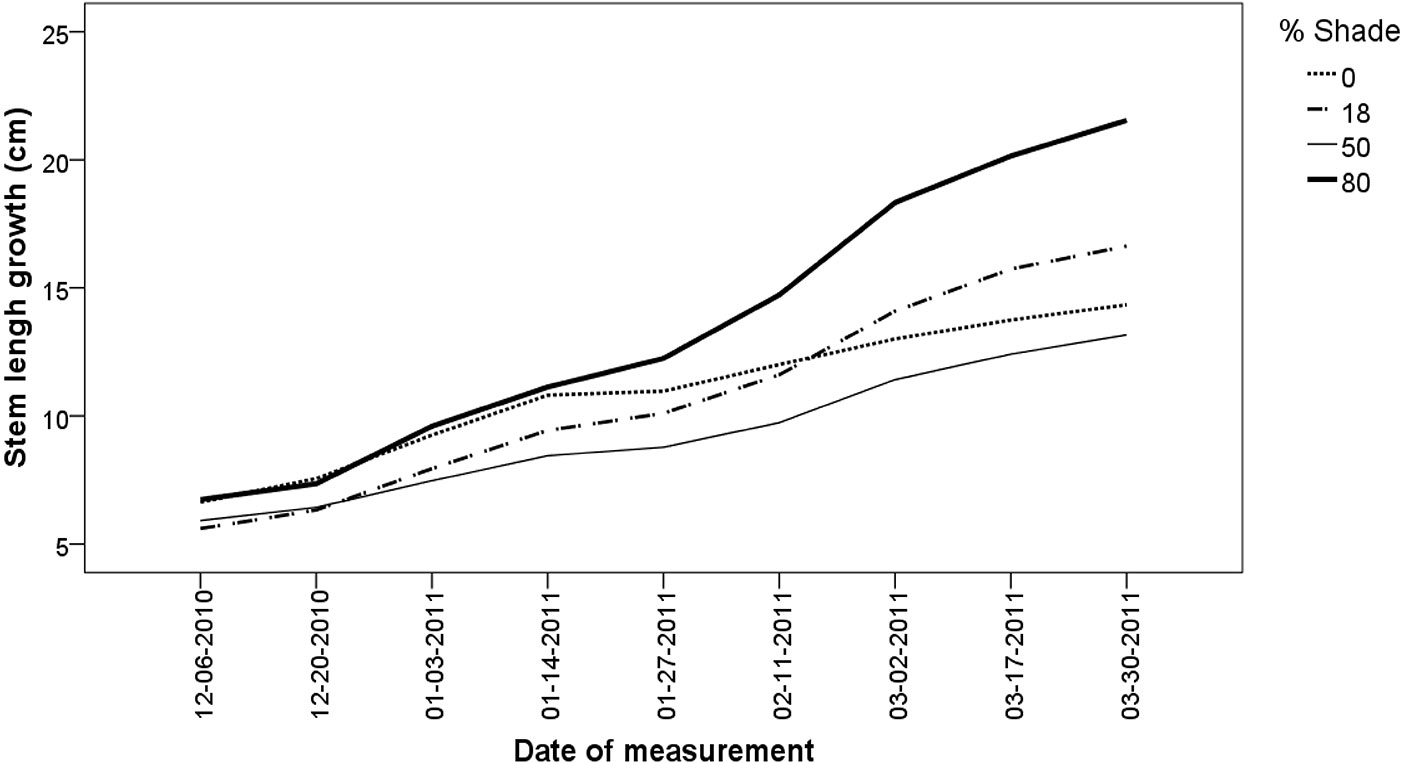
Shading resulted in significant differences in all morphological traits (Tab. 1). L differed significantly over time among shade treatments (p < 0.001), being higher under 80% shade and lower under 50% shade treatments (Fig. 1). Seedlings exposed to 18% shade showed the highest shoot and root biomass, while the seedlings exposed to 80% shade were the tallest. As the shade level increased, seedlings tended to be significantly more slender, with higher SLA (Tab. 2). It is noteworthy to mention that seedlings exposed to 18% shade had the better quality according to their mean DI values. Survival also differed according to the shade treatment. As expected, the lower survival was found in 0% shade treatment. Chlorophyll fluorescence increased as seedlings were exposed to higher lighting conditions.
Tab. 1 - Effect of shading on morphological attributes of container-grown N. alessandrii seedlings. Mean values with the same letter are not significantly different (p>0.05) after Tukey’s test. (PAR): photosynthetically active radiation; (SLA): Specific leaf area; (L): length of the stem; (D): root collar diameter.
| Shading (%) |
PAR (%) |
D (mm) |
L (cm) |
SLA (cm2 g-1) |
Biomass (g) | ||
|---|---|---|---|---|---|---|---|
| Shoot | Roots | Total | |||||
| 0 | 100 | 2.9 ± 0.14 a | 14.3 ± 0.95 b | 143.2 ± 7.9 b | 1.52 ± 0.14 ab | 0.83 ± 0.14 b | 2.35 ± 0.16 ab |
| 18 | 45 | 3.0 ± 0.13 a | 16.4 ± 1.02 b | 140.3 ± 7.3 b | 2.19 ± 0.49 a | 1.41 ± 0.12 a | 3.60 ± 0.54 a |
| 50 | 41 | 2.4 ± 0.09 b | 13.2 ± 0.87 c | 159.2 ± 14.2 b | 1.20 ± 0.15 b | 0.83 ± 0.13 b | 2.03 ± 0.12 b |
| 80 | 20 | 3.0 ± 0.10 a | 21.7 ± 1.11 a | 235.0 ± 6.8 a | 1.54 ± 0.14 ab | 0.80 ± 0.08 b | 2.34 ± 0.21 b |
Fig. 1 - Stem length of N. alessandrii seedlings exposed at three shade levels and to unshaded conditions over 32 weeks.
Tab. 2 - Effect of shading on quality indexes, survival and chlorophyll fluorescence of container-grown N. alessandrii seedlings. Mean values with the same letter are not significantly different (p>0.05) after Tukey’s test. (PAR): photosynthetically active radiation; (F); chlorophyll fluorescence.
| Shading (%) |
PAR (%) |
Survival (%) |
F (μmol photons m-2 s-1) |
Quality indexes | ||
|---|---|---|---|---|---|---|
| Slenderness | Shoot:root | Dickson | ||||
| 0 | 100 | 60 c | 2922 ± 138.9 c | 5.2 ± 0.65 b | 2.0 ± 0.76 b | 0.34 ± 0.04 ab |
| 18 | 45 | 73 b | 2437 ± 101.2 b | 6.5 ± 0.46 ab | 1.6 ± 0.32 b | 0.45 ± 0.07 a |
| 50 | 41 | 77 b | 2373 ± 142.8 b | 7.0 ± 0.50 ab | 1.6 ± 0.29 b | 0.24 ± 0.14 b |
| 80 | 20 | 92 a | 1406 ± 85.4 a | 8.1 ± 0.44 a | 2.3 ± 0.13 a | 0.24 ± 0.02 b |
Discussion
Effects of light and shade on survival and morphological responses of N. alessandrii
The growth rate of N. alessandrii in this study could be considered low with respect to Santelices et al. ([19]), who reported L values ranging from 28 to 32 cm and D values of 4 mm in containerized seedlings grown in 140 ml pots and exposed to 35 and 50% shade. The largest D growth was achieved by increasing light intensity, being clear that the light intensity influences nursery seedling growth. The results of this study agree with those of Santelices et al. ([19]), indicating that a shadow treatment that allows about 40% PAR could optimize N. alessandrii nursery growth.
As the seedlings received less PAR, there was an increase in SLA, which reinforces the need to provide shade in the early stages of ruil development. In Fagus sylvatica L. both an increase and a decrease in specific leaf weight and dry weights were observed, while there was an increase in light intensity ([13], [1]). The results of this study are in part similar to that reported by Ammer ([1]), who observed a decrease of SLA with increasing light intensity, and may be related to factors such as the origin of seeds and the degree of adaptability to light conditions in their natural distribution area.
A plant exposed to low light intensity will allocate more biomass to stems and leaves (i.e., shoots), consequently increasing its shoot:root ratio ([12]). This behavior was observed in the 20% PAR treatment. However, plants with a high shoot:root ratio show also some drawbacks, as they consume more water than plants with a low shoot:root ratio. The lower the ratio of these two components, the greater the chance of plant survival ([15]), especially in dry areas ([14]).
The results of the DI estimation shows that seedlings exposed to 18% shade had a better quality, mainly because the above index combines the SI and SRI, thereby observing a greater TB in seedlings exposed to 18% shade level according to Chirino et al. ([3]). In the case of SRI, Navarro-Cerrillo et al. ([14]) recommended a ratio close to the unit to ensure good survival in the early establishment of Q. ilex. In this study seedlings exposed to 18-50% shade showed more balanced SRI values (mean: 1.6).
Ecophysiological responses of N. alessandrii to shade increase
In the present study, differences in F were observed among treatments. The unshaded treatment showed the highest values for this variable, which indicates that N. alessandrii needs protection from direct sunlight in its early stages of development. The 20% PAR treatment had the lowest F values, indicating a low level of light stress and a coincidence with the biomass allocation analysis (i.e., SRI) and also with the survival analysis. Indeed, the lack of light is the limiting factor in this treatment, and the plant allocates more biomass to the shoots at the expense of the roots. Seedling survival decreases with light, which is consistent with studies on other Nothofagus species ([11], [4], [16]), where an improved survival at low light intensities was observed.
Our results show that the SLA is related to F, because in the lower light intensity treatment (20% of the PAR), the leaves became wider, which could indicate a high efficiency by N. alessandrii to capture and use limited amounts of light and then consume carbohydrates efficiently. This efficiency could be explained in part by the high SLA and SRI observed with that level of light intensity. A high SLA implies a greater number of chloroplasts and enzymes and an improved photosynthetic capacity per unit leaf area ([7]).
Conclusions
This study has shown that the shade level affects the morphological and chlorophyll response of container-grown N. alessandrii seedlings. Although the uniform shade environments created with shade-cloth in this study are not representative of the fluctuating light conditions occurring in the forest, our results indicate that N. alessandrii seedlings performed poorly in full sunlight (100% PAR), while with moderate shade (41-50% PAR), a better development is achieved (improved SI and DI). Chlorophyll fluorescence was inversely related to shade, probably indicating that photochemical efficiency of N. alessandrii was lower under full sunlight. These findings have considerable implications for ecosystem management. However, results of this study should be interpreted with caution, since the seedling response to shade may differ in diverse field conditions as a consequence of other environmental factors.
Acknowledgements
We are indebted with Dr. Anne Bliss, from the University of Colorado at Boulder (CO, USA), for her valuable corrections of English. Many thanks to Daniela Sánchez and Daniela Muñoz for data collection. We also thank the Universidad Católica del Maule (Talca, Chile) for funding this research.
References
Gscholar
Gscholar
Gscholar
Online | Gscholar
CrossRef | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Sergio Espinoza
Antonio María Cabrera
Universidad Católica del Maule, Centro de Desarrollo del Secano Interior, Avenida San Miguel 3605, Talca (Chile)
Corresponding author
Paper Info
Citation
Santelices R, Espinoza S, Cabrera AM (2015). Effect of four levels of shade on survival, morphology and chlorophyll fluorescence of Nothofagus alessandrii container-grown seedlings. iForest 8: 638-641. - doi: 10.3832/ifor1321-007
Academic Editor
Gianfranco Minotta
Paper history
Received: Apr 23, 2014
Accepted: Oct 06, 2014
First online: Jan 08, 2015
Publication Date: Oct 01, 2015
Publication Time: 3.13 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 54126
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 45634
Abstract Page Views: 3415
PDF Downloads: 3776
Citation/Reference Downloads: 19
XML Downloads: 1282
Web Metrics
Days since publication: 4071
Overall contacts: 54126
Avg. contacts per week: 93.07
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 8
Average cites per year: 0.73
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Conservation of Betula oycoviensis, an endangered rare taxon, using vegetative propagation methods
vol. 13, pp. 107-113 (online: 23 March 2020)
Technical Reports
Nursery practices increase seedling performance on nutrient-poor soils in Swietenia humilis
vol. 8, pp. 552-557 (online: 09 December 2014)
Research Articles
Forecasting the field performance of Austrian pine seedlings using morphological attributes
vol. 10, pp. 99-107 (online: 13 October 2016)
Research Articles
Use of alternative containers for promoting deep rooting of native forest species used for dryland restoration: the case of Acacia caven
vol. 10, pp. 776-782 (online: 02 September 2017)
Research Articles
Fertilisation of Quercus seedlings inoculated with Tuber melanosporum: effects on growth and mycorrhization of two host species and two inoculation methods
vol. 10, pp. 267-272 (online: 13 December 2016)
Research Articles
Seedling quality and short-term field performance of three Amazonian forest species as affected by site conditions
vol. 17, pp. 80-89 (online: 21 March 2024)
Research Articles
The effect of calcium on the growth of native species in a tropical forest hotspot
vol. 11, pp. 221-226 (online: 01 March 2018)
Research Articles
Controlled-release fertilizers combined with Pseudomonas fluorescens rhizobacteria inoculum improve growth in Pinus halepensis seedlings
vol. 8, pp. 12-18 (online: 12 May 2014)
Research Articles
Germination and seedling growth of holm oak (Quercus ilex L.): effects of provenance, temperature, and radicle pruning
vol. 7, pp. 103-109 (online: 18 December 2013)
Short Communications
The effects of salicylic acid, oxalic acid and chitosan on damping-off control and growth in Scots pine in a forest nursery
vol. 13, pp. 441-446 (online: 24 September 2020)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword