Comparison of commercial elm cultivars and promising unreleased Dutch clones for resistance to Ophiostoma novo-ulmi
iForest - Biogeosciences and Forestry, Volume 8, Issue 2, Pages 158-164 (2015)
doi: https://doi.org/10.3832/ifor1209-008
Published: Aug 07, 2014 - Copyright © 2015 SISEF
Research Articles
Collection/Special Issue: 3rd International Elm Conference, Florence (Italy - 2013)
The elms after 100 years of Dutch Elm disease
Guest Editors: A. Santini, L. Ghelardini, E. Collin, A. Solla, J. Brunet, M. Faccoli, A. Scala, S. De Vries, J. Buiteveld
Abstract
Elms, and especially Ulmus × hollandica have been dominant and very much appreciated trees in cities and rural landscape for centuries in the Netherlands. As a result of two Dutch Elm Disease (DED) epidemics in the 20th century these trees largely disappeared from the landscape. Despite the introduction of new cultivars with increased levels of DED-resistance, by the end of the 20th century the elm had disappeared from the top 20 list of trees produced by Dutch nurseries. New cultivars with increased resistance to DED are used to a limited extent only. Apparently the lasting problems with DED in old cultivars has led to a lack of confidence in the resistance of these latest released cultivars among urban foresters and landscape managers. This paper reports on a study that aims at restoring the position of the elm as a street tree in the Netherlands by providing information on resistance to O. novo-ulmi causing DED of the currently available cultivars. All elm cultivars currently on the Dutch market were compared in an inoculation test. In 2007 a field experiment of 18 cultivars, one species and 10 non-released clones from the Dutch elm breeding program was established. Two cultivars were used as reference clones: “Commelin” (relatively susceptible) and “Lobel” (relatively resistant). In 2008 and 2009 the elms were stem-inoculated with Ophiostoma novo-ulmi and disease development was assessed throughout the summer and the following year. Clear differences in resistance to O. novo-ulmi were found between the cultivars, with “Columella”, “Sapporo Autumn Gold”’ and “Rebella” being highly resistant and significantly different from “Lobel” and “Regal”, “Urban”, “Belgica”, “Den Haag” and the U. laevis seedlings being the most susceptible and comparable to “Commelin”. The non-released clones performed comparable to “Lobel’”or even better. The ranking of the cultivars based on their level of resistance to O. novo-ulmi in this field test corresponds well with experience in urban green practice. Our conclusion is that there is a wide range of cultivars available with a good to excellent level of resistance. The available cultivars have a broad genetic base due to different parentage and use of exotic germplasm in the crossings. This broad genetic background may contribute to the stability of resistance in case new forms of the disease appear. The non-released clones performed well compared to the released cultivars and give good opportunities to further broaden the current range of cultivars on the Dutch and European market.
Keywords
DED-resistance, Elm Cultivars, Ulmus, Inoculation Test, Ophiostoma novo-ulmi
Introduction
In the past centuries elms (Ulmus spp.) became a very important part of the landscape in large areas of the Netherlands. Elms have a combination of highly valued characteristics such as no specific requirements concerning soil type, the ability to resist (sea) winds, the very good recovery from mechanical damage, its adaptability to city conditions including its ability to endure de-icing salts and closed pavements and its much appreciated open crown. In fact, the elm is the ideal street and landscape tree in large parts of the Netherlands. This made the elm, and especially U. × hollandica cultivars, the dominant tree in the coastal areas and cities in the western part of the country at the beginning of the 20th century. The spread of Dutch elm disease (DED), caused by Ophiostoma ulmi (Buisman) and later Ophiostoma novo-ulmi (Brasier) during the 20th century however resulted in millions of trees being lost ([6]). Since 1928, elm breeding for resistance to DED has resulted in the release of several more or less resistant cultivars to the European market. Especially in the Netherlands - where the first cultivar (“C. Buisman”) was released in 1936 - and the USA, large and long-term selection programs have been performed resulting in a number of hybrid clones with moderate to very good resistance. Also, more recently, other European programs on elm breeding were set up, e.g., in Italy, Spain and France ([9]). Today a wide range of clones of different DED-resistance and different parentage is available on the market. However, the use of these new elm cultivars in the Netherlands is still limited. “Vegeta” and “Commelin” elms that were planted in very large numbers in the second part of the twentieth century because of their level of resistance to DED caused by O. ulmi were attacked by O. novo-ulmi. Apparently, the lasting problems with DED in these earlier released cultivars, such as the sanitation costs for removing and replacing diseased and dead trees, has led to a lack of confidence in the resistance of newly released cultivars among urban foresters and landscape managers. As a result and despite its glorious past, around the year 2000 the elm was not even listed in the top 20 of street trees produced by Dutch tree nurseries ([1]). However, the outcome of a survey among Dutch trees nurseries was that there is no other species that can replace the elm and it was concluded that confidence in the elm should be restored. None of the substitute tree species has the same set of characteristics that makes the elm so well-suited as a street tree; therefore the best replacement for the classical Dutch elm is still an elm, provided it is resistant to DED ([6]). This paper reports on a study aimed at restoring the position of the elm as a street tree in the Netherlands by providing information on the level of DED-resistance of the currently available elm cultivars. The objective was to compare in one inoculation test all cultivars released from the Dutch breeding program and from foreign breeding programs available in the Dutch tree nursery market. From the Dutch elm breeding program which was put on hold in 1992, six cultivars issued between 1963 and 1989 with moderate to very high resistance, were included ([4]). The USA breeding programs also have resulted in new cultivars ([9], [17]), with several of them being used in the Netherlands lately in landscape and urban forestry. At the start of the study in 2005 eight cultivars from the USA were available in the Netherlands, which were all included in the study, together with an unnamed clone being considered for release. These are believed to have a high resistance based on tests in the USA, but have never been tested under Dutch conditions or compared to such a large number of Dutch cultivars in one test. More recently, several new cultivars with good resistance were issued in Italy ([13], [15], [16]) and Spain ([8]). However, in 2005 these cultivars were not yet available on the Dutch market and subsequently not included. In 2002 and 2006, INRA (France) released the cultivars “Wanoux” and “Nanguen” ([11], [10]). Both originate from the Dutch breeding program, but were not released due to the fact that they were thought to be of less interest for the Netherlands based on results from previous adaptability trials. As a consequence they were not available in Dutch tree nurseries in 2005 and therefore not included in the test. Additionally, 10 unreleased selections from the Dutch breeding program were compared with the available cultivars in order to investigate whether these clones have an increased resistance compared to the currently available cultivars.
Material and Methods
Plant material and experimental design
The field test comprised of 18 cultivars, one species and 10 new unreleased selections from the Dutch breeding program (Alterra, formerly known as the Dorschkamp Institute). Cultivars included were all those with moderate to high DED-resistance and available in 2005 in the Dutch tree nursery market. These included nine hybrid cultivars developed in the USA, but not yet tested under Dutch conditions and six hybrid cultivars from the Dutch breeding program. Additionally, the historical elm cultivar “Den Haag” was included, as it is highly appreciated by some city foresters in the Netherlands because of good field performance, and U. laevis as it is rather popular in the Netherlands. It has some form of field resistance due to its unattractiveness to the beetle, which acts as a vector for the fungus. For reasons of comparison, the old historical non-resistant cultivar “Belgica” was also added. Finally, 10 new and not yet released selections, mainly complex hybrids from the Dutch breeding program ([4]) and currently tested in adaptability tests by Alterra were included in the trial. The two Dutch hybrid clones “Lobel” and “Commelin”, with known DED response from earlier inoculations tests performed by Heybroek ([4]) were added as reference clones for respectively good and low disease resistance. The selected cultivars and clones and their parentage are shown in Tab. 1. The plants were propagated by soft cuttings (USA cultivars, as these cultivars are always sold as own-rooted plants) or graftings on an U. glabra rootstock (all other cultivars and the new Alterra selections) in 2006. For the species U. laevis seedlings were used. In April 2007 these plants were planted in a field trial at a tree nursery in Ommeren, the Netherlands (51° 56’ N, 5° 29’ E) on a clay soil (river deposit) at a spacing of 1 m within and 1.35 m between rows. The plants were planted in a randomized block design with nine blocks and four ramets of each clone per block. After planting the plants were watered several times, nevertheless the very dry weather conditions in the spring of 2007 resulted in a substantial loss of plants in the first season. These plants were replaced by spare plants from another part of the field in April 2008. The soil was kept free from weeds by using a herbicide until the canopy closed. No regular pruning was done, only some low branches were removed to enable better access to the field and when needed to maintain stability of the trees. Finally, at the start of the experiment 976 plants in total were available for inoculation.
Tab. 1 - Cultivars and clones included in the trial and their parentage. (*): According to Heybroek et al. ([5]) and references herein: 5 = very good; 4 = good, 3 = moderate, 2 = low, 1 = minimal, n.a. = not available. (**): Good field resistance due to unattractiveness to the DED vector (Scolytus).
| Clone/ Species |
Origin | Provenance or parentage | Year released |
Resistance to DED* |
|---|---|---|---|---|
| Commelin | NL | U. x hollandica “Vegeta” x U. minor | 1960 | 2 |
| Groeneveld | NL | U. glabra (no. 49) x U. minor no. 1 | 1963 | 3 |
| Lobel | NL | (U. wallichiana x U. glabra “Exoniensis”) x “Bea Schwartz” selfed) | 1973 | 4 |
| Dodoens | NL | (U. glabra “Exoniensis” x U. wallichiana) selfed | 1973 | 4 |
| Plantijn | NL | (U. glabra “Exoniensis” x U. wallichiana) x U. minor | 1973 | 4 |
| Clusius | NL | (U. glabra “Exoniensis” x U. wallichiana) x “Bea Schwartz” selfed | 1983 | 4 |
| Columella | NL | Plantijn selfed? | 1989 | 5 |
| 1028 | NL | ((U. wallichiana x U. minor) x (U. pumila x U. minor)) x Plantijn |
- | n.a. |
| 1043 | NL | U. davidiana var. japonica selection | - | n.a. |
| 1111 | NL | Plantijn x Dodoens | - | n.a. |
| 1236 | NL | Plantijn x Wredei | - | n.a. |
| 1241 | NL | Lobel x ((U. pumila x U. davidiana var. japonica) x U. pumila)) | - | n.a. |
| 1304 | NL | (U. glabra x U. minor) x Commelin | - | n.a. |
| 1312 | NL | ((U. pumila x U. minor) x Commelin)) OP | - | n.a. |
| 1315 | NL | ((U. wallichiana x Commelin) x (Dodoens x (U. glabra x U. minor)) | - | n.a. |
| 1322 | NL | U. minor x (U. pumila x U. minor) x U. minor | - | n.a. |
| 1324 | NL | (U. wallichiana x Commelin) x Dodoens x (U. glabra x U. minor) | - | n.a. |
| Belgica | B | U. x hollandica | - | 1 |
| Den Haag | NL | U. pumila x “Belgica” | 1936 | 3 |
| Cathedral | US | U. pumila x U. davidiana var. japonica | 1994 | 4-5 |
| New Horizon | US | U. davidiana var. japonica x U. pumila | 1995 | 5 |
| Regal | US | Commelin x (U. pumila x U. minor Hoersholmiensis) | 1983 | 4 |
| Rebona | US | U. davidiana var. japonica x U. pumila | 1994 | 5 |
| Rebella | US | U. america x U. parvifolia | 2011 | n.a. |
| Sapporo Autumn Gold | US | U. pumila x U. davidiana var. japonica | 1975 | 4 |
| Homestead | US | U. pumila x ((Commelin) x (U. pumila x “Hoersholmiensis”)) | 1984 | 4-5 |
| Pioneer | US | U. x hollandica selection | 1984 | 4 |
| Urban | US | (U. x hollandica “Vegeta” x U. minor) x U. pumila | 1976 | 3 |
| U. laevis | NL | The Netherlands | - | ** |
Inoculations
Four independent inoculations with Ophiostoma novo-ulmi were performed on different replicates of the same plant material, thus each tree was inoculated once. In both 2008 and 2009 two inoculations were carried out, the first in June and the second in July, coinciding with the period in which elms in the Netherlands are at their greatest susceptibility to the fungus. For each inoculation, 9 plants per clone were used (one in each block). Inoculations were performed with an aggressive well-known tester isolate H328 belonging to the subspecies O. novo-ulmi ssp. novo-ulmi, formerly known as the EAN race (kindly provided by C. M. Brasier, Forestry Commission, UK). The conidial suspension was prepared according to the method by Tchernoff ([21]), modified according to Sutherland et al. ([19]). The inoculum consisted of a conidial suspension of O. novo-ulmi (106 spores ml-1). Inoculations were performed using the Dutch inoculation method ([4]), by forcing a knife with a drop of 0.12 ml inoculum on it into the vascular xylem of the lower stem at 30-50 cm height. This severe method ensures that even clones with reasonable resistance may develop some disease symptoms and that maximum differentiation in resistance level between clones can be obtained. Inoculations were performed in the morning only on dry and sunny days to be sure that the inoculum was absorbed by the trees very easily. Knowing that symptoms are influenced by the vigor of the clone and ramet ([14]), care was taken to inoculate well established and vigorously growing plants only. Trees with low vigor (shorter < 1 m) or incomplete bud burst were excluded from inoculation and dropped from the experiment. Also replacement trees, planted in 2008, were not inoculated in 2008 but left for inoculation in 2009. The average stem diameter of the clones measured at 1m height in April 2008 prior to inoculation varied from 4.5 cm to 17.5 cm. Eventually, 116 of the 976 plants available in Spring 2008 were not inoculated, because they died during the experiment prior to inoculation or showed low vigor (shorter < 1m). During the first inoculation, a number of spare plants at another part of the field were inoculated with water.
Assessments
Typical symptoms for Dutch elm disease are wilting, yellowing of the leaves, leaf fall, formation of “shepherd’s crooks” and dieback of twigs. To evaluate symptom development in the trees, the percentage of defoliation 4 and 8 weeks after inoculation, disease index 4 and 8 weeks after inoculation, and recovery ability of trees one year after inoculation were assessed. The disease index was based on a scale with 9 infection classes, expressing the extent of wilting and twig dieback in the tree. Classes were defined as follows: 0 = no symptoms; 0.5 = start of leaf necrosis; 1 = yellowing and/or wilting of leaves, but no dieback of twigs; 1.5 = severe leaf fall and/or dieback of the tips of the twigs or inner twigs of the crown; 2 = partly or completely dieback of young top twigs with or without formation of “shephard’s crooks”; 2.5 = dieback to the base of this year’s shoots and/or massive occurrence of diseased twigs; 3 = dieback of 1-year old shoots as well as partly two-year-old shoots (up to halfway); 3.5 = dieback of 1-year old shoots as well as older shoots; 4 = severe dieback (more than half of the tree is dead). During the next growing season following inoculation of trees, the ability to recover was assessed based on the percentage of defoliation of the restored foliage and proportion of crown mortality. Mortality in the crown was assessed using 5 classes: 0 = healthy, no crown dieback; 1 = dieback in < 25 % of the crown; 2 = 25-50%; 3 = 51-75%; and 4 ≥ 75 % of the crown shows dieback.
As symptoms in the more susceptible clones usually increase with time, symptoms were also recorded 12 weeks after the first inoculation. In the following three inoculations, defoliation and disease index were only assessed 4 and 8 weeks after inoculations.
Data analysis
The disease index, defoliation (%, rounded to nearest decimal) and crown mortality observations are ordinal variables and to test differences between clones would normally be analyzed with a Chi square test or generalized (log) linear or ordinal-logistic model (GLM). However, the counts in some combinations (e.g., clone x disease index class) were very low or even zero, which hamper such analysis. Additionally, our interest lies in the ranking and grouping of the clones based on their level of resistance. Therefore, in order to analyze the ranking and grouping of the clones according to their performance for defoliation, disease index and crown mortality a Monte-Carlo randomization test was used ([7]). A χ2 statistic was calculated (χ2observed) on the basis of observed data in a two-way table, obtaining an estimated probability for each class. Using the clone totals for each and the calculated probabilities, simulated frequencies were calculated. For each test this was done 10000 times to get an simulated distribution of χ2. If the χ2observed was in the upper or lower 2.5% tail then significance was inferred (p < 0.05, two-tailed). In order to rank the clones they were classified according to their average score for disease index, defoliation or crown mortality. To make groupings, inclusion or exclusion of only adjacent individuals were considered. Using the above tests, maximum sized groups of clones containing no significant differences between the clones within that group were obtained. All symptom assessments were performed by two independent observers. For the statistical analysis only the maximum (index, percentage) value was used (bias to classify clones to be more susceptible). Initially, the analysis was carried out separately for each inoculation. However, in the third inoculation (performed in June 2009), the two control cultivars “Lobel” and “Commelin” with known levels of resistance which were included in the trial to investigate whether the four inoculations were successful, showed substantially less symptoms than expected. Subsequently, it was concluded that the third inoculation was not successful and the analysis was performed excluding this inoculation (a total of 668 plants). Additionally, Spearman’s rank correlation coefficient was calculated for the different parameters considered (percentage of defoliation, disease index, crown dieback and diameter of the plants).
Results
Methodological aspects
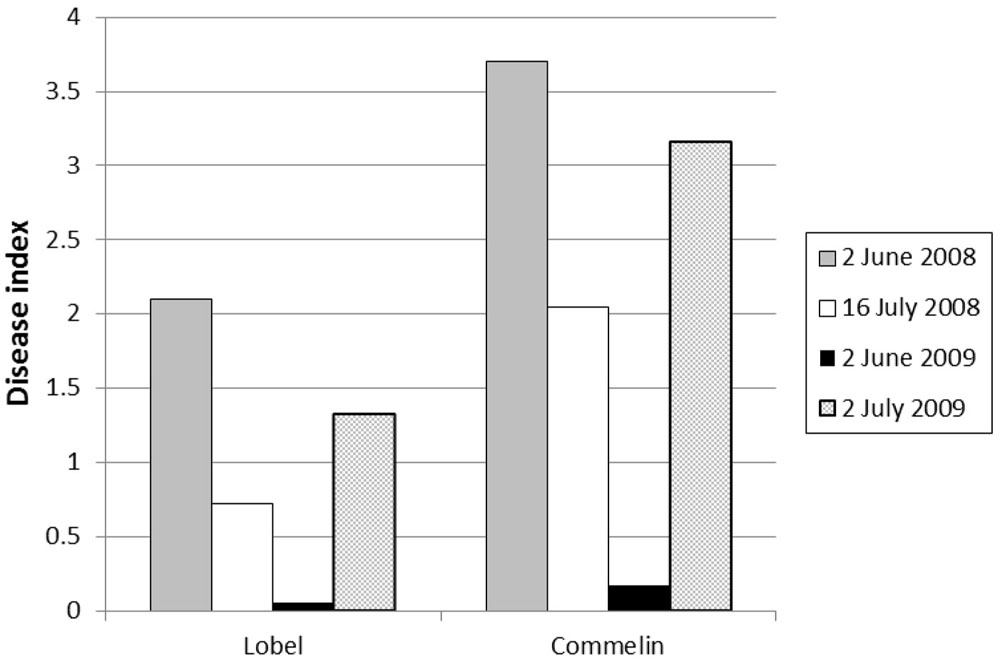
Symptoms developed only in inoculated trees, while none of the untreated control trees (inoculated with water) showed disease symptoms. One of the four independent inoculations performed (2 June 2009) was not successful, resulting in substantially less symptom development in both “Lobel” and “Commelin” reference cultivars as compared to the other tree inoculations (Fig. 1). Furthermore, their symptom development was less than expected based on results from previous inoculation tests performed in the Dutch breeding program (disease index range over the past 5 inoculation tests: “Lobel” d.i. = 1.3-3.0 and “Commelin” d.i. = 2.2-3.3, unpublished results). The limited symptom development after the third inoculation may be likely due to the conidial suspension used, whose concentration was considerably lower than in the other three inoculations (8·104 con. ml-1 instead of 1·106 con. ml-1). Because of the limited symptom development, data of this third inoculation were discarded from further analyses.
Fig. 1 - Differences in symptom expression (disease index after 8 weeks) between the four inoculation dates for the two control clones “Lobel” and “Commelin”.
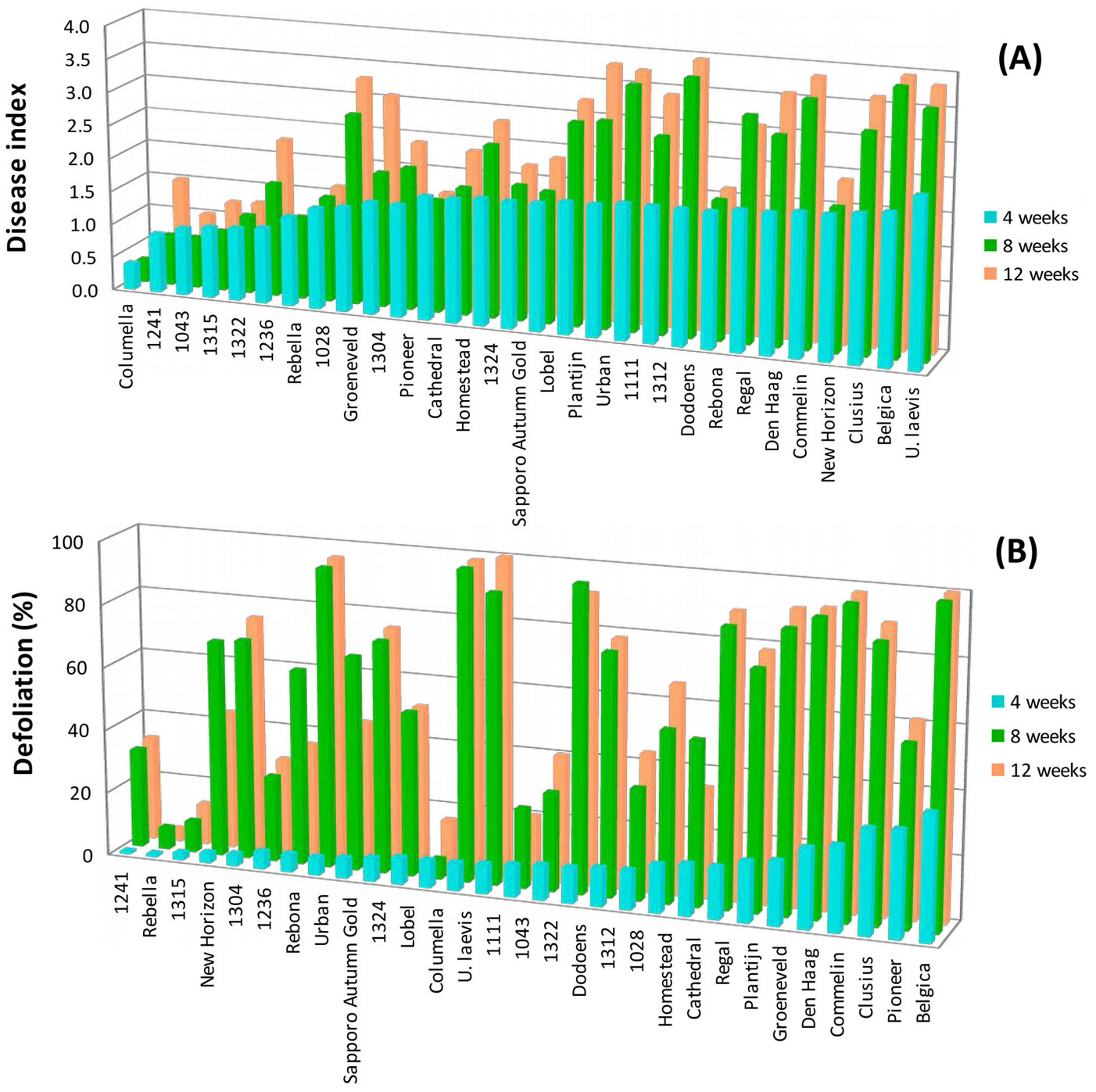
When comparing the three dates of symptom assessment, first symptoms were visible in most clones 4 weeks after inoculations, which further developed in some of these clones. Differences in symptom expression between the clones were very clear 8 weeks after inoculations (Fig. 2a, Fig. 2b). Since symptoms were rather constant 8 weeks after inoculation and the assessment date corresponded with evaluation dates reported by other studies ([14], [12], [18]), it was decided to assess symptoms after 4 and 8 weeks in the subsequent inoculations.
Fig. 2 - Disease index (A) and defoliation (B) assessed 4, 8 and 12 weeks after inoculation (2 June 2008).
Differences in DED symptom expression between clones
Defoliation and disease index
Clear differences in the percentages of defoliation were already visible among clones 4 weeks after inoculation (Tab. 2). “Columella”, “Sapporo Autumn Gold” and most of the Dutch unreleased clones showed less than 10% leaf fall, while the susceptible old cultivar “Belgica” already showed more than 50% leaf fall. Such differences became more pronounced after 8 weeks (Tab. 2). “Columella” and the Alterra clone 1315 still showed hardly any leaf fall, while leaf fall in the susceptible clones, such as “Belgica”, “Commelin” and the U. laevis seedlings, became very severe with percentages up to 90. Changes in ranking after 8 weeks were limited, except for “Rebella” that did not show increased defoliation after 8 weeks, and moved 15 places upward in the ranking. Comparison of the disease index (combined rating of wilting and twig dieback) after 4 and 8 weeks (see Tab. S1 in Appendix 1) also showed that in most clones symptoms progressed with time. After 8 weeks pronounced differences among clones in disease index were visible, ranging from clones with almost no or very limited symptoms to clones with severe symptoms. The resistant cultivar “Columella” and several Alterra clones (“1241”, “1322”, “1315”, “1043”, “1304”, “1028”), which hardly showed any symptoms or only wilting and leaf necrosis after 4 weeks, still were hardly diseased after 8 weeks (disease index < 1). As expected, very susceptible clones such as “Belgica” and “Commelin” and the U. laevis seedlings showed severe symptoms after 8 weeks (disease index > 2.5). Again, differences in clone ranking after 4 and 8 weeks were limited, except for a few clones. “Rebella”, “New Horizon”, “Cathedral” and “Rebona” did not show any substantial increase in disease index score and rose in ranking after 8 weeks, while “Dodoens”, “1111” and “1312” descended in ranking.
Tab. 2 - Average percentage of defoliation of the clones tested 4 weeks, 8 weeks and one year after inoculation (average of 3 inoculations). Values with the same letter do not differ significantly (p < 0.05).
| Clone | # of plants | Defoliation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| after 4 weeks | after 8 weeks | after 1 year | ||||||||
| % | Sign. | Rank | % | Sign. | Rank | % | Sign. | Rank | ||
| 1241 | 8 | 0 | a | 1 | 17.5 | ab | 4 | 16.3 | abcd | 5 |
| 1315 | 26 | 2.7 | a | 2 | 5 | ab | 2 | 5 | a | 1 |
| 1304 | 27 | 4.4 | ab | 3 | 26.7 | ab | 9 | 24.8 | de | 13 |
| Columella | 26 | 5.8 | abc | 4 | 4.6 | a | 1 | 5.4 | ab | 2 |
| 1322 | 15 | 6 | abc | 5 | 20 | ab | 7 | 22.7 | bcd | 11 |
| 1043 | 3 | 6.7 | abc | 6 | 16.7 | ab | 3 | 13.3 | abcd | 4 |
| 1111 | 27 | 8.1 | abc | 7 | 41.1 | c | 14 | 44.4 | efgh | 17 |
| 1028 | 22 | 8.2 | abc | 8 | 19.5 | ab | 6 | 19.1 | abcd | 7 |
| Sapporo Autumn Gold | 22 | 8.6 | abc | 9 | 29.1 | ab | 10 | 18.2 | abcd | 6 |
| 1236 | 20 | 9 | abcd | 10 | 23.5 | ab | 8 | 19.5 | abcd | 8 |
| 1324 | 20 | 10.5 | abcd | 11 | 40 | bc | 13 | 37 | ef | 15 |
| Lobel | 24 | 10.8 | abcd | 12 | 32.9 | ab | 11 | 35.4 | def | 14 |
| New Horizon | 27 | 14.8 | abcd | 13 | 35.9 | b | 12 | 21.1 | abcd | 9 |
| Plantijn | 28 | 15.7 | abcd | 14 | 45.4 | defg | 19 | 51.1 | efghij | 21 |
| Homestead | 24 | 15.8 | abcde | 15 | 42.5 | d | 16 | 46.7 | efghi | 18 |
| Dodoens | 27 | 16.7 | abcde | 16 | 55.2 | fgh | 22 | 55.9 | efghij | 22 |
| Clusius | 24 | 18.3 | abcde | 17 | 46.7 | efgh | 21 | 48.3 | efghi | 19 |
| 1312 | 14 | 19.3 | abcde | 18 | 46.4 | defgh | 20 | 49.3 | efghi | 20 |
| Rebona | 24 | 20.8 | abcde | 19 | 44.6 | de | 17 | 21.3 | bcd | 10 |
| Rebella | 27 | 21.1 | bcde | 20 | 18.9 | ab | 5 | 5.6 | abc | 3 |
| Cathedral | 24 | 29.6 | cdef | 21 | 41.3 | d | 15 | 22.9 | cd | 12 |
| Pioneer | 26 | 30.8 | cdef | 22 | 44.6 | def | 18 | 43.1 | efg | 16 |
| Groeneveld | 27 | 31.1 | cdef | 23 | 61.9 | fghi | 23 | 60.7 | efghij | 23 |
| Regal | 24 | 32.9 | def | 24 | 72.9 | fghij | 24 | 72.5 | fghij | 25 |
| Urban | 27 | 33.3 | ef | 25 | 73.7 | ghij | 25 | 71.9 | fghij | 24 |
| U. laevis | 26 | 36.2 | ef | 26 | 90.8 | j | 29 | 90.8 | j | 29 |
| Commelin | 27 | 43.7 | fg | 27 | 84.4 | ij | 27 | 84.8 | hij | 27 |
| Den Haag | 26 | 49.6 | fg | 28 | 83.1 | hij | 26 | 82.3 | ghij | 26 |
| Belgica | 26 | 60.4 | g | 29 | 88.8 | j | 28 | 88.8 | ij | 28 |
Recovery
Assessment of defoliation in the next year (including branches with no leaves because of death of shoots) showed that most clones had a similar percentage of affected (defoliated and dead) shoots, as in the first year after 8 weeks. Most clones with clear symptoms in the season of inoculation were not able to form new foliage in the next season. Clones hardly showing any leaf fall in the year of inoculation, neither did show symptoms in the subsequent year. In a few American clones (“Rebella”, “New Horizon”, “Sapporo Autumn Gold”, “Rebona” and “Cathedral”) part of the affected branches recovered in the next growing season (Tab. 2). A similar picture may be drawn from the data for crown dieback after 1 year (Tab. S1 in Appendix 1). The same five cultivars were able to produce new shoots and showed flushing in at least 75% of the crown. However, complete recovery was not seen in these clones, as part of the affected branches died. Eight weeks after inoculations, Alterra clone “1241” expressed less symptoms than many other clones, but one year later its crown dieback did not significantly differ from many other clones. “Columella” showed significantly less crown dieback than many others (Tab. S1 in Appendix 1), and performs similar to “Sapporo Autumn Gold”, “Rebella”, “Rebona”, “Cathedral” and “New Horizon” and some of the Alterra clones (“1043”, “1315” and “1322”).
Correlations between different parameters
A strong Spearman’s rank correlation coefficient was found between the percentage of defoliation and disease index assessed after 8 weeks (rs = 0.975, p < 0.001), indicating that clones with a high score for percentage defoliation in general also had a high score for disease index. Disease index after 8 weeks and crown dieback in the next season were also significantly correlated (rs = 0.847, p < 0.001). Thus both parameters are good for ranking the level of resistance to O. novo-ulmi in individual clones. Also, a weak and positive (though not significant) relationship was found between the mean pre-inoculation diamater of clones (June 2008) and 8-weeks disease index and crown dieback after one year (rs = 0.32, p > 0.05 and rs = 0.18, p > 0.10, respectively).
Discussion
This inoculation test is one of the few studies that compared a large number of well-known cultivars for resistance to O. novo- ulmi in a common field experiment. Disease scores from different experiments are difficult to compare because the variation in disease symptoms may be caused by many factors, e.g., age of material tested, isolate of the fungus, inoculation method, tree growth and weather conditions ([9], [20]). By combining all available cultivars and clones in one field test many of these factors were excluded. Moreover, to further reduce the amount of variation even more clones were propagated under the same conditions as far as possible, material of the same age was used, and all plants were infected with the same isolate. Although variation in symptom development in field tests might still be large as compared to controlled conditions ([17]), it is assumed that the differences observed in this field test among the inoculated clones are related to their genetic background. Despite the limitations of the study, such as the use of only one isolate and the limited number of plants for some clones, our results clearly revealed large differences in the resistance level between individual cultivars and clones. The ranking of cultivars for their level of resistance to O. novo-ulmi confirms, with a few exceptions, earlier evidence from the literature and experience from plantings in the Netherlands, as summarized in Heybroek et al. ([5]). The few exceptions are “Regal”, “Den Haag” and the U. laevis seedlings. “Den Haag”’ and the U. laevis seedlings are known as tolerant elms in Dutch plantings in practice. In our study, U. laevis seedlings were not resistant to O. novo-ulmi; the relatively high field resistance of U. laevis is due to other factors such as their unattractiveness to the vector Scolytus beetle ([22]). “Regal” is known as a cultivar with very good resistance in the USA ([3]) or as good as “Lobel” in France ([10]). Nonetheless, “Regal” significantly underperformed “Lobel” in this study. On the other hand, the superiority of “Sapporo Autumn Gold” over the Dutch cultivars “Lobel”, “Plantijn”, “Clusius” and “Dodoens”, which are known as cultivars with good resistance, is in agreement with earlier tests ([2], [11]). Assessment based on 4- or 8-weeks disease symptoms compared to symptoms in the following year gives a slightly different ranking because different patterns of disease development are visible. A large group of cultivars and clones showed the same ranking over the whole period of assessment. Another group of cultivars, namely “Cathedral”, “New Horizon”, “Rebona”, “Rebella” and “Sapporo Autumn Gold”, from the University of Wisconsin (USA) breeding program clearly behaved differently. They initially react with relatively high disease index and high levels of defoliation (4-weeks after inoculation). However, these cultivars recovered well later on in the same growing season, showing flushing of dormant secondary buds and hardly exhibiting crown dieback in the following year. The most remarkable recovery was shown by “Rebella”. In urban plantings the degree of crown damage in the following year is the principal trait of interest for managers, thus the initial disease response in these five cultivars, especially “Rebella”, should be of less concern and they could be considered as highly resistant. As our main objective was to rank cultivars for their resistance to O. novo-ulmi instead of assessing their level of resistance in real situations, a severe method of inoculation was chosen. The advantage of the Dutch method (wherein a high-dose inoculum is directly brought into the xylem of the lower part of the trunk) is that it leads to strong symptoms, thereby giving conservative estimates for the level of resistance. This also means that, in practice, when these clones are infected in a natural way, they are likely to show even less symptoms than in this test. Consequently, this study has shown that in the present-day assortment there are many cultivars with a good or very good (i.e., better than the reference clone “Lobel”) level of resistance.
Resistance to DED is under control of many genes and can be accumulated through subsequent crossings and back crossings. This study suggests that the general level of resistance of released cultivars has increased over time. Comparison of the crown dieback index with the date of release of the cultivars shows a moderate negative correlation (rs = -0.71, p<0.005), suggesting that cultivars released lately have indeed a higher level of resistance. Whether this trend is due to the incorporation and/or accumulation of resistance genes from the Asian germplasm through the production of more complex hybrids, is difficult to say. For instance, the Dutch breeding program initially focused on crossing native European species (U. glabra and U. minor) and their hybrids (U. × hollandica), and the first releases were first-generation hybrids or backcrosses between these species (“Groeneveld” and “Commelin”). Lately, advanced generations of hybrids were produced including Asian germplasm. This next generation of released hybrids (namely “Clusius”, “Lobel”, “Dodoens” and “Plantijn”) all have an U. wallichiana grandparent (1/4). Also the latest cultivar of the Dutch breeding program, the highly resistant “Columella”, is an offspring from “Plantijn” and a next-generation hybrid with 50% U. minor, 25% U. glabra and 25% U. wallichiana parentage ([4]). Furthermore, “Urban”, “Regal” and “Homestead” cultivars are complex hybrids obtained by crossing U. glabra and U. minor in combination with U. pumila as a source of resistance. However, these hybrids have varying success for DED-resistance. As a consequence, it may be hypothesized that the improved resistance showed by the newly released cultivars could be due to the enhanced use of Asian germplasm in the recent decades. We see that the most recently released cultivars are mainly F1 hybrids between Asian species, namely the American cultivars “Rebona”, “Cathedral”, “New Horizon” and “Rebella”, which have been released since the nineties and have a comparable level of resistance as “Columella”. All are products of crossings between U. pumila and U. davidiana var. japonica or U. americana and U. parvifolia. Of the 18 elm cultivars tested in this study, eight have U. pumila parentage varying from 1/4 to 5/8. In these cultivars U. pumila is used as a source of resistance genes, although from different accessions or provenances. Our results revealed a substantial difference in resistance to O. novo-ulmi among the above cultivars.
Variation in DED-resistance is large within most Asian species ([17]). This study confirms that the use of U. pumila not necessarily lead to highly resistant cultivars, depending on the accession used as a source of resistance. Also the non-released Alterra selections included in this test are almost all complex hybrids obtained from crossings between native and Asian species (mainly U. wallichiana - Tab. 1). One out of this group (clone “1304”) has a parentage with only U. glabra and U. minor, demonstrating that it is possible to improve disease resistance by making complex hybrids of purely native European elms.
Conclusion
The Dutch and American cultivars tested in this study show a large variation in resistance to O. novo-ulmi. In general, their level of resistance corresponds well with the results from breeding trials, other inoculation tests and experiences from plantings in urban green. Furthermore, a considerable number of cultivars with good to excellent resistance to O. novo-ulmi are available from the Dutch nursery market. This provides good opportunities for the use of elms as street tree in the future. The widely varying genetic background of these cultivars is a positive aspect concerning the stability of the observed resistance against O. novo-ulmi in the case new forms of the disease appear. Additionally, several of the unnamed Alterra clones performed well as for resistance compared to the currently available cultivars in the tree nursery market. Such clones have been under evaluation in adaptation trials over the past two decades. Some of these clones have nice architectural characteristics and may be released in the future to further broaden the current range of cultivars for the Dutch and European market.
Acknowledgments
This study was funded by the Dutch Product Board of Horticulture. Plant material was kindly provided by tree nursery “Bonte Hoek Kwekerijen B.V.”. We thank Ronnie Nijboer for arranging this. Thanks are also due to Bram Versprille (Stichting Vermeerderingstuinen Nederland) for providing part of the propagation material and to Gerrit Schalk for assistance in the propagation of some cultivars. We thank Toon Helmink and Jan van Leijden for their technical assistance in performing the inoculation trial and Jacco Alblas for technical assistance in maintaining the experiment. Finally, we want to thank Sven de Vries for his comments on an earlier version of the manuscript.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Bert Van Der Werf
Alterra, Wageningen UR, P.O. box 47, 6700AA Wageningen (The Netherlands)
Applied Plant Research, Wageningen UR, P.O. Box 85, 2160 AB Lisse (The Netherlands)
Corresponding author
Paper Info
Citation
Buiteveld J, Van Der Werf B, Hiemstra JA (2015). Comparison of commercial elm cultivars and promising unreleased Dutch clones for resistance to Ophiostoma novo-ulmi. iForest 8: 158-164. - doi: 10.3832/ifor1209-008
Academic Editor
Alberto Santini
Paper history
Received: Dec 20, 2013
Accepted: May 18, 2014
First online: Aug 07, 2014
Publication Date: Apr 01, 2015
Publication Time: 2.70 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 54343
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 44727
Abstract Page Views: 3544
PDF Downloads: 4667
Citation/Reference Downloads: 18
XML Downloads: 1387
Web Metrics
Days since publication: 4151
Overall contacts: 54343
Avg. contacts per week: 91.64
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 21
Average cites per year: 1.91
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Seven Ulmus minor clones tolerant to Ophiostoma novo-ulmi registered as forest reproductive material in Spain
vol. 8, pp. 172-180 (online: 13 August 2014)
Research Articles
Genetic variation and heritability estimates of Ulmus minor and Ulmus pumila hybrids for budburst, growth and tolerance to Ophiostoma novo-ulmi
vol. 8, pp. 422-430 (online: 15 December 2014)
Research Articles
Monitoring of the incidence of Dutch Elm Disease and mortality in experimental plantations of French Ulmus minor clones
vol. 15, pp. 289-298 (online: 29 July 2022)
Review Papers
Genomics of the Dutch elm disease pathosystem: are we there yet?
vol. 8, pp. 149-157 (online: 07 August 2014)
Research Articles
Outcome of Ceratocystis platani inoculations in Platanus × acerifolia in relation to season and inoculum dose
vol. 9, pp. 608-617 (online: 17 March 2016)
Review Papers
Dutch elm disease and elm bark beetles: a century of association
vol. 8, pp. 126-134 (online: 07 August 2014)
Review Papers
Avoidance by early flushing: a new perspective on Dutch elm disease research
vol. 2, pp. 143-153 (online: 30 July 2009)
Research Articles
Effects of substrate and ectomycorrhizal inoculation on the development of two-years-old container-grown Norway spruce (Picea abies Karst.) seedlings
vol. 8, pp. 487-496 (online: 10 November 2014)
Review Papers
Ulmus laevis in the Iberian Peninsula: a review of its ecology and conservation
vol. 8, pp. 135-142 (online: 07 August 2014)
Research Articles
Variations in the performance of hybrid poplars subjected to the inoculation of a microbial consortium and water restriction
vol. 16, pp. 352-360 (online: 13 December 2023)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword