Distribution and abundance of the alien Xylosandrus germanus and other ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) in different forest stands in central Slovenia
iForest - Biogeosciences and Forestry, Volume 12, Issue 5, Pages 451-458 (2019)
doi: https://doi.org/10.3832/ifor3114-012
Published: Sep 29, 2019 - Copyright © 2019 SISEF
Research Articles
Abstract
The East Asian ambrosia beetle (Xylosandrus germanus - Blandford 1894) is an invasive species that has become successfully established in Europe and North America. In Slovenia, X. germanus was first recorded in 2000 in the western part of the country, and since 2008 the species has also been identified in other parts of Slovenia. The first economic damage was recorded in 2016 after a massive attack on recently felled logs of different tree species, spurring research into this non-native invasive species. To examine the distribution and abundance of X. germanus compared to other ambrosia beetles and to determine voltinism and the flight period of the species in our climatic conditions, we deployed 19 ethanol-baited traps from March to November 2017 in oak-, beech- and fir-dominated forest stands in central Slovenia. To verify the vertical distribution of X. germanus, traps were installed at altitudes ranging from 303 m to 941 m a.s.l. Furthermore, the impact of the ice storm that hit Slovenia in 2014 on the abundance of X. germanus was also studied. Non-native X. germanus represented 71.8% of the total catch and was significantly more abundant than the other five most common species: Xyleborinus saxesenii (20.0%), Xyleborus monographus (3.6%), Anisandrus dispar (2.5%), Trypodendron domesticum (1.2%) and Trypodendron signatum (0.6%). X. germanus was most abundant in beech-dominated stands, but the differences between forest types were not significant. The species was found along the entire altitudinal gradient. Our results indicate that the swarming of X. germanus in lowland forests may already occur by the middle of March. Maximum flying activity was observed in May and early June in forests below 600 m a.s.l. and at the end of May and in June in forests above 700 m a.s.l. Only one generation per year was observed. The ice storm positively affected the abundance of X. germanus, especially in areas where sanitary logging was delayed. Xyleborinus attenuatus was detected for the first time in Slovenia.
Keywords
Xylosandrus germanus, Ambrosia Beetles, Black Timber Bark Beetle, Invasive Species, Habitat Preference, Monitoring, Ethanol-baited Traps
Introduction
Ambrosia beetles are weevils from the Scolytinae and Platypodinae subfamilies (Coleoptera: Curculionidae) that are obligately associated with nutritional fungal symbionts ([23]). They usually excavate tunnels in the wood of dead or stressed trees in which they actively cultivate ambrosia fungi. Because of their hidden way of life (most of the time they reside within the wood), they can be easily and imperceptibly transported to new environments ([38]). Many species are polyphagous and have haplodiploid reproduction and sibling mating behavior. All these features allow small founder populations to rapidly establish and spread in new environments ([29]). Therefore, ambrosia beetles are one of the most successful groups of invasive species worldwide ([28]).
The black timber bark beetle Xylosandrus germanus (Blandford, 1894) is native to East Asia ([28]). In Europe, it was first recorded in 1952 in Germany ([16]). In the last two decades of the 20th century, the species was also recorded in Austria, Belgium, France, Italy, Poland and Switzerland. After the year 2000 X. germanus spread throughout Europe and is now present in Croatia, the Czech Republic, Denmark, Hungary, the Netherlands, Romania, the Russian Federation, Slovakia, Slovenia, Spain, Sweden, Turkey, the United Kingdom and Ukraine ([3], [8], [13]).
In Slovenia, X. germanus was recorded for the first time in 2000. It was found on an infested sweet chestnut tree (Castanea sativa Mill.) in the western part of the country ([25], [42]). Since 2008, the species has also been identified in other parts of Slovenia. It has often been caught in traps intended for monitoring Monochamus beetles (Coleoptera: Cerambycidae), and in a few cases, infestations of visually healthy trees have been found ([25], [26], [27]).
The first economic damage was recorded in 2016, when X. germanus infested a large amount of timber that was not removed from the forest immediately after logging ([19]). In addition to finding X. germanus in sweet chestnut trees, we have also found infestations on Norway spruce (Picea abies [L.] H. Karst), silver fir (Abies alba Mill.), Scots pine (Pinus sylvestris L.), common beech (Fagus sylvatica L.) and sessile oak (Quercus petraea [Mattuschka] Liebl.).
In North America, where the species was introduced in 1932, X. germanus is one of the most economically important ambrosia beetle pests in nurseries ([37]), while in Europe it is considered a secondary pest ([20], [32]). However, in some parts of Europe, X. germanus has become one of the most common scolytid species ([17], [20], [5], [21], [38]) and is considered to have the potential to affect native scolytid communities ([20]).
X. germanus is extremely polyphagous ([44]). However, in Italy, the forest type was recently found to have a significant effect on the activity-density of the species ([38]). The abundance of X. germanus in ethanol-baited traps was significantly higher in chestnut-dominated (C. sativa) forests than in forests dominated by hop hornbeam (Ostrya carpinifolia Scop.) and beech (F. sylvatica). Furthermore, temperature was also found to have a positive impact on the activity-density of X. germanus, meaning that low temperatures limit its spread to high-elevation forests ([38]). In Europe an elevation limit of 500 m a.s.l. was proposed by Bruge ([6]), but a study from Romania ([34]) states that X. germanus was found at an altitude of 760 to 900 m a.s.l. Similar findings have recently been reported from Slovakia ([13]). The number of generations per year also depends primarily on environmental temperatures. In Central Europe, X. germanus is considered to be monovoltine ([6], [20]), but in Italy, two generations per year have been observed ([39]).
The main aim of this study was to examine the distribution and abundance of X. germanus and other ambrosia beetles in different forest stands dominated by different tree species and at different altitudes in the central part of Slovenia. Furthermore, we also wanted to determine voltinism and the flight period of X. germanus in our climatic conditions. Forests damaged due to natural disasters are an appropriate habitat for the development of the species ([25]); therefore, the impact of the 2014 ice storm on the abundance of X. germanus was also studied.
Materials and methods
Research area
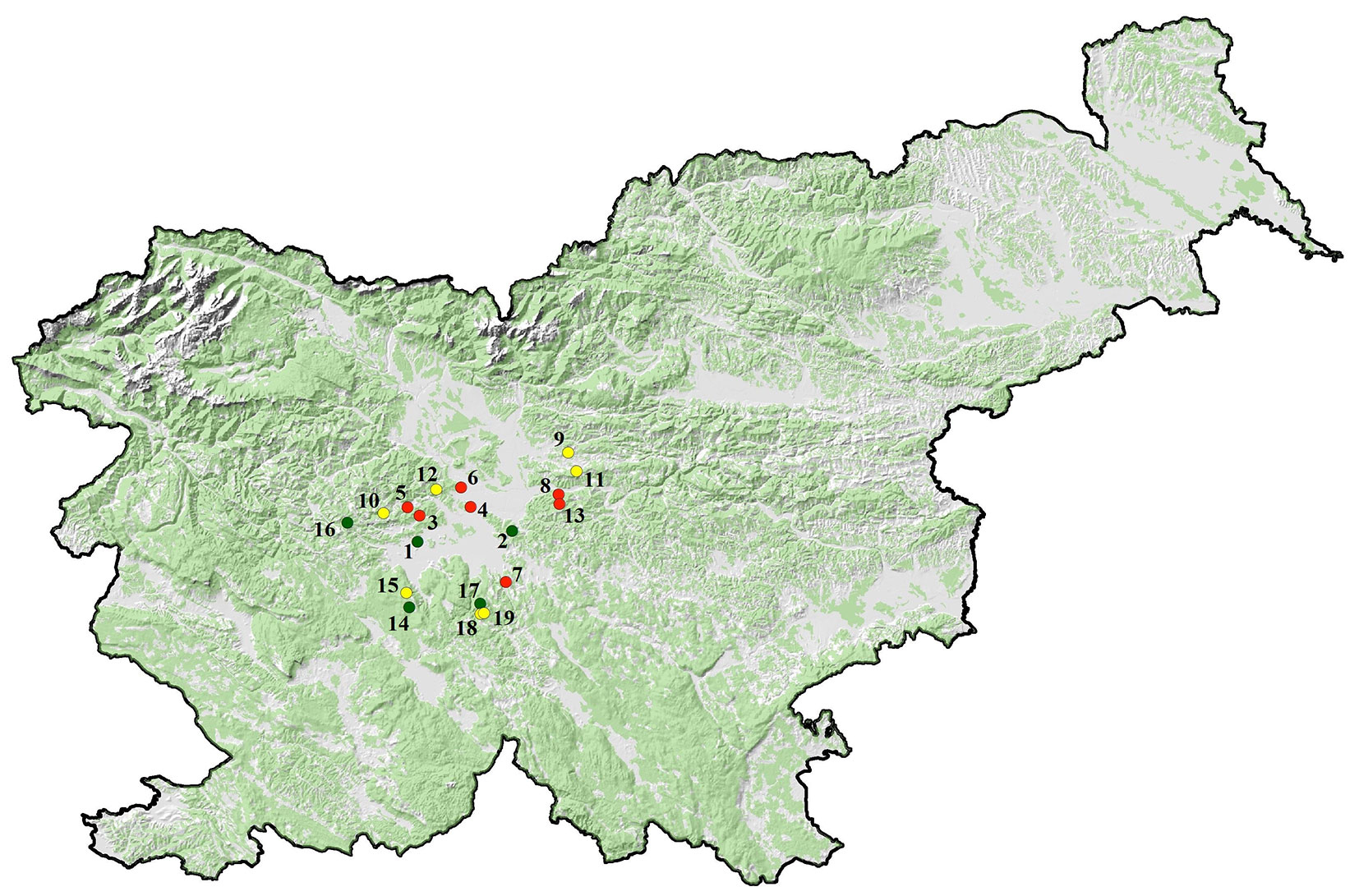
The study was conducted in 19 different forest stands located in the wider area of the capital city of Ljubljana (Fig. 1). The selected stands are dominated (i.e., more than 50% of trees belong to a given species) by common beech (F. sylvatica, 7 stands), sessile oak (Q. petraea, 7 stands) and silver fir (A. alba, 5 stands). The stands are located at altitudes ranging from 303 m to 941 m a.s.l. and were therefore divided into three altitudinal groups: (I) lower altitudes, below 450 m a.s.l.; (II) medium altitudes, between 450 and 600 m a.s.l.; (III) higher altitudes, above 700 m a.s.l. (Tab. 1).
Fig. 1 - Trap locations in stands of common beech (yellow), sessile oak (red) and silver fir (green). The numbers on the map match the stand numbers in Tab. 1.
Tab. 1 - Data on trap locations.
| Stand no. | Local unit | Latitude | Longitude | Dominant tree species | Altitude (m a.s.l.) |
Altitude group |
|---|---|---|---|---|---|---|
| 1 | Vrhnika | 45°59′ 52″ | 14°21′ 42″ | Abies alba | 303 | I |
| 2 | Domžale | 46°01′ 03″ | 14°35′ 24″ | Abies alba | 328 | I |
| 3 | Vrhnika | 46°00′ 20″ | 14°20′ 11″ | Quercus petraea | 329 | I |
| 4 | Ljubljana | 46°03′ 27″ | 14°29′ 20″ | Quercus petraea | 335 | I |
| 5 | Ljubljana | 46°02′ 32″ | 14°21′ 58″ | Quercus petraea | 344 | I |
| 6 | Ljubljana | 46°05′ 26″ | 14°27′ 57″ | Quercus petraea | 357 | I |
| 7 | Škofljica | 45°55′ 53″ | 14°34′ 32″ | Quercus petraea | 413 | I |
| 8 | Domžale | 46°04′ 45″ | 14°42′ 07″ | Quercus petraea | 452 | II |
| 9 | Domžale | 46°08′ 59″ | 14°43′ 31″ | Fagus sylvatica | 499 | II |
| 10 | Ljubljana | 46°02′ 45″ | 14°16′ 42″ | Fagus sylvatica | 502 | II |
| 11 | Domžale | 46°07′ 07″ | 14°44′ 41″ | Fagus sylvatica | 529 | II |
| 12 | Ljubljana | 46°05′ 12″ | 14°24′ 20″ | Fagus sylvatica | 574 | II |
| 13 | Domžale | 46°03′ 48″ | 14°42′ 14″ | Quercus petraea | 589 | II |
| 14 | Vrhnika | 45°53′ 14″ | 14°20′ 35″ | Abies alba | 713 | III |
| 15 | Vrhnika | 45°54′ 42″ | 14°20′ 06″ | Fagus sylvatica | 749 | III |
| 16 | Ljubljana | 46°01′ 45″ | 14°11′ 29″ | Abies alba | 801 | III |
| 17 | Škofljica | 45°53′ 41″ | 14°30′ 49″ | Abies alba | 860 | III |
| 18 | Škofljica | 45°52′ 39″ | 14°30′ 53″ | Fagus sylvatica | 900 | III |
| 19 | Škofljica | 45°52′ 43″ | 14°31′ 24″ | Fagus sylvatica | 941 | III |
Traps, lures and sampling
In each stand, one black cross-vane panel trap (WitaPrall IntPt - Nassfalle, Witasek PflanzenSchutz GmbH, Germany) was placed in the middle of a plot of at least 2 ha where a particular tree species dominates. However, these plots are a part of a larger forest area where a particular species is the most common tree species. Traps were hung on a tree branch approximately two meters above the ground and at least 50 m from the forest edge. All traps were equipped with a half-liter plastic bottle without a cap, containing 200 ml of 96% ethanol as an attractant. The bottles were attached in the upper half of the traps. Furthermore, wet collecting cups filled with 200 ml of antifreeze (80-98% ethylene glycol - Arteco, Belgium) to preserve the collected entomofauna were positioned at the bottom of the traps. Traps were set in the period between 20 February 2017 and 7 March 2017. On 10 March 2017, traps were visited for the first time, and since then, traps were visited at 14-day intervals until 3 November 2017. Due to the different time settings of the traps, catch data from the period before 10 March 2017 were not included in the analysis.
On each visit, samples were collected from each trap. In total, 17 samples per trap were collected in the entire sampling period. After every sampling, the collecting cups were refilled with fresh ethylene glycol. On each visit, the amount of evaporated ethanol was also measured, and the same amount of fresh pure ethanol was added to the ethanol-containing bottle. The mean release rate of ethanol during the sampling period was 1.65 ml day-1.
Samples were analyzed using an Olympus SZX12® stereo microscope, and ambrosia beetles were identified according to the insect identification keys presented by Grüne ([18]), Pfeffer ([35]) and Faccoli ([9]).
Statistical analyses
For each species, analyses were performed on the total cumulative number of beetles captured during the trapping period per trap. Data were analyzed using the statistical package SPSS® ver. 25 (IBM, Armonk, NY, USA). The Kolmogorov-Smirnov test was performed to check the normality of the data. For non-normally distributed data, log(x+1) transformations were used to normalize them. A one-way ANOVA was used to test the differences in ambrosia beetle species abundance (only six of the most common species were included in the analysis) and to separately test the differences in the abundance of species between different forest types and between different altitudinal groups. To detect differences in abundance between pairs of samples, Games-Howell post-hoc test was used. The correlation between sanitary felling and the abundance of X. germanus was tested by the Pearson’s correlation coefficient. Data on the quantity of sanitary logging due to the ice storm in 2014 at individual trap locations were obtained from the Slovenia Forest Service database ([46]).
Results
Distribution and abundance of X. germanus and other ambrosia bark beetles
We trapped eleven species of ambrosia beetles: three non-native and eight native to Europe (Tab. 2). There were significant differences in beetle abundance between the different species (one-way ANOVA: F[5, 108]=34.026, p<0.001). Non-native X. germanus represented 71.8% of the total catch, and the results of the post-hoc test (Tab. 2) show that it was significantly more abundant (p<0.05 - Games-Howell post-hoc test) than the other five most common species: Xyleborinus saxesenii (Ratzeburg, 1837) at 20.0%, Xyleborus monographus (Fabricius, 1792) at 3.6%, Anisandrus dispar (Fabricius, 1792) at 2.5%, Trypodendron domesticum (Linnaeus, 1758) at 1.2% and Trypodendron signatum (Fabricius, 1787) at 0.6%. X. germanus was the most abundant species at 18 of the 19 locations (at one location, it was outcompeted by X. saxesenii). The other two non-native species were rare. Twelve specimens of Gnathotrichus materiarius (Fitch, 1858) and one specimen of Xyleborinus attenuatus (Blandford, 1894) were caught. Four species, namely, X. germanus, X. saxesenii, A. dispar and T. signatum, were caught at all 19 sampled locations.
Tab. 2 - Species of ambrosia beetles caught in ethanol-baited traps. The total and mean number ± standard error (SE) of individuals and number of sites (out of 19) where each species was trapped are shown. Means followed by the same letter were not significantly different (p>0.05) after Games-Howell post-hoc test. (*): Non-native species.
| Species | No. of sites |
Total no. of individuals |
Mean ± SE |
|---|---|---|---|
| Xylosandrus germanus * | 19 | 67592 | 3557.5 ± 1611.5 a |
| Xyleborinus saxesenii | 19 | 18825 | 990.8 ± 220.7 b |
| Xyleborus monographus | 14 | 3385 | 178.2 ± 138.1 c |
| Anisandrus dispar | 19 | 2336 | 122.9 ± 20.5 d |
| Trypodendron domesticum | 17 | 1127 | 59.3 ± 15.9 c,d |
| Trypodendron signatum | 19 | 558 | 29.4 ± 5.3 d |
| Xyleborus dryographus | 11 | 208 | 10.9 ± 4.5 |
| Trypodendron lineatum | 6 | 59 | 3.1 ± 1.5 |
| Gnathotrichus materiarius * | 4 | 12 | 0.6 ± 0.4 |
| Xyleborus cryptographus | 1 | 1 | 0.1 ± 0.1 |
| Xyleborinus attenuatus * | 1 | 1 | 0.1 ± 0.1 |
| Total | 19 | 94104 | 4952.8 ± 1802.9 |
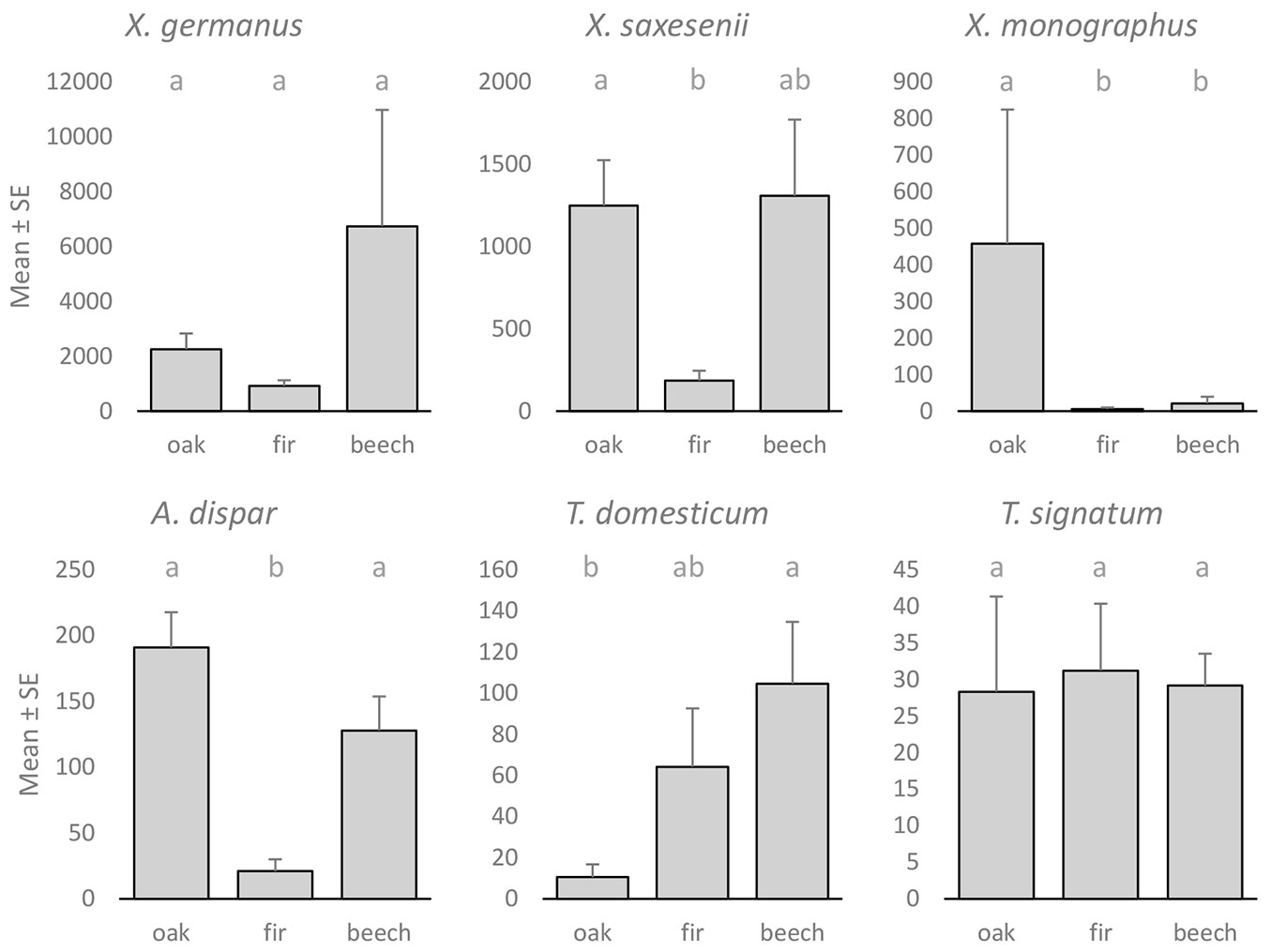
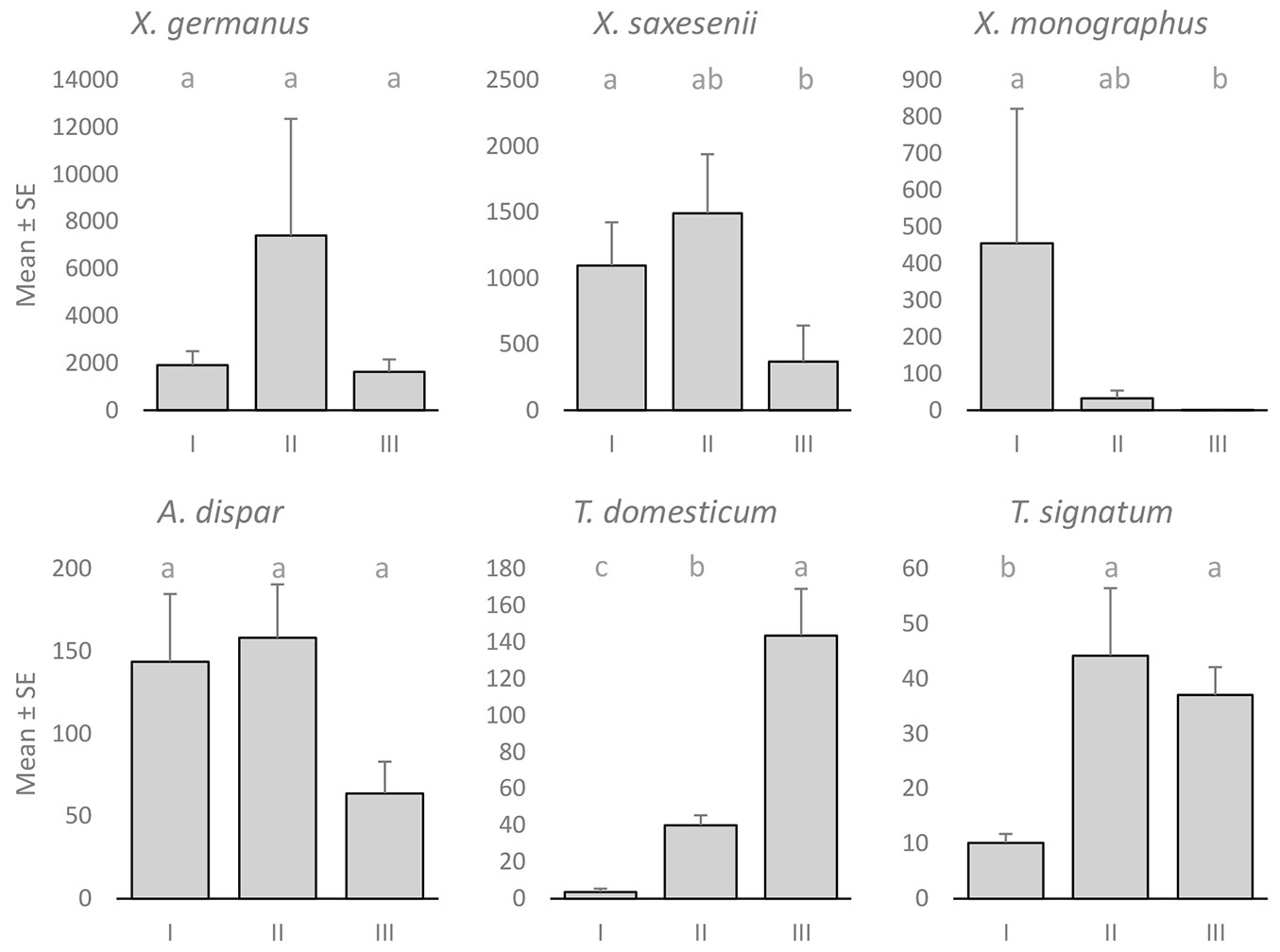
X. germanus was most abundant in beech-dominated stands (Fig. 2) and at medium altitudes (Fig. 3). Although the mean catch in beech stands was almost three times larger than that in oak stands and more than seven times larger than that in fir stands, the differences were not significant (one-way ANOVA: F[2, 16]=3.346, p=0.061). Similarly, the mean catch at medium altitudes was more than three times larger than that at lower altitudes and more than four times larger than that at higher altitudes, but again, the differences were not significant (one-way ANOVA: F[2, 16]=1.829, p=0.193). However, it should be pointed out that 47.3% of X. germanus specimens were caught at one beech stand at a medium altitude. If we eliminate this location from the analysis, the differences between altitudes were even more non-significant (p=0.506 - Games-Howell post-hoc test), while the differences between different forest types remained at a similar level (p=0.063). Although the differences were not significant (oak-fir: p=0.122; beech-fir: p=0.063 - Games-Howell post-hoc test), fir stands proved to be the least suitable for X. germanus. The abundance of the species in oak and beech stands was quite similar (p=0.568 - Games-Howell post-hoc test), especially if we removed the extreme beech location from the data (p=0.909 - Games-Howell post-hoc test).
Fig. 2 - Mean catch ± standard error (SE) of different ambrosia beetle species in three forest types. Different lowercase letters indicate significant differences (p<0.05) between forest types after Games-Howell post-hoc test.
Fig. 3 - Mean catch ± standard error (SE) of different ambrosia beetle species at different altitudes. Different lowercase letters indicate significant differences (p<0.05) between altitudes after Games-Howell post-hoc test.
Significant differences in the abundance of X. saxesenii between different forest types (one-way ANOVA: F[2, 16]=4.235, p=0.033) and different altitudes (one-way ANOVA: F[2, 16]=7.839, p=0.004) were found. The species was least common in fir stands (Fig. 2) and at higher altitudes (Fig. 3). Significant differences in abundance between fir and oak stands (p=0.009 - Games-Howell post-hoc test) and lower and higher altitudes (p=0.019 - Games-Howell post-hoc test) were also observed.
X. monographus was almost exclusively trapped in oak-dominated stands (Fig. 2) and at lower altitudes (Fig. 3). Nearly 95% of all X. monographus specimens were trapped in oak-dominated stands; however, 78.0% were trapped at only one location. Differences in abundance between forest types were significant (one-way ANOVA: F[2, 16]=5.911, p=0.012). Furthermore, significant differences between different altitudes (one-way ANOVA: F[2, 16]=11.581, p=0.001) were also observed.
The abundance of A. dispar in fir-dominated stands was significantly lower than that in oak- (p=0.009 - Games-Howell post-hoc test) and beech-dominated stands (p=0.019 - Games-Howell post-hoc test - Fig. 2). The species was less abundant at higher altitudes (Fig. 3), but the differences between different altitudes were not significant (one-way ANOVA: F[2, 16]=1.421, p=0.270).
Both Trypodendron species, T. domesticum and T. signatum, were more abundant at higher altitudes (Fig. 3). The catch of T. domesticum at higher altitudes was significantly larger than that at medium (p=0.002 - Games-Howell post-hoc test) and lower altitudes (p<0.001 - Games-Howell post-hoc test), and the difference was also significant between medium and lower altitudes (p=0.001 - Games-Howell post-hoc test). The abundance of T. signatum at lower altitudes was significantly lower than that at medium (p=0.007 - Games-Howell post-hoc test) and higher altitudes (p<0.001 - Games-Howell post-hoc test), but there was no significant difference (p=0.989 - Games-Howell post-hoc test) between medium and higher altitudes. The catch of T. signatum was evenly distributed between different forest types (Fig. 2), while a significant difference (one-way ANOVA: F[2, 16]=6.794, p=0.007) in the abundance of T. domesticum between different forest types was found. There was a significant difference between beech- and oak-dominated stands (p=0.002 - Games-Howell post-hoc test).
Flight period of X. germanus
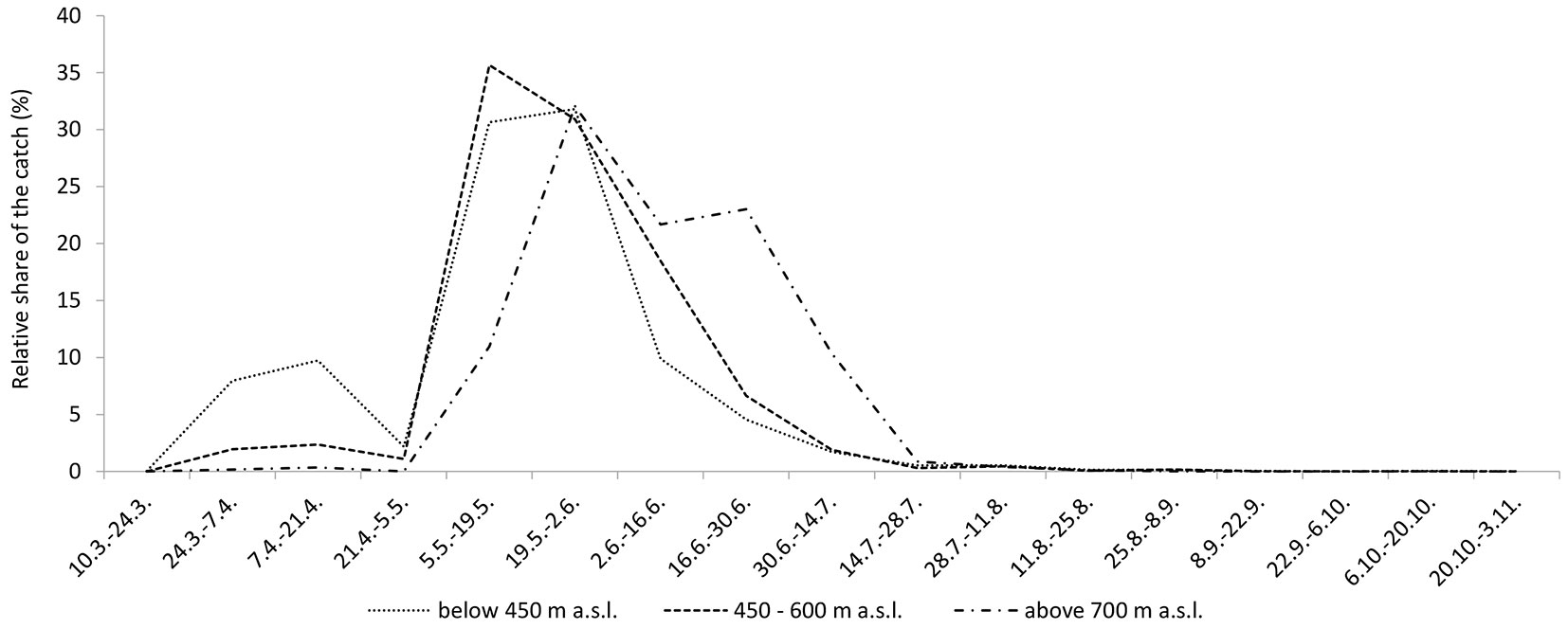
The first X. germanus specimens were trapped in the first monitoring period (Fig. 4), between 10 and 24 March, but only in three stands at lower altitudes. In the second monitoring period, the species was trapped at 12 additional locations. At the locations above 800 m a.s.l., X. germanus appeared in the third monitoring period (2 locations) or, even later, in the fifth monitoring period (2 locations).
Fig. 4 - Relative share of the catch (number of caught specimens in particular period and particular altitude group / number of caught specimens in entire monitoring period at particular altitude group) of X. germanus in different periods and at different altitudes.
The flight period at the end of April was interrupted due to cold and wet weather (Fig. 4). At lower and medium altitudes, peak flight activity appeared between 5 May and 2 June. At higher altitudes (above 700 m a.s.l.), peak flight activity appeared between 19 May and 2 June, but the catch was relatively large until the end of June. By the end of July, more than 99.0% of all X. germanus specimens had been trapped. A second peak in flight activity, which would indicate the development of the second generation, was not observed. However, X. germanus specimens were still sporadically caught in September and October.
Ice storm impact on the abundance of X. germanus
A catastrophic ice storm damaged Slovenian forests in February 2014. The positive correlation between the amount of sanitary felling in the period from 2014 to 2017 and the abundance of Xylosandrus germanus was not significant (one-tailed Pearson’s correlation coefficient: r=0.308, p=0.10). However, if we excluded the year 2014 and considered only the period from 2015 to 2017, the positive correlation was higher and significant (one-tailed Pearson’s correlation coefficient: r=0.41, p=0.04). Furthermore, the catch of X. germanus at one beech stand was extremely large. If we remove this data, the positive correlation was even higher and more significant (one-tailed Pearson’s coefficient: r=0.481, p=0.022).
Discussion
Less than 10 years after its first discovery, X. germanus is already the most abundant ambrosia beetle species in the research area. The species was trapped at all 19 sampled locations. It was the most common species at all locations except one and represented 71.8% of all ambrosia beetles caught in this study. Similar dominance has also been reported for other parts of Europe. In Belgium, X. germanus constituted 79.6% and 84.0% of all trapped scolytids in two monitoring studies in beech forest stands near Brussels ([17]). In a large monitoring project of saproxylic beetles near Vienna in Austria, X. germanus represented 70% of all identified individuals ([21]). Most recently, in a study located in northeastern Italy, X. germanus was the most commonly trapped ambrosia bark beetle (58.7% - [38]). Our results refer only to ambrosia bark beetles (Tab. 2); however, if we include the data on other beetles, individuals of X. germanus represented 71.6% of all bark beetles and 65.4% of all beetles. In addition to Curculionidae, Salpingidae was the most commonly trapped beetle family in our study.
Klimetzek et al. ([30]) tested different ethanol release rates and found a positive correlation between the ambrosia beetle catch and ethanol release rates. However, some authors have reported that high ethanol release rates may have an inhibitory effect and reduce the catch of ambrosia beetles ([41], [36], [12]). The release rate in our study was relatively high and could have had a negative impact on some species. However, based on unpublished data from experiments in which we used nonbaited traps, we believe that a lower ethanol release rate would not have affected the proportion of X. germanus in the catches.
As in some other studies in Europe ([33], [12], [38]), X. saxesenii was the most common native ambrosia beetle. It was especially abundant at lower and medium altitudes, but the proportion of this species in the catch was significantly reduced at higher altitudes (Fig. 3). In contrast, the catch of T. domesticum at higher altitudes was significantly larger than that at medium and lower altitudes, and therefore, at the three higher locations, T. domesticum was the most common native species. However, when interpreting the results for T. domesticum, it is necessary to stress that we missed the beginning and the peak of flight activity at lower and medium altitudes. In the local literature ([43], [24]), there is information that the species begins to fly in the second half of March, when ambient temperatures reach 13-14 °C, but our study (especially trap catches before 10 March 2017 - data not included in analysis) showed that in the lowlands, T. domesticum already had a peak occurrence before 10 March. According to temperature data from nearby meteorological stations ([1]), it is assumed that the temperature swarming threshold for T. domesticum is lower than that mentioned before, and it could be similar to that in Croatia, where it was found that flight activity starts when daily temperatures rise above 9 °C ([10]). However, the abundance of T. domesticum and, to a lesser extent, T. signatum in our lowland forests is certainly greater than our results showed.
In North America X. germanus did not exhibit flight activity until one or two days with a maximum daily temperature of at least 20 °C ([40]). According to data from the official meteorological station in Ljubljana ([1]), similar maximum daily temperatures were also measured in the second half of the first monitoring period (between 10 and 24 March). This turned out to be sufficient to induce flight activity in the first X. germanus specimens in nearby oak stands with a southern exposition. In the middle of the second monitoring period (between 24 March and 6 April), maximum daily temperatures also exceeded 20 °C at higher altitudes, and the first X. germanus specimens were also trapped at all other traps located below 800 m a.s.l. On this basis, we assume that the temperature threshold of 20 °C is also valid in our climatic conditions.
The flight activity of beetles varies from season to season due to different weather conditions in every year. However, Graf & Manser ([15]) reported that the flight activity of X. germanus in Switzerland started at the beginning of May, with a maximum in June and July. Similar observations were also made in Romania ([34]). Earlier flight activity of the species was observed in Croatia ([11]), where swarming began in the beginning of April, with peak flight activity already occurring at the end of April. On the basis of our results, it could be argued that the swarming of the species in lowland forests may take place by the middle or end of March. Maximum flight activity in our study was observed in May and early June in forests below 600 m a.s.l. and at the end of May and in June in forests above 700 m a.s.l. However, we assume that the cold and wet weather at the end of April prevented an even earlier occurrence of peak flight activity and that the peak occurrence of X. germanus could occur as soon as the end of April, as formerly recorded in Croatia.
In Central Europe X. germanus has one generation per year ([6], [20]), while in Italy two generations per year have been observed ([39]). The first peak of occurrence in Italy is in the middle of May and the second at end of July or at the beginning of August (M. Faccoli, personal communication). Because we observed the first peak in May, we also expected the occurrence of a second peak in July or August, but it obviously did not appear (Fig. 4). Therefore, we assume that only one generation per year develops in central Slovenia.
Minimum winter temperatures are presumably the key limiting factor for the survival of X. germanus ([6], [20], [13]). According to Slovenian Environment Agency data ([7]), January 2017 was the coldest January in the last three decades. The average monthly temperature in Ljubljana was -3.1 °C, which is 3.5 °C lower than the average of the period 1981-2010. The abundance of X. germanus in our traps after such exceptional conditions indicates that winter temperatures in the lowlands of Slovenia are most likely not a real limiting factor for the species. However, at higher altitudes, conditions are more extreme, and the vertical spread of the species is certainly limited. In our research, X. germanus was found along the entire altitudinal gradient between 303 and 941 m. Our results thus confirm the findings from Slovakia ([13]) and Romania ([34]), where the species appeared at altitudes of approximately 900 m. However, the catch of X. germanus in the trap at the highest altitude (941 m a.s.l.) was relatively large (2060 specimens). We therefore assume that we did not reach the upper boundary of the appearance of the species in Slovenia. In Slovakia, X. germanus was found to attack spruce logs at an altitude of 1020 m, but it has not yet been found in forests above 1100 m a.s.l. ([13]).
In Slovakia, altitude had a significant effect and forest type (oak, beech, spruce) had a nonsignificant effect on the abundance of X. germanus ([13]). However, the results from Slovakia suggest that beech forests between approximately 500 and 700 m a.s.l. are optimal for the species. Warmer and dryer oak forests at lower altitudes, and cool submontane mountain forests are presumably less suitable for the beetle. Our findings are to some extent also in accordance with the suggested optimal site conditions. The abundance of X. germanus in our study was also greatest at medium altitudes (450-600 m a.s.l.) and in beech stands (Fig. 2, Fig. 3). However, the differences between altitude groups and forest types in our study were not significant. Our results actually show that lower elevation oak forests are equally suitable for X. germanus compared to higher elevation beech forests, while fir stands proved to be the least suitable for X. germanus. The abundance of X. germanus in Italy ([38]) was significantly higher in sweet chestnut-dominated forests than in hop-hornbeam- and beech-dominated forests. Therefore, the relatively large admixture of sweet chestnut in the oak stands in our study could have contributed to the large abundance of the species in oak stands. Indeed, ongoing research work in Slovenia also shows the massive presence of X. germanus in other forest types, demonstrating that the species is a habitat generalist.
In most cases, X. germanus attacks hosts that are stressed, dying or recently dead ([37]). Forests damaged by natural disasters are therefore a suitable environment for the development of the species ([25]). In February 2014, Slovenia was hit by an ice storm that damaged approximately 9.3 million m3 of trees over an area of more than 600.000 ha ([45]). The forests in our research area were also damaged, but to varying degrees. The measure of damage is the amount of sanitary logging carried out in the period after the ice storm. If we take into account the entire period (2014-2017), the positive correlation between the amount of sanitary felling and the abundance of X. germanus is weak to moderate and not significant. However, if we exclude 2014, the positive correlation is higher and significant. Thus, we can conclude that the ice storm had a positive effect on the abundance of the species, especially in areas where sanitary logging was delayed.
In addition to X. germanus, two other non-native ambrosia beetle species were trapped in our study. G. materiarius is native to North America and was introduced to Europe in the 1930s ([2]). In Slovenia, it was first found in 2003 during an inventory of fauna at Brdo pri Kranju ([14]). Since then, it has often been found in traps intended for monitoring of Monochamus beetles in pine stands around Slovenia ([27]). Damage caused by this species has not yet been recorded. To our knowledge, the specimen of X. attenuatus caught in our study is the first finding of this species in Slovenia. It was trapped in the period between 14 and 28 July in an oak-dominated forest in the outskirts of Ljubljana. In another monitoring program that took place in 2018, another specimen of X. attenuatus was caught in an ethanol-baited trap located near Tolmin in the western part of Slovenia. However, this species was first found in Europe in 1987 ([31]). Its exact distribution is not clear because of confusion with the closely related X. saxesenii; nevertheless, it is estimated to be widely distributed within the EU ([4]).
Conclusions
The spread of X. germanus in Europe has accelerated since 2000 ([13]). Based on the results of this study and observations in other parts of Slovenia, we can conclude that the species has become established in Slovenia. Moreover, as in some other countries in Europe ([17], [21], [38], [13]), monitoring programs using ethanol-baited traps show that X. germanus has become a dominant ambrosia beetle species regardless of forest type. Findings of the species at higher altitudes ([34], [13], and this study) indicate the vertical spread of the species in Europe, which is most likely the result of climate change.
Despite the widespread and massive presence of the species in our forests, damage to standing trees, with rare exceptions for individual trees, has not yet been recorded. In fact, it is very difficult to find signs of the presence of the species in the forest, although we know that the X. germanus population is large. Therefore, despite the damage recorded in 2016 when X. germanus infested a larger amount of timber that was not removed from the forest immediately after logging ([19]), we agree with Lakatos & Kajimura ([32]) who stated that X. germanus in Europe is a secondary pest that does not cause remarkable damage. However, there are some examples of beetle-fungus symbioses that have shifted from being a secondary pest on a wide range of hosts to becoming a primary pest on a narrow range of hosts in their introduced ranges ([22]). In some regions of North America, a similar shift may have already occurred for X. germanus ([3]); therefore, there is a risk that the species will become an important pest in European orchards, plantations, nurseries and vineyards, as is currently the case in North America ([13]). Furthermore, with climate change, we can expect more stressful events and large-scale disruptions in forests and, consequently, more attacks of X. germanus on weakened or fallen trees.
Maintaining tree health is essential to prevent X. germanus attacks ([37]). This is, for example, easier to achieve in forest nurseries than in forest stands. The most important measure to prevent attacks and economic damage in forests is to transport felled logs from the forests immediately after harvesting and to store the wood in places that allow for proper drying ([15]). Another option is to carry out logging, transport and storage of lumber outside of the main flight period of the species ([11]).
Acknowledgements
This research was financed by the Pahernik Foundation and the Slovenian Research Agency through research program P4-0059. We are grateful to Daniel Borkovič (Department of Forestry and Renewable Forest Resources, Biotechnical Faculty, University of Ljubljana, Slovenia) for his participation in the fieldwork.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Roman Pavlin
Petra Grošelj 0000-0001-9363-9783
Maja Jurc 0000-0002-8666-1453
Department of Forestry and Renewable Forest Resources, Biotechnical Faculty, University of Ljubljana, Večna pot 83, SI-1000 Ljubljana (Slovenia)
Corresponding author
Paper Info
Citation
Hauptman T, Pavlin R, Grošelj P, Jurc M (2019). Distribution and abundance of the alien Xylosandrus germanus and other ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) in different forest stands in central Slovenia. iForest 12: 451-458. - doi: 10.3832/ifor3114-012
Academic Editor
Massimo Faccoli
Paper history
Received: Mar 29, 2019
Accepted: Jul 27, 2019
First online: Sep 29, 2019
Publication Date: Oct 31, 2019
Publication Time: 2.13 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2019
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 50929
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 36844
Abstract Page Views: 9806
PDF Downloads: 3410
Citation/Reference Downloads: 9
XML Downloads: 860
Web Metrics
Days since publication: 2317
Overall contacts: 50929
Avg. contacts per week: 153.86
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2019): 12
Average cites per year: 1.71
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Distribution and habitat suitability of two rare saproxylic beetles in Croatia - a piece of puzzle missing for South-Eastern Europe
vol. 11, pp. 765-774 (online: 28 November 2018)
Research Articles
A bark beetle infestation predictive model based on satellite data in the frame of decision support system TANABBO
vol. 13, pp. 215-223 (online: 06 June 2020)
Research Articles
Determination of differences in temperature regimes on healthy and bark-beetle colonised spruce trees using a handheld thermal camera
vol. 14, pp. 203-211 (online: 02 May 2021)
Progress Reports
Landscape-scale Ips typographus attack dynamics: from monitoring plots to GIS-based disturbance models
vol. 4, pp. 256-261 (online: 13 December 2011)
Research Articles
Discovering interaction between oaks and carabid beetles on a local scale by point pattern analysis
vol. 9, pp. 618-625 (online: 06 May 2016)
Review Papers
Dutch elm disease and elm bark beetles: a century of association
vol. 8, pp. 126-134 (online: 07 August 2014)
Research Articles
Identification of the ambrosia beetle Anisandrus dispar (Fabricius) (Coleoptera Curculionidae Scolytinae) using TaqMan™ probe assay on biological samples
vol. 16, pp. 182-187 (online: 30 June 2023)
Short Communications
Effective monitoring as a basis for adaptive management: a case history of mountain pine beetle in Greater Yellowstone Ecosystem whitebark pine
vol. 2, pp. 19-22 (online: 21 January 2009)
Review Papers
Forest health under climate change: impact of insect pests
vol. 17, pp. 295-299 (online: 30 September 2024)
Research Articles
Long-term changes in surface-active beetle communities in a post-fire successional gradient in Pinus brutia forests
vol. 10, pp. 376-382 (online: 16 March 2017)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword