Effects of drought and nutrient deficiency on grafts originating from sound and shaken sweet chestnut trees (Castanea sativa Mill.)
iForest - Biogeosciences and Forestry, Volume 9, Issue 1, Pages 109-114 (2015)
doi: https://doi.org/10.3832/ifor1572-008
Published: Jul 19, 2015 - Copyright © 2015 SISEF
Research Articles
Abstract
Scions taken from felled, shaken or sound sweet chestnut trees (Castanea sativa Mill.) were grafted and grown for one year in a polythene tunnel in order to compare their responses to water and nutrient stresses. Phenological characteristics of the original trees were strongly reproduced in the grafts grown both in this controlled environment and later on in the field. Grafts originating from shaken trees flushed up to six days later, senesced earlier and produced larger spring vessels. Artificially imposed drought reduced stomatal densities by 5.6% and xylem vessel diameters by up to 35%. Fertiliser additions significantly increased stem increments and promoted earlier flowering, with hermaphrodite flowering filaments more common in grafts from shaken trees. It is considered that, because of their larger spring vessels, shaken trees may be more vulnerable to cavitation and therefore to drought, even though moisture stress is mitigated by some plasticity in earlywood vessel diameter.
Keywords
Shake Defect, Castanea Sativa, Moisture Stress, Soil Fertility
Introduction
Ring shake in chestnut, the separation of annual rings at planes of tangential weakness, is a major timber defect, leading to a decrease of the economic value of standing trees. The predisposing factors are unknown, but it is likely that the genetic background, edaphic conditions and environmental stresses may all be involved ([18]), besides mechanical drying and inherent growth stresses. Shake usually manifests itself as fractures across the large earlywood vessels of this ring-porous species or, less commonly, through the separation of earlywood from latewood ([12]). The uniseriate rays may resist this, as shaken trees have been shown to develop greater ray volumes ([13]). Chemical attributes of the wood, such as the higher concentration of phenol homosyringaldehyde in the lignin of shaken trees, may be another factor ([30]).
Shake-prone trees may be relatively more susceptible or less well-adapted to moisture stresses than sound trees. Low soil water availability on freely draining sites may predispose such trees, while fluctuations in soil moisture, for example in gleyed soil profiles, are also thought to exacerbate shake in the similarly ring-porous Quercus robur and Q. petrea ([28]). Summer drought stress is a major growth limitation affecting chestnut plantations in some parts of the Mediterranean ([1]), where moisture stress can be mitigated by management treatments such as thinning, removal of competing vegetation or lengthening of the rotation ([23], [24], [7], [3]), thus giving more soil resources per tree. In England, Henman & Denne ([16]) recommended planting oak (Quercus spp.) on deep soils with good moisture retention to avoid shake.
Under moisture stress, the xylem vessels are more likely to cavitate and lose their hydraulic conductivity through leakage at the pit membranes ([4], [19]). A trade-off therefore exists between wide-diameter earlywood vessels that transport most water early in the growing season ([6]) and the risk of embolisms occurring later during drought episodes. The wider vessels associated with shaken chestnut individuals may thus be more prone to blockage by cavitation ([20]), although Sperry et al. ([29]) found that some ring-porous species could tolerate cavitation or rely on new xylem production to restore hydraulic conductance. Furthermore, vessel size has been shown to acclimate to the availability of spring and summer precipitation in Quercus robur ([14]). Similar responses in the Mediterranean region have been found in both the diffuse-porous Q. ilex ([5], [2]) and the ring-porous Q. pyrenaica ([6]).
Shake may also be related to shortage of nutrients on the poor soils that commonly support chestnut plantations. Ranger & Colin-Belgrand ([22]) reported that decreasing the chestnut rotation length might deplete forest soil nutrients, a conclusion also reached by Rubio & Escudero ([25]). In Britain, Rackham ([21]) pointed out that continual coppicing cycles of 8-30 years, removing 2-4 dry tones ha-1 yr-1, might result in net nutrient export. However, in a fertilization experiment conducted at Kings forest in Kent, England, Evans ([8]) reported no growth increases of chestnut coppice. Here the addition of lime significantly depressed growth, but small significant increases in foliar phosphate levels resulted from the addition of mineral phosphate.
However, responses to fertiliser on poor substrates may be significant. In former agricultural soils, Alvarez-Alvarez et al. ([1]) found superior yields of plantations of Castanea sativa and the hybrid C. × coudercii where deeper profiles and the supplies of available phosphorus, Ca and K were greater than in forest soils. Laroche et al. ([17]) believed that calcium, an important element stabilising the middle lamella of cell walls, might be implicated in chestnut ring shake. In a glasshouse experiment using as a growth medium the C horizon of a mesotrophic brown soil, they found that one-year chestnut trees were able to grow for at least two years on this impoverished material. NPK fertiliser improved growth, as did further additions of calcium and magnesium, but liming did not depress growth. Fonti et al. ([10]) suggested that shake might be due to difficulties in calcium absorption, rather than the absolute amount of calcium.
Clearly, drought and nutrient treatments cannot be applied directly to trees growing in the field where their shake status is yet unknown. Instead, this investigation took grafts from trees known to be shaken or sound and subjected them to controlled environmental conditions. The specific questions were: (I) is the growth of sweet chestnut scions influenced by their background and origin, or by stressed soil conditions such as low moisture and nutrients, or both? (ii) Could the growth responses and characteristics of the trees be helpful to foresters in identifying those that might be susceptible to shake?
Methods
Scions for grafting were taken from the upper crown of selected shaken or sound chestnut trees at five field sites in Kent, England, during March and April 2002: Brickhouse (51o 18′ N, 1o 5′ E), Godinton (51o 9′ N, 0o 50′ E), Three Wents (51o 5′ N, 0o 42′ E), Larkey Valley (51o 15′ N, 1o 2′ E) and Postling (51o 7′ N, 1o 4′ E). Shake was confirmed by observing 5 cm thick green discs, cut from the base of each tree. These were mostly coppice stems but also included some maidens and were of differing age, ranging from 32 to 102 years and from 22-86 cm diameter at breast height (1.3 m above ground). The scions were approximately 10-15 cm long, containing on average five buds. They were immediately dispatched to Horticulture Research International (HRI), East Malling, for grafting on to commercial nursery seedling stock.
After a season’s growth at HRI East Malling, the grafts were re-potted into 25 cm diameter, 7.5 litre pots in an unheated polythene tunnel at the Wye Campus of Imperial College in the winter of 2003 (Fig. 1). Plastic saucers were placed underneath to conserve water and nutrients. Of 288 grafts, 90 from each category (shaken and sound) were selected from the mid-size range. The growing medium, an infertile, acidic sandy silt loam (Tab. SM1 in Appendix 1) was taken from the top 25-30 cm of the profile of a field at Godinton Park (51o 9′ N, 0o 51′ E). Large stones and weeds were removed by hand, and the soil thoroughly mixed.
Fig. 1 - Layout of the pot experiment in a polythene tunnel, using one year-old grafted saplings, either of shaken or sound origin.
In mid-March 15th 2003, five pots containing the re-potted grafts were soaked in a water- filled container and saturated for 4-5 hours. The pot surface was covered with black polythene to prevent evaporation and afterwards allowed to drain. Field capacity was determined after the third day, when Time Domain Reflectometry (TDR) indicated an average of 35% volumetric water content. After 5 days without watering, the plants started to wilt at TDR readings of 10-11% volumetric water content; thereafter, 11% volumetric water content was taken as the critical baseline to avoid wilting and plant death.
Experimental design
The experiment was a completely randomised design, with three levels of moisture stress and three of NPK nutrients applied to 10 replicate grafts of either sound or shaken trees. There were thus 18 treatments and 180 experimental units.
Three soil moisture treatments were applied. Control grafts received no water stress and were maintained at field capacity throughout. Medium water stress was applied at 19-27% TDR and high stress at 11-19% volumetric water content. Tap water was applied to maintain each of these three regimes every 2-5 days, depending on the weather, and triggered by soil moisture levels indicated by continuous TDR readings. The TDR instrument (Soil Moisture Equipment Corp., Santa Barbara, CA, USA) used two horizontally inserted wave-guides 7 cm from the bottom of the pot; 40% of all replicates had wave-guides inserted.
Three nutrient levels were applied once to the pot surfaces as Growmore mini granules (an NPK 7:7:7 fertiliser) in late March 2003. The highest level of 4.94 g per pot was the equivalent of 23.8 kg N, 8.8 kg P and 21.4 kg K ha-1, while the medium level had half this amount (2.47 g per pot) and the control had no applied fertiliser.
Grafts were grown in treatments for a full growing season in the polythene tunnel. Flushing times were noted between March 15th and May 31st, with assessments carried out every other day, using the following scores:
- 0: buds closed;
- 1: <50% of buds breaking;
- 2: c. 50% of buds breaking;
- 3: all buds breaking;
- 4: unexpanded leaves visible at c. 50% of bud sites;
- 5: unexpanded leaves visible at all bud sites;
- 6: leaves half expanded;
- 7: leaves fully expanded.
Leaf retention at the end of the growing season was also observed over a period of 30 days from October 10th to November 10th, every five days, counting all remaining leaves on the grafts. The percentage of leaf fall was calculated from the difference between the first and last counts.
Stem volume increments were calculated at the beginning and the end of the experiment, i.e., in March and October respectively. Leaf areas were assessed in mid-August, when two mature leaves were taken from the mid-crown of the leading graft shoot, using a leaf area meter (Delta-T Devices, Burwell, Cambridge, UK). In the same month stomatal densities were determined from two mature leaves, drawn from the mid-crown of two randomly selected grafts per treatment, using dental silicone rubber painted with nail varnish.
Flowering characteristics were recorded in June and included flower development, types of flowers (whether hermaphrodite or not), catkin length and filament length, fruiting and vessel size. Fruiting was assessed at the end of the growing season. Fruit diameters were grouped into three sizes, ≥6 cm, ≥3-<6 cm, and <3 cm in diameter.
At the conclusion of the experiment, in the winter of 2004, 165 grafts consisting of approximately equal numbers of shaken and sound trees, were planted outside into a nearby experimental plot and spaced 1.5 m apart, in a fertile, silty clay loam soil. Twigs from 40 of these grafts were then collected after one growing season in November 2004, to assess the annual rings formed in 2003 and 2004. These consisted of equal numbers of both shaken and sound origins and represented high and non-water stressed treatments in the original high-nutrient regime only. Xylem vessel diameters in the spring vessels were then determined microscopically from microtomed twig sections, stained with Safranin O, Fast Green and Crystal Violet ([15]).
Finally, leaf flushing scores of leaves on trees growing in the outdoor experimental plot were recorded on May 3rd 2008 and again on May 6th 2014.
Statistical analysis
The completely randomised experimental design was tested using the Analysis of Variance (ANOVA) for all main growth parameters, except for t-tests on vessel sizes formed respectively in 2003 and 2004. Flushing scores in 2008 and 2014 also used t-tests. The potential effects of physiological age on graft performance were tested using a Multivariate ANOVA or MANOVA ([9]).
Linear regression was applied to examine the relationship between vessel diameter and physiological tree age.
Results
Generally, grafts taken from sound trees showed superior performance to those of shaken origin, the relative differences depending on the level of water stress and nutrients applied.
Flushing and leaf retention
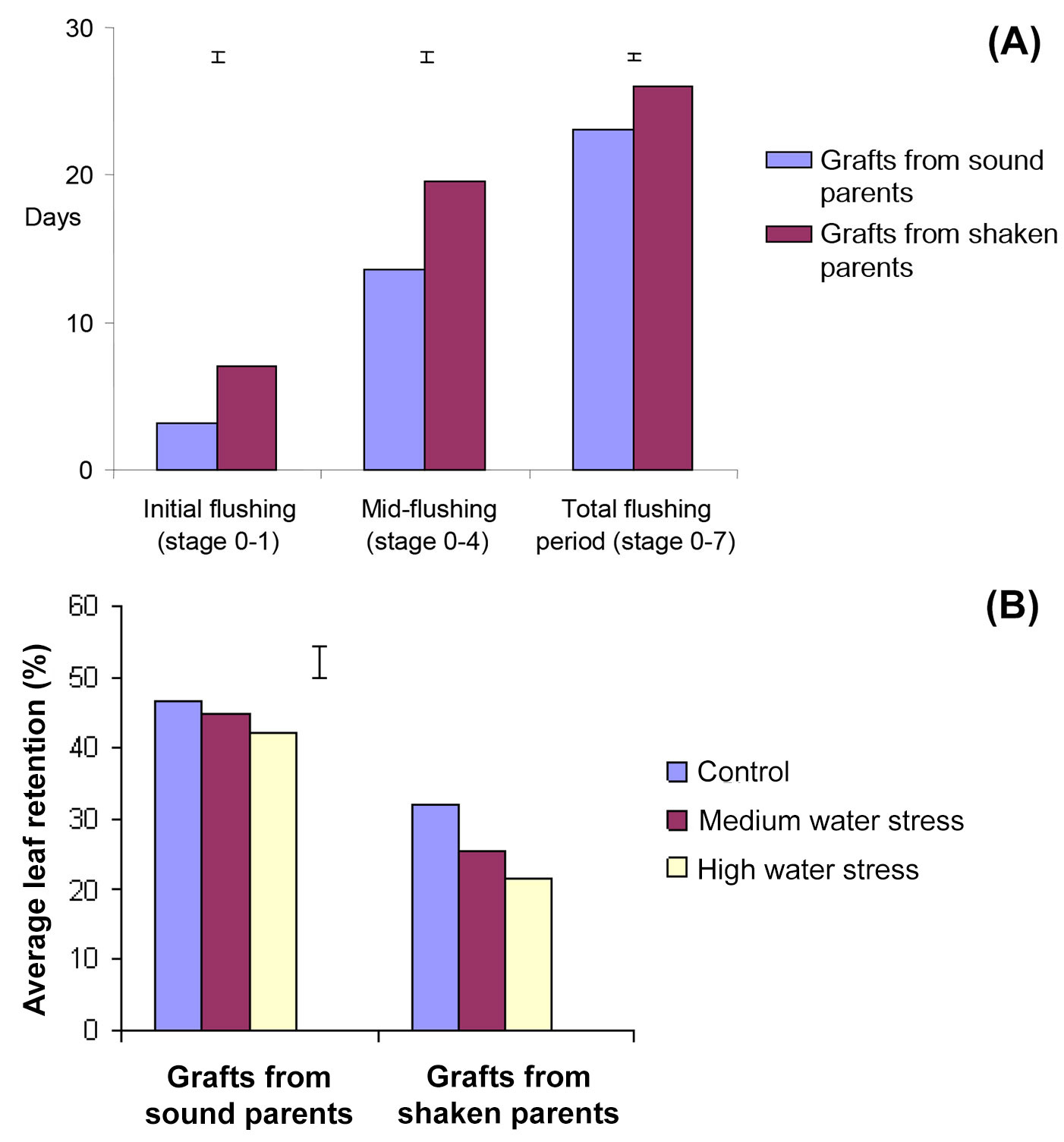
Flushing periods from sound or shaken trees, stages 0-1 (beginning), 0-4 (mid-flushing) and the total period (0-7) were significantly different. The ANOVA confirmed that sound tree grafts flushed earlier than shaken ones (p<0.01), used fewer days to reach mid-flushing stage (13.6 vs. 19.6 days, p<0.001) and completed all flushing stages within 23 days, at least three days earlier (p<0.001 - Fig. 2a). Grafts growing in the highest fertiliser regime also flushed earlier than unfertilised controls and had shorter flushing periods (p<0.001). Water stress reduced leaf retention generally (Tab. 1). Grafts from shaken trees lost their leaves sooner (26.3%) but had little effect on grafts from sound ones (44.6%, p=<0.001 - Fig. 2b).
Fig. 2 - (A) Effect of graft origin on different stages of flushing. Bars are standard errors of the mean difference between days from the start of first flushing. (B) The interaction between graft origin and water regime on leaf retention percentage during senescence at the beginning of autumn (20 September - 20 October 2003). The error bar is the standard error of the difference between treatment means.
Tab. 1 - Effects of different watering regimes on graft leaf area, leaf retention, stomatal density and fruiting during the growing season. Fruit sizes: (category 1): large fruits (diameter ≥ 6cm); (category 2): medium fruits (3 cm ≤ diameter < 6cm); (category 3): small fruits (diameter < 3cm).
| Variables | Water stress treatment | P | SED | LSD | ||
|---|---|---|---|---|---|---|
| None | Medium | High | ||||
| Leaf area (cm2) | 73.60 | 70.40 | 70.50 | 0.065 | 3.380 | 6.690 |
| Stomatal density mm-2 | 165.25 | 162.08 | 156.08 | 0.001 | 2.253 | 4.590 |
| Number of female flowers | 0.91 | 0.26 | 0.49 | 0.061 | 0.271 | 0.539 |
| Number of fruits category 1 | 1.77 | 1.37 | 0.93 | 0.106 | 0.387 | 0.784 |
| Number of fruits, category 2 | 4.68 | 1.51 | 1.06 | <0.001 | 0.728 | 1.474 |
| Number of fruits,category 3 | 2.29 | 4.87 | 2.71 | 0.024 | 0.960 | 1.946 |
| Total number of fruits | 8.75 | 7.75 | 4.72 | <0.001 | 1.018 | 2.066 |
| Leaf retention % (10 October - 10 November 2003) |
38.44 | 36.12 | 31.73 | <0.001 | 1.576 | 3.116 |
In 2008 and 2014 flushing stage scores were again used to compare the performance trees growing outside in the experimental plot. In 2008, four years after their removal from the polythene tunnel, trees of sound origin still flushed earlier than shaken trees (mean scores: 3.23 and 2.59, respectively - p<0.05, n=75). In 2014, after 11 years, taken in a slightly earlier growing season, the flushing scores were 5.19 for “sound” and 4.23 for “shaken” trees (p<0.01, n=69).
Overall stem increment
The average stem increment of grafts from sound or shaken trees was similar (39.1 cm3 and 34.5 cm3) and not significantly different (p=0.114) according to the analysis of variance. However, stem increments of grafts from both origins were considerably increased by fertiliser treatments (p<0.001) compared with unfertilised controls (Tab. 2). Stem increment growth was unaffected by water stress.
Tab. 2 - Effect of nutrient regime on flushing, leaf area, leaf petiole length and stem increment of grafts during the growing season.
| Variables | NPK (4.94g pot-1) |
NPK (2.47g pot-1) |
No NPK (control) |
p-value | SED | LSD |
|---|---|---|---|---|---|---|
| Beginning of flushing (days) | 4.53 | 4.77 | 6.00 | <0.001 | 0.412 | 0.813 |
| Flushing period (days) | 24.08 | 24.19 | 25.37 | <0.001 | 0.310 | 0.612 |
| Leaf area (cm2) | 72.40 | 68.00 | 64.10 | 0.052 | 3.380 | 6.690 |
| Petiole length (cm) | 1.98 | 1.82 | 2.01 | 0.029 | 0.075 | 0.149 |
| Stem increment (cm3) | 42.90 | 39.90 | 27.60 | <0.001 | 3.500 | 6.930 |
Leaf area
Mean leaf areas of grafts taken from sound trees (76.50 cm2) were significantly greater than from shaken ones (59.80 cm2; p<0.01). Leaves from fertilised grafts were only marginally larger (p=0.052) and were unaffected by water stress (p=0.065 - Tab. 2). However, petiole lengths were shorter in middle fertiliser treatment, contrasting with the others (p=0.029). Water stress had little effect on leaf areas (Tab. 1).
Stomatal density
Stomatal densities were reduced under conditions of water stress (p=0.001 - Tab. 1), especially in the highly stressed treatment, where the average density was 156.1 mm-2, a 5.6% reduction compared with the control. The densities on leaves of grafts taken from sound trees (163.8 mm-2) was marginally greater than those shaken ones (158.4 mm-2, p=0.020).
Flowering and fruiting
More than half of the grafts (59.4%) flowered during the growing season. Significant differences were found between graft origin, with shaken origins having a greater tendency to flower, to flower earlier, and to produce a number of hermaphrodite filaments (Tab. 3), although these only accounted for 9% of the total. In contrast, grafts from sound trees had longer catkins and filaments and produced greater numbers of female flowers. Grafts from shaken trees produced larger numbers of small fruits (<3 cm diameter) than from sound trees (Tab. 4). The total number of fruits produced was greatest when grafts were grown at field capacity (p<0.001 - Tab. 1), mainly in sound-origin grafts, which also produced most large (category 1) fruits (Tab. 4).
Tab. 3 - Effects of graft origin, from sound or shaken trees, on flowering.
| Variables | Sound | Shaken | p-value | SED | LSD |
|---|---|---|---|---|---|
| Proportion of flowering grafts (%) | 57.20 | 72.80 | 0.031 | 0.072 | 0.142 |
| Beginning of flowering (days) | 12.42 | 8.73 | <0.001 | 0.565 | 1.124 |
| Female flowers (number per graft) | 0.91 | 0.20 | 0.002 | 0.221 | 0.440 |
| Catkin length (cm) | 8.79 | 4.97 | <0.001 | 0.450 | 0.896 |
| Filament length (mm) | 3.54 | 3.08 | 0.017 | 0.191 | 0.380 |
| Flowers with hermaphrodite filaments (number per graft) | 0.10 | 0.55 | 0.003 | 0.146 | 0.290 |
Tab. 4 - Effects of water regimes on flowering and fruiting in grafts of sound or shaken origin: mean figures per graft. Fruit sizes: (category 1): large fruits (diameter ≥ 6cm); (category 2): medium fruits (3 cm ≤ diameter < 6cm); (category 3): small fruits (diameter < 3cm).
| Parameter | Water stress | Graft origin | p | SED | LSD | |
|---|---|---|---|---|---|---|
| Sound trees | Shaken trees | |||||
| Female flowers (number) | None | 1.5 | 0.31 | 0.073 | 0.383 | 0.762 |
| Medium | 0.26 | 0.27 | ||||
| High | 0.96 | 0.03 | ||||
| Hermaphrodite flowers (number) | None | 0.11 | 0.93 | 0.074 | 0.253 | 0.503 |
| Medium | 0.03 | 0.55 | ||||
| High | 0.17 | 0.17 | ||||
| Catkin length (cm) | None | 7.95 | 5.47 | 0.080 | 0.78 | 1.551 |
| Medium | 9.15 | 5.14 | ||||
| High | 9.27 | 4.28 | ||||
| Fruits, category 1 (number) | None | 2.64 | 0.91 | 0.014 | 0.547 | 1.109 |
| Medium | 1.3 | 1.44 | ||||
| High | 0.69 | 1.19 | ||||
| Fruits, category 2 (number) | None | 6.15 | 3.21 | 0.083 | 1.029 | 2.085 |
| Medium | 1.50 | 1.52 | ||||
| High | 1.11 | 1.02 | ||||
| Fruits, category 3 (number) | None | 2.59 | 2 | 0.076 | 1.358 | 2.752 |
| Medium | 2.91 | 6.83 | ||||
| High | 1.92 | 3.51 | ||||
| Total fruits (number) | None | 11.39 | 6.11 | <0.001 | 1.442 | 2.921 |
| Medium | 5.72 | 9.78 | ||||
| High | 3.71 | 5.72 | ||||
Fertilised grafts flowered on average earlier (p=0.005) and earlier still in grafts taken from shaken individuals (Tab. 5). Interestingly, there were more medium-sized (category 2 fruits) produced by sound grafts growing in unfertilised pots, but more such fruits on shaken grafts in fertilised pots. Some interactions between water and nutrient availability were difficult to interpret. Catkins were shorter in high water stress, high fertiliser treatments. There were more medium-sized fruits produced at low water stress, irrespective of fertiliser status, and more small fruits in unfertilised controls at medium water stress (Tab. 6).
Tab. 5 - Effects of nutrient regimes on flowering and fruiting in grafts of sound or shaken origin: mean figures per graft. Fruit sizes: (category 2): medium fruits (3 cm ≤ diameter < 6cm); (category 3): small fruits (diameter < 3cm).
| Parameter | Nutrient Regime | Graft origin | p | SED | LSD | |
|---|---|---|---|---|---|---|
| Sound trees | Shaken trees | |||||
| Beginning of flowering (days) | NPK (4.94g pot-1) | 14.59 | 7.95 | 0.041 | 0.078 | 1.947 |
| NPK (2.47g pot-1 ) | 11.86 | 9.00 | ||||
| No added NPK | 10.82 | 9.24 | ||||
| Catkin length (cm) |
NPK (4.94g pot-1 ) | 7.67 | 5.25 | 0.082 | 0.780 | 1.551 |
| NPK (2.47g pot-1) | 9.17 | 4.93 | ||||
| No added NPK | 9.53 | 4.71 | ||||
| Fruits, category 2 (number) | NPK (4.94g pot-1) | 1.75 | 2.65 | 0.017 | 1.029 | 2.085 |
| NPK (2.47g pot-1 ) | 2.49 | 1.98 | ||||
| No added NPK | 4.52 | 1.11 | ||||
| Fruits, category 3 (number) | NPK (4.94g pot-1) | 2.15 | 4.01 | 0.058 | 1.358 | 2.752 |
| NPK (2.47g pot-1) | 3.76 | 2.91 | ||||
| No added NPK | 1.52 | 5.42 | ||||
Tab. 6 - Interactions between water and nutrient regimes on flowering and fruiting of grafts. Fruit sizes: (category 2): medium fruits (3 cm ≤ diameter < 6cm); (category 3): small fruits (diameter < 3cm).
| Parameter | Water stress | NPK (g pot-1) | p | SED | LSD | ||
|---|---|---|---|---|---|---|---|
| 44.5g | 2.47g | Control | |||||
| Petiole length (cm) | None | 2.05 | 1.67 | 2.09 | 0.091 | 0.13 | 0.258 |
| Medium | 2.00 | 1.87 | 2.07 | ||||
| High | 1.89 | 1.93 | 1.87 | ||||
| Catkin length (cm) |
None | 6.31 | 6.72 | 7.10 | 0.010 | 0.955 | 1.9 |
| Medium | 8.15 | 6.92 | 6.36 | ||||
| High | 4.92 | 7.5 | 7.9 | ||||
| Fruits, category 2 (number) | None | 5.40 | 3.29 | 5.35 | 0.020 | 1.26 | 2.554 |
| Medium | 0.25 | 3.44 | 0.84 | ||||
| High | 0.95 | 0.02 | 2.26 | ||||
| Fruits, category 3 (number) | None | 2.53 | 3.5 | 0.85 | 0.010 | 1.664 | 3.371 |
| Medium | 3.71 | 2.84 | 8.06 | ||||
| High | 3.00 | 3.66 | 1.49 | ||||
Vessel size
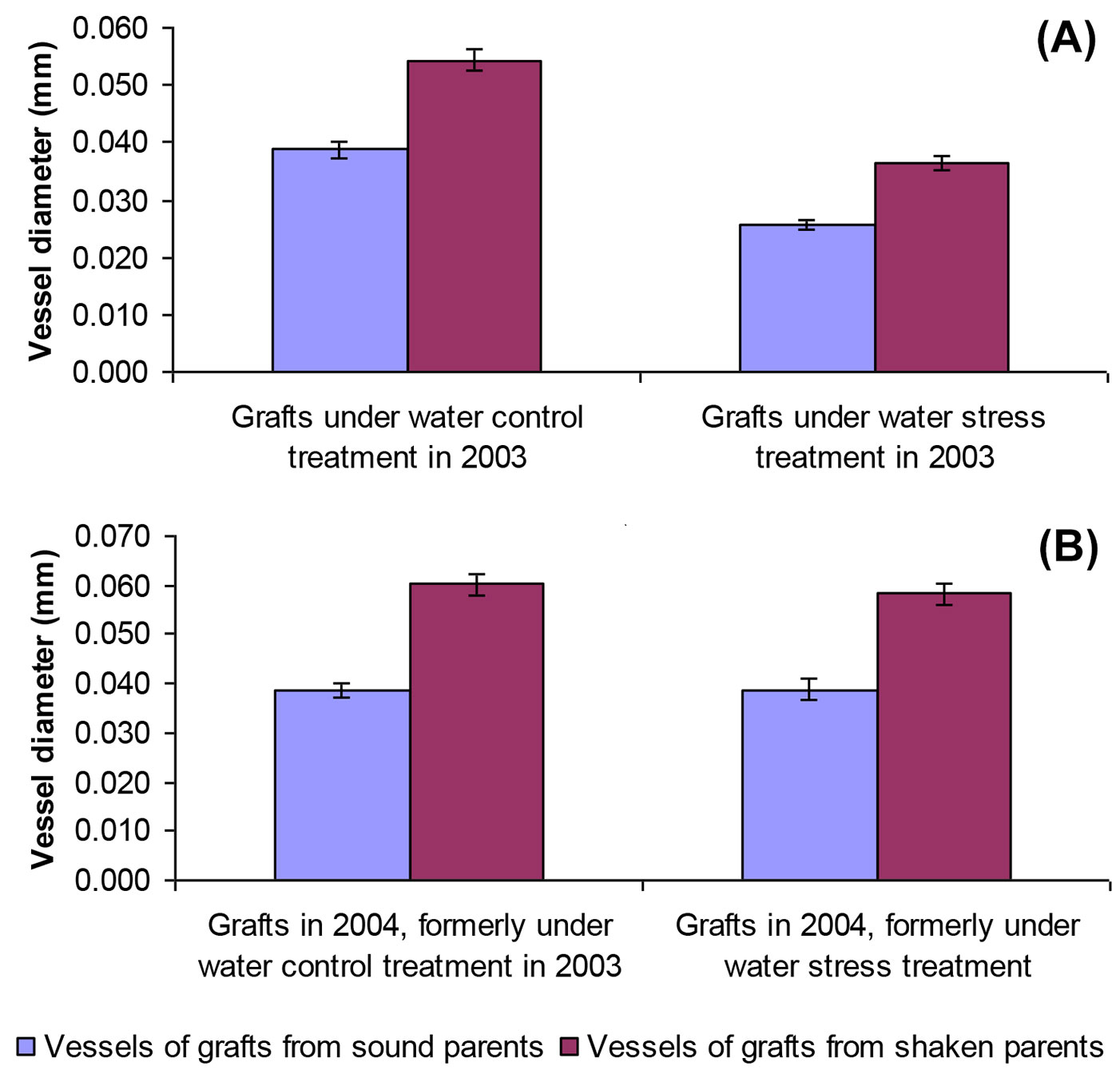
The mean vessel diameters of grafts grown in 2003 with no water stress (0.048 mm) was significantly greater than in stressed treatments (0.031 mm), confirmed by t-testing (p<0.001 - Fig. 3). With no water stress, the average vessel diameter of grafts from sound trees was significantly wider (0.035 mm) than those formed under stress (0.026 mm, p<0.001 - t-test). A similar trend was observed with grafts of shaken origin (means of 0.054 mm compared with 0.037 mm, p<0.001). Mean vessel diameter was always wider in shaken grafts: pooling water stress treatments, those from shaken trees (0.046 mm) were significantly narrower than from sound trees (0.030 mm, p<0.001 - Fig. 3).
Fig. 3 - Comparison of mean earlywood vessel diameters in grafts from sound and shaken trees under control and water stress treatments in (A) 2003 and (B) in the same grafts in 2004, when no water stress was applied. Error bars are standard errors of the means (SE).
Grafts grown outside in the experimental plot in 2004, where soil moisture was theoretically consistent, formed xylem vessels of similar diameter irrespective of water stress treatments applied a year earlier (Fig. 3). Once again, t-testing showed that average vessel diameters originating from shaken trees (0.059 mm) were significantly wider than those from sound trees (0.039 mm, p<0.001). Vessel lumens developed outside during 2004 were also significantly wider than their 2003 counterpart: those from sound origins were 0.026 and 0.037 mm in 2003 and 2004, respectively (p< 0.001), compared with 0.037 and 0.058 mm for plants from shaken trees (p<0.001).
Discussion
Physiological age of graft material
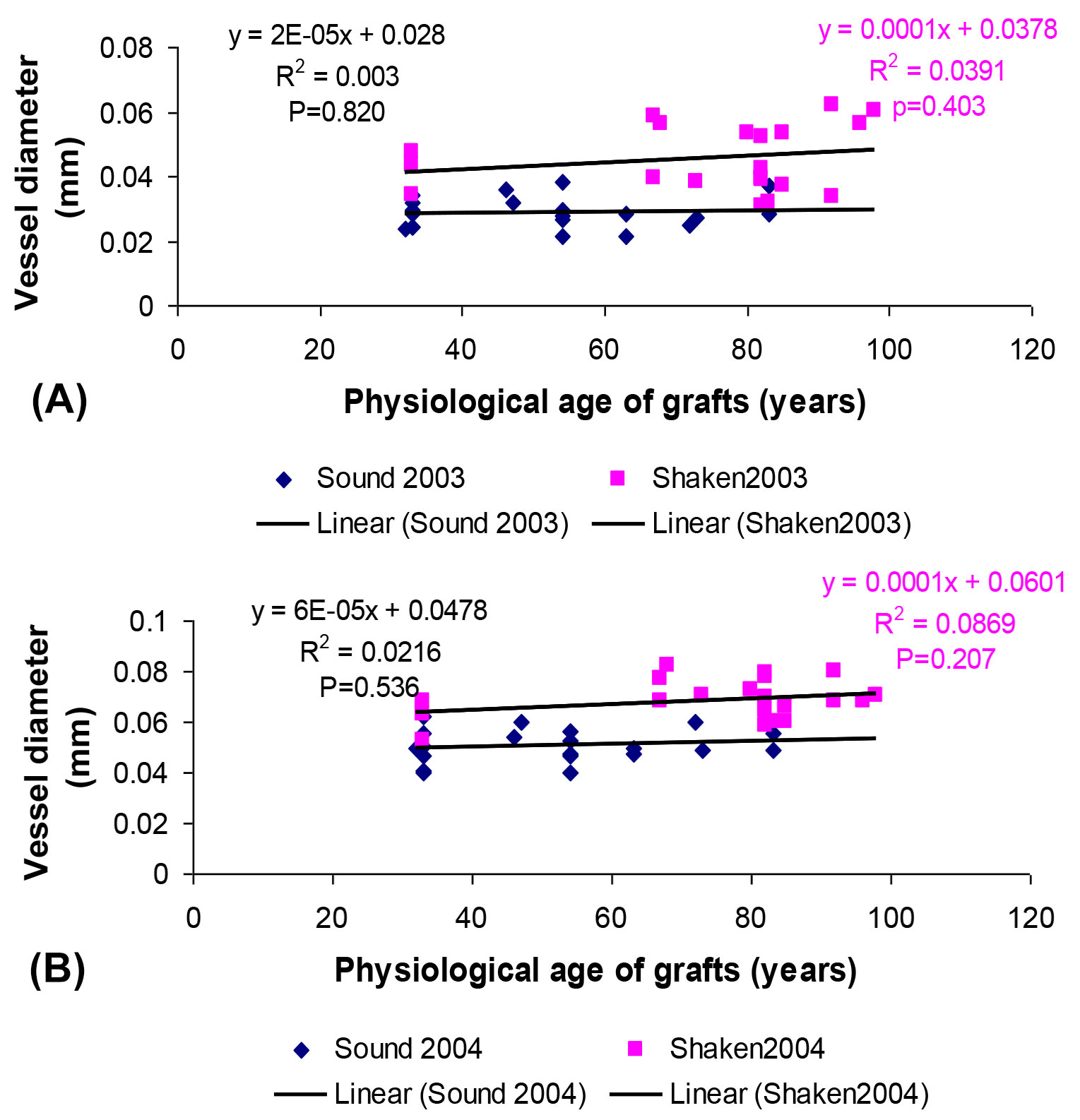
As the incidence of shake tended to be greater in older trees ([20]), it was important to test whether responses to water and nutrients was influenced by the physiological age of grafts. The MANOVA analysis, however, showed only marginal effects on age on performance. Graft stem increment, leaf retention and most flowering and fruiting parameters were largely independent of original tree age. The observed differences between graft origins were therefore reliable for these parameters, but for vessel diameters formed in 2003 there was a tendency for stressed grafts from older trees to produce larger early vessels. When pooling water stress treatments, however, the regressions analysis showed vessel diameter to be independent of age, with shaken origins showing wider diameters (Fig. 4). Similarly, no relationship between vessel size and original tree age was found in 2004.
Fig. 4 - Relationship between vessel diameter and physiological age of grafts taken from sound and shaken trees, showing the consistently larger vessels in shaken grafts in (A) 2003 and (B) 2004, combining water stress treatments.
Phenology
In most respects the performance of grafts from sound trees was superior to that of shaken grafts, irrespective to the treatment. Grafts from sound trees flushed earlier, had marginally larger leaf areas, higher densities of stomata and retained their leaves for longer than those from shaken trees, generally agreeing with observations made on the original trees in the field in 2001 ([20]). Flushing scores taken in 2008 and 2014, four and 11 years respectively after the polythene tunnel experiment, showed that sound grafts still flushed earlier than shaken ones, implying that leaf phenology is under strong genetic control. Late flushing thus appears to be the most promising visual characteristic that foresters and timber merchants could use to distinguish sound from shaken trees.
Treatment
Significant growth resulted from nutrient additions, with larger average leaf areas and stem increments, but with no major differences between grafts taken from shaken or sound trees. Although the general trend conflicts with findings by Evans ([8]) of little fertiliser response in commercial chestnut coppices at Kings Wood, the soil medium in that case was relatively fertile loamy-clay plateau drift, unlike the growth medium used in this experiment. The contrast here is analogous to the superior growth of chestnut plantations on ex-agricultural, rather than forest soils described by Alvarez-Alvarez et al. ([1]).
Flowering and fruiting also differed between sound and shaken graft origins. Flowering frequency was greater in shaken-origin grafts, which also flowered earlier and with a significantly greater number of hermaphrodite filaments. Water stress reduced the total number of their fruits (most notably in the middle and smallest size ranges), while fruiting tended to increase with added fertiliser. However, grafts from sound trees produced fewer flowers under water stress and least when nutrient and water stress was applied. The opposite occurred in shaken grafts, with more flowering occurring and giving rise to medium-sized fruits when conditions were limiting. This suggests that shaken trees may be more tolerant of low fertility. In terms of visual cues, foresters may select against copious flowering in potentially shaken trees, but hermaphrodite flowers are probably too infrequent to be used as indicators.
The lack to response of the grafts to drought treatments suggests either innate tolerance, or that, by avoiding wilting, the lowest water regime was not severe enough. Water stress did reduce the number of stomata, presumably in response to drought, consistently with Salisbury & Ross ([26]) and Woodward ([31]) who showed that the density is controlled by endogenous and environmental factors. Water stress also appeared to reduce vessel size, in agreement with observations by Sass & Eckstein ([27]) and Corcuera et al. ([5]) on other tree species. While vessel sizes remained constant in the outside plot, the smaller diameter vessels observed under imposed drought treatments in the polythene tunnel indicated a plastic response to environmental conditions. However, wider diameter vessels always occurred in grafts from shaken trees, both inside and outside the controlled tunnel environment, contradicting the findings of Fonti et al. ([11]), who observed that in shake-prone stems of chestnut earlywood the vessels were neither more numerous nor wider.
Conclusion
Factors such as vessel diameter, flushing time, flowering type and flowering incidence appeared to be under strong genetic control in chestnut, of which late flushing is the most useful to foresters and timber merchants as a possible indicator of shake in standing trees. While there was evidence that the plant could control soil water stresses to an extent by reducing earlywood vessel sizes and leaf stomata densities, the differences in vessel diameter between shaken and sound tree origins remained, leaving the latter potentially more susceptible to cavitation during summer drought. Growth was enhanced by moderate levels of fertiliser on an infertile growth medium, with the grafts of shaken trees possibly less affected. The growth of both shaken and sound trees could potentially be improved either by fertiliser addition on poor soils, or by a more fertile situation.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Supplementary Material
Authors’ Info
Authors’ Affiliation
51 Hurst Road, Ashford, Kent TN24 9RS (UK)
40 Milton Road, East Harnham, Salisbury SP2 8AX (UK)
8 Long Row, Mersham, Ashford, Kent TN25 7HD (UK)
Corresponding author
Paper Info
Citation
Mutabaruka C, Cook HF, Buckley GP (2015). Effects of drought and nutrient deficiency on grafts originating from sound and shaken sweet chestnut trees (Castanea sativa Mill.). iForest 9: 109-114. - doi: 10.3832/ifor1572-008
Academic Editor
Tamir Klein
Paper history
Received: Jan 23, 2015
Accepted: Jun 03, 2015
First online: Jul 19, 2015
Publication Date: Feb 21, 2016
Publication Time: 1.53 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 51537
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 42904
Abstract Page Views: 3129
PDF Downloads: 4131
Citation/Reference Downloads: 22
XML Downloads: 1351
Web Metrics
Days since publication: 3880
Overall contacts: 51537
Avg. contacts per week: 92.98
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2016): 1
Average cites per year: 0.10
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Does management improve the state of chestnut (Castanea sativa L.) on Belasitsa Mountain, southwest Bulgaria?
vol. 8, pp. 860-865 (online: 27 April 2015)
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Research Articles
Evaluation of fast growing tree water use under different soil moisture regimes using wick lysimeters
vol. 6, pp. 190-200 (online: 08 May 2013)
Research Articles
Environmental niche and distribution of six deciduous tree species in the Spanish Atlantic region
vol. 8, pp. 214-221 (online: 28 August 2014)
Research Articles
The responses of soil microbial community and enzyme activities of Phoebe zhennan cultivated under different soil moisture conditions to phosphorus addition
vol. 11, pp. 751-756 (online: 15 November 2018)
Research Articles
The complexity of mycobiota associated with chestnut galls induced by Dryocosmus kuriphilus in Galicia (Northwestern Spain)
vol. 17, pp. 378-385 (online: 14 December 2024)
Research Articles
Investigations on yellowing of chestnut crowns in Trentino (Alps, Northern Italy)
vol. 13, pp. 466-472 (online: 07 October 2020)
Research Articles
Case study of a new method for the classification and analysis of Dryocosmus kuriphilus Yasumatsu damage to young chestnut sprouts
vol. 5, pp. 50-59 (online: 10 April 2012)
Research Articles
Growth dynamics and productivity of pure and mixed Castanea sativa Mill. and Pseudotsuga menziesii (Mirb.) Franco plantations in northern Portugal
vol. 7, pp. 92-102 (online: 18 December 2013)
Research Articles
Fungal community of necrotic and healthy galls in chestnut trees colonized by Dryocosmus kuriphilus (Hymenoptera, Cynipidae)
vol. 12, pp. 411-417 (online: 13 August 2019)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword