Day and night respiration of three tree species in a temperate forest of northeastern China
iForest - Biogeosciences and Forestry, Volume 8, Issue 1, Pages 25-32 (2015)
doi: https://doi.org/10.3832/ifor0982-007
Published: May 26, 2014 - Copyright © 2015 SISEF
Research Articles
Abstract
Leaf day respiration is one of the most fundamental plant physiological processes and plays a vital role in the plant carbon cycle. However, day respiration is inherently complex and difficult to measure. In this study, the Kok method and the Laisk method were used to measure leaf day respiration for the saplings of one evergreen conifer species (Pinus koraiensis) and two deciduous broadleaved species (Tilia amurensis and Fraxinus mandshurica) in a temperate forest. Results show that discrepancy between the corrected day respiration values estimated by the Kok and Laisk methods was only 4% for the three tree species. On average, day respiration was 55.9% and 52.6% lower compared to night respiration for the three tree species, as measured by the Kok and Laisk method, respectively. Day respiration of the evergreen conifer species estimated by the Kok method was 31.7% lower while that estimated by the Laisk method was 36.8% lower than that of the deciduous broadleaved species. Night respiration of the evergreen conifer trees was 40.7% lower than those of the deciduous broadleaved trees. Day respiration rate is positively correlated with night respiration rate. Notably, day respiration rate decreased with increased photosynthetic photon flux density, and even a small amount of light significantly inhibited leaf day respiration in all three species.
Keywords
Dark Respiration, Deciduous Broadleaved Tree, Evergreen Conifer Tree, Gross Primary Production, Light Inhibition, Temperate Forest
Introduction
Dark respiration (non-photorespiratory respiration), which occurs both in light and in darkness, has a critical function in modulating the carbon balance of plants and terrestrial ecosystems. Ryan ([41]) showed that the proportion of carbon consumption by respiration is nearly 70% of the total photosynthetic carbon fixation, and leaf respiration accounts for approximately 50% of total plant respiration ([35]). At the ecosystem level, CO2 emission from plant respiration accounts for 30-70% of the total ecosystem CO2 exchange ([2]). Plant respiration releases 60 Gt C yr-1 into the atmosphere ([42]). Therefore, understanding the carbon cycle of terrestrial ecosystems requires a more thorough understanding of the characteristics of leaf respiration.
Due to difficulty in directly measuring leaf day respiration (RL), it is often assumed to be equal to night respiration ([34], [36], [10], [4]). However, Kok ([22]) found that net CO2 assimilation rate (An) rapidly decreased near the light compensation point, leading to the conclusion that leaf respiration may vary with light intensity. Indeed, McCashin et al. ([28]) found that 14CO2 production during tricarboxylic acid cycle is approximately 20% lower in light than in darkness, which indicates that day respiration is inhibited in light. Subsequent studies confirmed that day respiration rates are lower than night respiration rates. Using stable 12C/13C isotope techniques, Tcherkez et al. ([46]) found that two main processes (glycolysis and the Krebs cycle) of day respiration are strongly inhibited in illuminated leaves of French bean. Thus, plant respiration in light is overestimated if RL is assessed using data obtained during nighttime ([15], [44], [21], [23], [5], [17], [6]).
Plant respiration is a critical component of the gross primary production (GPP). Therefore, it is important to take into account the inhibitory effect of light on leaf respiration in order to obtain sound estimates of GPP. Wohlfahrt et al. ([56]) estimated a reduction of 11-17% in GPP due to light inhibition in a mountain meadow, as compared to GPP model based on night respiration. Janssens et al. ([20]) proposed that assessing day respiration by extrapolating night respiration results in an overestimation of ~15% of the total ecosystem respiration. Bruhn et al. ([8]) recently reported that GPP estimation after taking into account light inhibition on leaf respiration for the average day is 76% of the GPP estimation taken without consideration of reduction in leaf respiration due to illumination. Thus, accurate estimates of the GPP requires the inhibition of leaf dark respiration by light to be considered.
The Kok and Laisk methods are the two main methods used to estimate leaf day respiration. Kok ([22]) analyzed the response of net photosynthesis rate to low light intensities and showed that the slope abruptly increases when the light intensity declines to the light compensation point - a phenomenon now known as the “Kok effect”. Extrapolation of the linear section of the light response curve before the slope change to a light intensity of zero gives an estimate of RL. This technique has been widely used to analyze leaf respiration ([44], [51], [8]). Laisk ([24]) proposed a different RL estimation method which was later extended by Brooks & Farquhar ([7]). Such method uses the response of An to a series of low intercellular CO2 concentrations (Ci) with two or more varying light intensities. Then, the CO2 compensation point (at which the CO2 released from photorespiration equals the CO2 uptake by photosynthesis) and RL are estimated by calculating the intersection points of the An-Ci curves under different light intensities.
Based on the aforementioned methods, a number of experiments have been performed to study the degree of respiration inhibition by light in different plant species. Villar et al. ([51], [52]) found that the mean inhibition of RL is 55% for two woody species, and that the light inhibition of respiration is greater in mature leaves (81%) than in young leaves (36%). Several studies further explored the variation in RL associated with physiological factors, including photosynthetic rate, specific leaf area, leaf age and relative growth rate ([37], [52], [5], [39]), as well as environmental factors, namely, CO2 concentration, temperature, and drought ([9], [29], [31], [53], [55], [48], [12], [58]). However, our understanding of the inhibition of plant day respiration is still limited compared with that of night respiration, and the degree of light inhibition and the many variables that may influence it are not clear. This is particularly true for trees of temperate forests, in spite of their fundamental contribution to the global carbon budget.
In this study, the Kok and Laisk methods were used to estimate the leaf day respiration for three dominant tree species (Pinus koraiensis, Tilia amurensis and Fraxinus mandshurica) in the Changbai mountain forest, which is a typical temperate natural forest in northeastern Asia. The major aim was to explore leaf respiration and the light inhibition degree of day respiration of evergreen conifer and deciduous broadleaved species in a temperate forest. Our findings may contribute to a better understanding of the biological processes of plant photosynthesis and respiration for the selected species, as well as to facilitate the accuracy of GPP estimates in temperate forests.
Materials and Methods
Experimental field and plant materials
The experiments were performed in the Changbai Mountain region of the Jilin province (northeastern China - 42° 24′ N, 128° 06′ E; elevation: 738 m a.s.l.). This region has a humid subalpine climate, and the growing season is from May to September. The average annual temperature is 3.6 °C and the total precipitation 695 mm. The soil is classified as dark brown forest soil.
Five-year-old saplings of three main tree species (P. koraiensis, T. amurensis, and F. mandshurica) were used in the experiment. The mean height of the potted saplings was 1.5 m, and its averaged basal diameter was about 2 cm. Data were collected from leaves of two deciduous broadleaved species (T. amurensis, and F. mandshurica) and two-years-old leaves of an evergreen conifer species (P. koraiensis).
Measurements and data processing
A portable photosynthesis system (LI-6400, LI-COR, Lincoln, NE, USA) was used to measure the net photosynthetic rate (An) of foliage under different photosynthetic photon flux density (PPFD) values and CO2 concentration levels. A 2×3 cm cuvette was used in the gas-exchange measurements, with the flow rate set to 500 μmol s-1. During the measurements, leaf temperature and relative humidity were controlled at approx. 25 °C and 60%, respectively. Measurements were taken from 08:00 to 12:00 on sunny days from July to September. For each treatment condition, three to four fully expanded and healthy representative leaves were randomly selected on saplings and considered as replicates. The sampled leaves were treated for each series gradient of PPFD values or CO2 concentrations under controlled optimal conditions using the portable photosynthesis system.
As for the Kok method, the sampled leaves were exposed to a range of PPFD values (150, 120, 90, 60, 50, 40, 30, 20, 10, 5, and 0 μmol m-2 s-1) at a normal ambient CO2 concentration (approximately 380 μmol mol-1). As for the Laisk method, each selected leaf was exposed to a series of CO2 concentrations (150, 120, 90, 70, 60, 50, and 40 μmol mol-1), and PPFD set at 50, 100, and 150 μmol m-2 s-1, respectively.
To compare RL and night respiration (RD), RD measurements were conducted after 20 min of dark acclimation at CO2 concentration of 45 and 380 μmol mol-1 in order to determine differences in respiration under low and ambient CO2 condition. To determine the effect of light intensity on day respiration, seven PPFD levels (50, 100, 150, 210, 300, 600, and 800 μmol m-2 s-1) and CO2 concentration series (150, 120, 90, 70, 60, 50, and 40 μmol mol-1) were set for leaves of P. koraiensis in August and September. An was measured under different CO2 concentrations for each PPFD level. For each level of PPFD the linear regression of An over Ci was performed. RL was estimated to be the value of the intercepted point of two regression lines established at each two ambient PPFD values. The measured data were then used to obtain RL values by the Laisk method, and each RL value corresponded to the averaged value of the two PPFD values. Additionally, determination for leaf nitrogen content was based on the Kjeldahl nitrogen method ([13]).
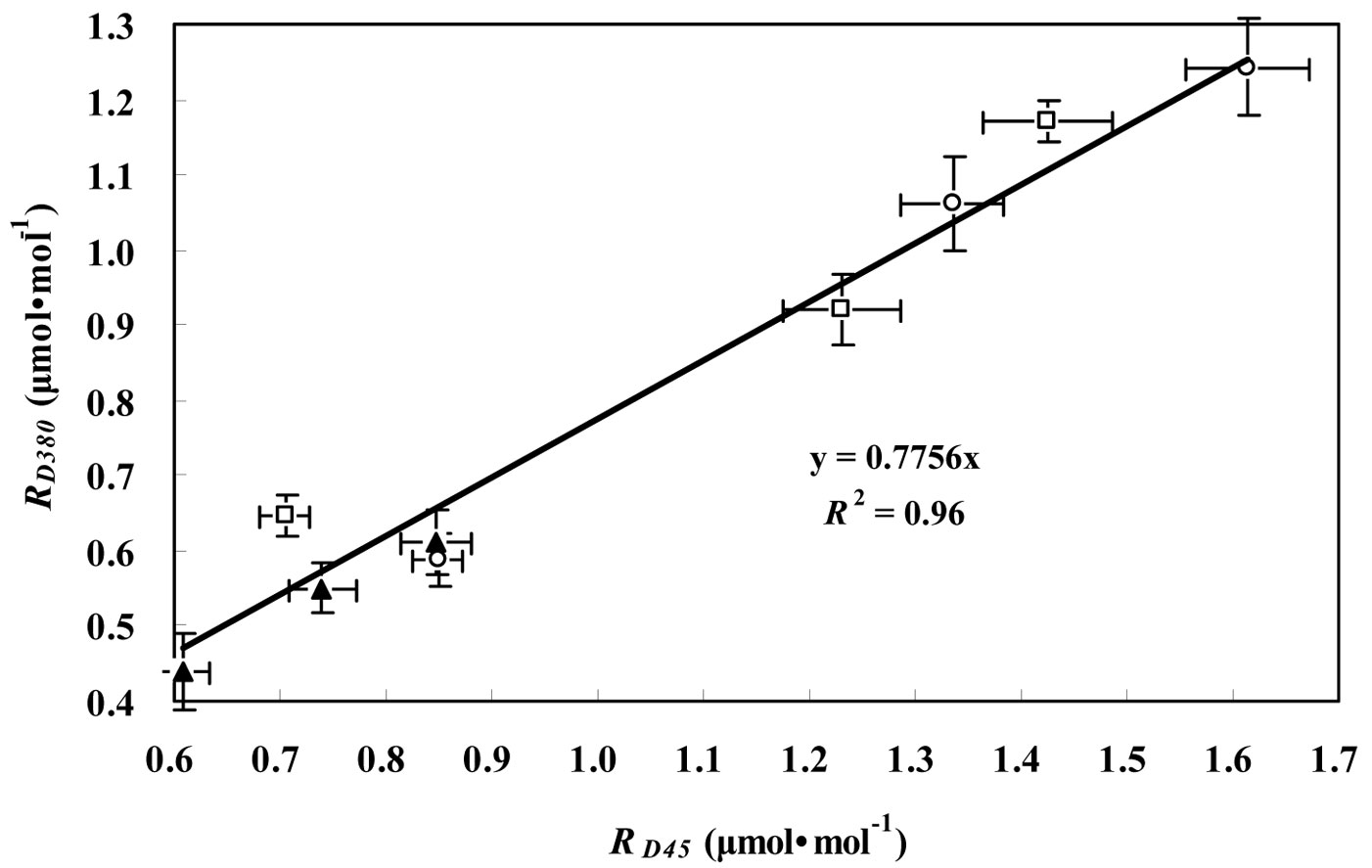
Fig. 1 - Relationship between RD45 and RD380 for the three tree species considered. RD45 and RD380 represent the leaf respiration in darkness measured at CO2 concentrations of 45 μmol mol-1 and 380 μmol mol-1, respectively, for P. koraiensis (black triangles), T. amurensis (white squares) and F. mandshurica (white circles). Data are presented as mean ± SD.
In the Kok method, the curve departed from a straight line when PPFD decreased to light compensation point. RL was obtained by extrapolating the line to zero while ignoring the points below the light compensation point. Day respiration estimated by the Kok method was corrected based on Kirschbaum & Farquhar ([21]). In the Laisk method, linear regressions of the An-Ci curves were obtained at three light levels, and the leaf dark respiration was estimated by the value of the point of intersection of those lines in the An coordinate. However, the Laisk method required the measurement to be carried out at very low CO2 concentration. To determine the effect of low CO2 concentrations on leaf respiration, the CO2 concentration was controlled at 45 or 380 μmol mol-1 during RD measurements for the three tree species. Fig. 1 shows the positive relationship between RD45 and RD380. The rates of RD at low CO2 concentration were always higher than the corresponding values at high CO2 concentration. Their relationship was expressed by a linear equation following the suggestion of Villar et al. ([51], [52] - eqn. 1):
(R2 = 0.96, P < 0.01) where RD380 and RD45 represent RD in the ambient CO2 concentration of 380 and 45 μmol mol-1, respectively. RD380 was about 23% lower than RD45 (P < 0.05). Therefore, the respiration rate measured at low CO2 concentration was generally overestimated. A correction for leakage was necessary because it is hard to avoid CO2 exchange or leakage between the infrared gas analyzer (IRGA) chamber and the surrounding air ([33], [43], [14], [40]). We corrected for CO2 leakage by diffusion in the Laisk method based on the following equation (eqn. 2) which was derived from the instruction manual of Li-Cor Inc ([26]):
where K is the CO2 diffusion flow rate (μmol s-1), and ui is the flow rate into the leaf chamber (μmol s-1). CS, CR and CA indicates sample, reference and ambient CO2 concentration (μmol mol-1), respectively.
Statistical analysis was performed using SPSS® version 17.0 (SPSS, Chicago, IL, USA). Differences among the three species analyzed were tested by one-way ANOVA (Duncan test) for the parameter of RL estimated independently by the Kok and the Laisk methods, RD, and degree of light inhibition of RL. Student’s t-test was used to evaluate the differences between RL estimated by the two above methods used, as well as the differences between RL and RD for the three tree species. Relationships were fitted with linear or polynomial functions which provided a simple and good description of the phenomenon. All the tests were based on a significance level of 0.05.
Results
Leaf dark respiration
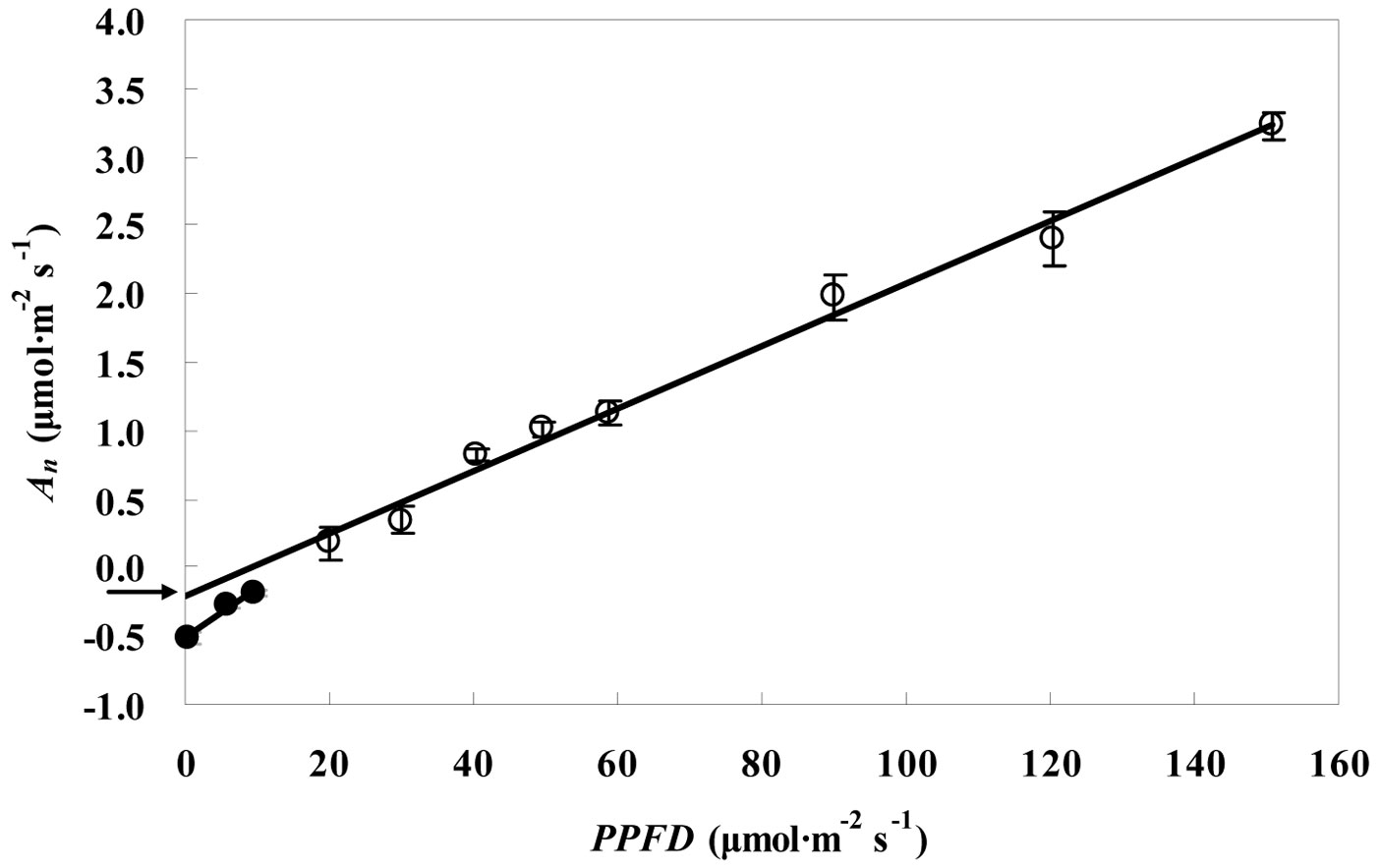
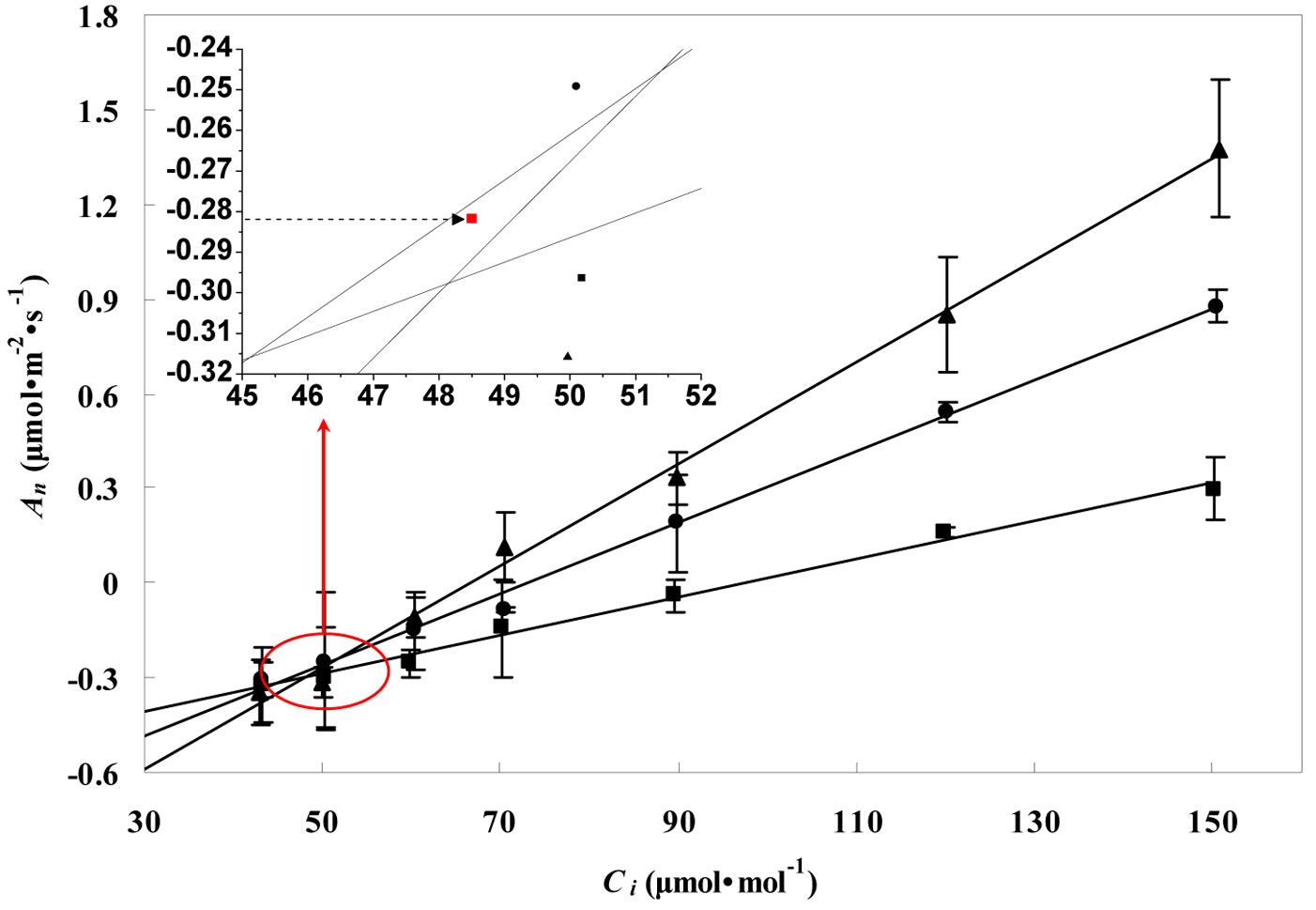
Fig. 2 and Fig. 3 show the RL values obtained for the leaves of P. koraiensis by the Kok and the Laisk methods, respectively. The black arrow in Fig. 2 indicates the apparent rate of day respiration (0.21 μmol m-2 s-1). As for the Laisk method, three linear regressions of the An-Ci curves intersected with each other within a centralized triangle range (Fig. 3). The central point (marked with red square in Fig. 3) represents the mean value of the three intersections of the triangle extended to the An axis and provides the value of day respiration. The intersection of the three lines obtained using the Laisk method formed a triangle instead of being a single point. The apparent lack of convergence of the three lines might result from measurement error arising from CO2 leak through the gaskets or from the influence of the respired CO2 on the leaf under the gaskets. Therefore, the day respiration rates estimated by the Kok and the Laisk methods were corrected. As for the Kok method, the corrected values of day respiration (RLK) were 18% higher (P < 0.05) than the apparent values, while for the Laisk method, the corrected values of day respiration (RLL) decreased 22% (P < 0.05) compared with the apparent values. Tab. 1 showed the RL values estimated by the Kok and the Laisk methods for the three tree species during the peak growing season. On average, RLK values obtained for the evergreen conifer (P. koraiensis) were 31.7% lower (P < 0.05) while the RLL values was 36.8% lower (P < 0.05) than those obtained for the deciduous broadleaved trees (T. amurensis and F. mandshurica). The difference between RLK and RLL values was about 4% for the three tree species.
Fig. 2 - Linear relationship between the net CO2 assimilation rate (An) and low PPFD values (Kok method) for P. koraiensis saplings in the Changbai station in July. The slope of the regression line of black circles (R2 = 0.98, P< 0.05) is higher than that of the linear regression line of white circles (R2 = 0.91, P< 0.05). Arrow indicates the value of day respiration. Data are presented as mean ± SD.
Fig. 3 - Linear regressions of the net CO2 assimilation rate (An) vs. the intercellular CO2 concentrations (Ci) at three PPFD values of 50 (black squares), 100 (black circles), and 150 (black triangles) μmol m-2 s-1 for leaves of P. koraiensis saplings in the Changbai Mountain in July (Laisk method). Day respiration value was calculated as the barycenter (the dotted arrow pointed) of the triangle formed by the intersection of the three lines. Data are presented as mean ± SD.
Tab. 1 - Leaf day respiration RL (RLK and RLL) and night respiration RD (μmol·m-2·s-1) for the leaves of the three species studied (P. koraiensis, T. amurensis and F. mandshurica) from July to September 2011. Data are presented as mean ± residual standard deviation (RSD) for RLK and RLL, and mean ± SD for RD. RLK and RLL are leaf day respiration values estimated by the Kok method and the Laisk method, respectively. (*): RSD of RLL was calculated by averaging the RSD values of three linear regressions.
| Species | Month | R L | R D | |
|---|---|---|---|---|
| R LK | R LL | |||
| Pinus koriaensis | July | 0.26 ± 0.0113 | 0.22 ± 0.0309 | 0.44 ± 0.1208 |
| August | 0.28 ± 0.0267 | 0.42 ± 0.0395 | 0.61 ± 0.0737 | |
| September | 0.22 ± 0.0277 | 0.18 ± 0.0371 | 0.55 ± 0.1071 | |
| Tilia amurensis | July | 0.33 ± 0.0261 | 0.35 ± 0.0699 | 0.65 ± 0.0924 |
| August | 0.52 ± 0.0407 | 0.59 ± 0.0294 | 1.17 ± 0.1104 | |
| September | 0.31 ± 0.0599 | 0.29 ± 0.0472 | 0.92 ± 0.1269 | |
| Fraxinus mandshurica | July | 0.30 ± 0.0362 | 0.36 ± 0.0818 | 0.59 ± 0.0640 |
| August | 0.56 ± 0.0278 | 0.59 ± 0.0529 | 1.24 ± 0.1593 | |
| September | 0.31 ± 0.0256 | 0.33 ± 0.0680 | 1.06 ± 0.1467 | |
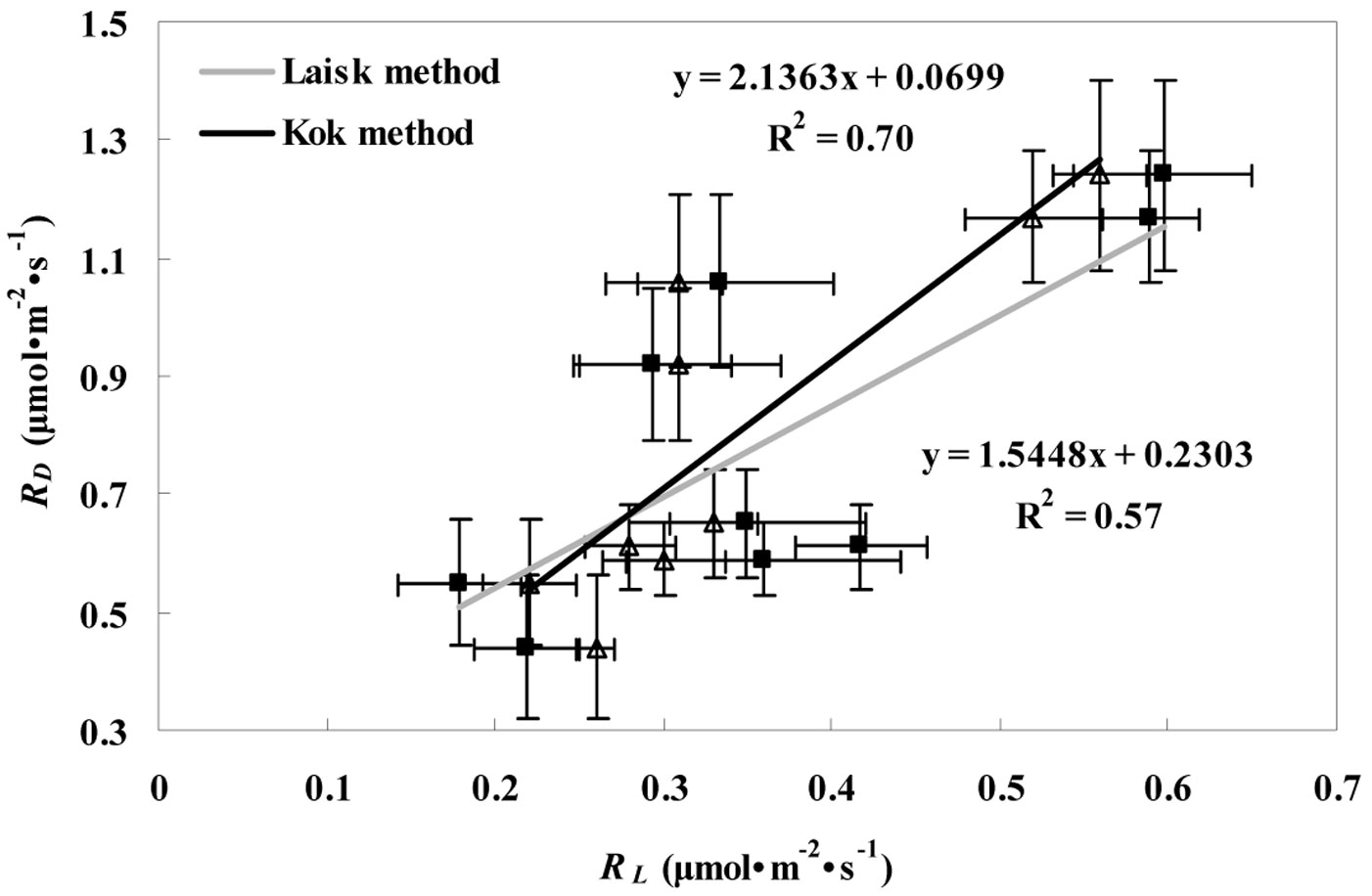
Also, the RD value of the conifer was lower than those of the deciduous trees (Tab. 1), and the averaged RD values for the conifer over the whole study period were 40.7% lower (P < 0.05) than those obtained for the broadleaves. As displayed in Fig. 4, a significant positive correlation was found between RD and RL estimated by the Kok (R2=0.70, P < 0.05) and the Laisk methods (R2=0.57, P < 0.05). The differences between RL and RD were significant for all three tree species (P < 0.05).
Fig. 4 - Relationship between RD and RL values estimated by Kok (RLK - triangles) and Laisk methods (RLL - squares) for the three tree species (P. koraiensis, T. amurensis, and F. mandshurica). Linear relationship estimated by the Kok and the Laisk method is represented with the black and gray line, respectively.
Relationship between day respiration and leaf nitrogen content
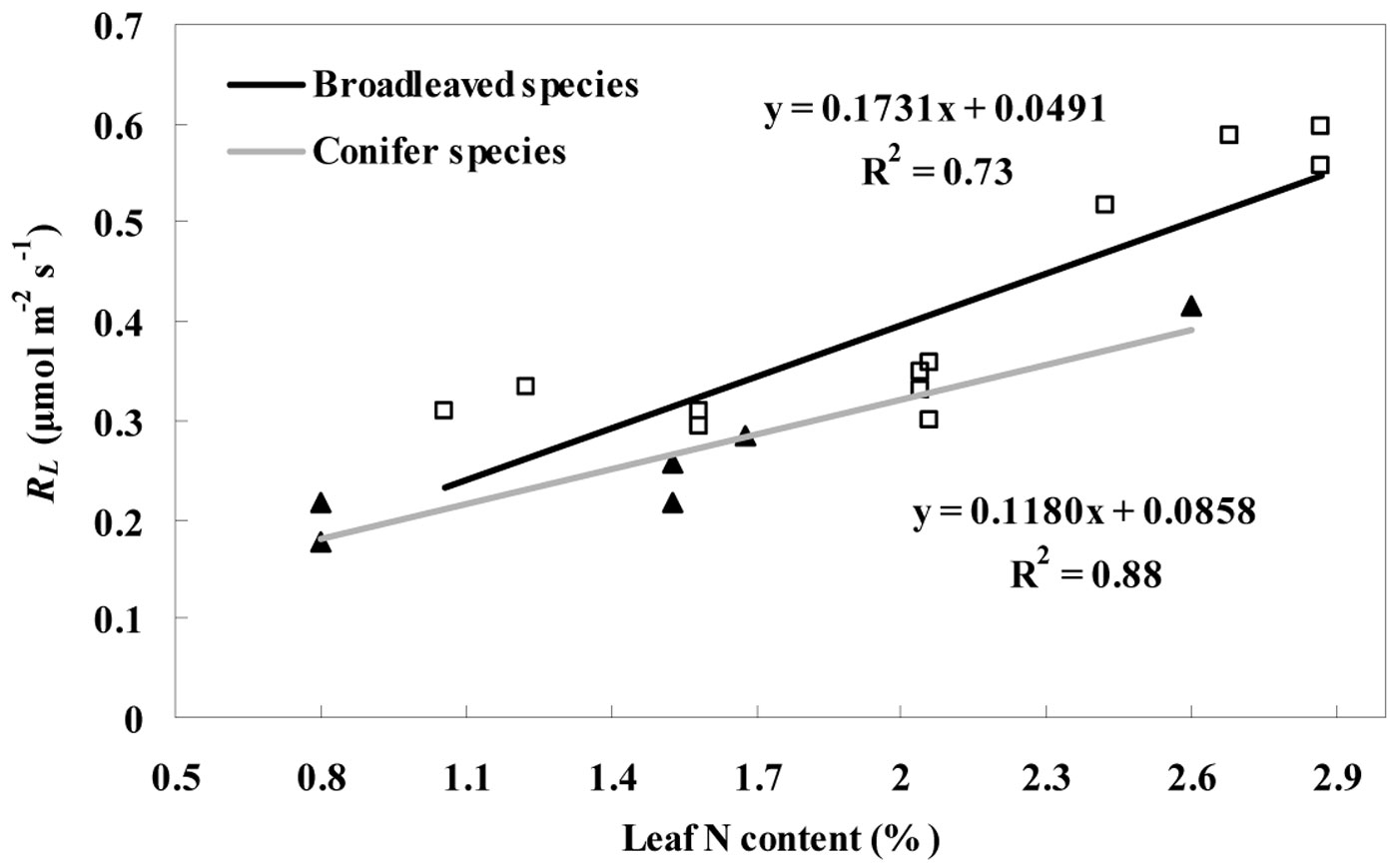
Fig. 5 - Variation in RL with leaf nitrogen content for the two deciduous broadleaved species (white squares) and one evergreen conifer species (black triangles). The linear regression for broadleaved species and conifer species was showed with the black and gray line, respectively. RL represents the leaf day respiration which includes the value estimated by the Kok (RLK) and Laisk methods (RLL) from July to September.
The average leaf N concentration for the evergreen conifer tree (P. koraiensis) was 27.1% lower (P < 0.05) than for the two deciduous broadleaved trees (F. mandshurica and T. amurensis). Moreover, a strong positive correlation between leaf nitrogen content and day respiration was found (Fig. 5). The correlation between RL and leaf nitrogen content is expressed as eqn. 3 for the conifer species (R2 = 0.88, P < 0.05) and as eqn. 4 (R2 = 0.73, P < 0.05) for the broadleaved species:
where RLec and RLdb are the day respiration rates of the evergreen conifer species and two deciduous broadleaved trees, respectively, and N is the leaf nitrogen content.
Light inhibition of day respiration
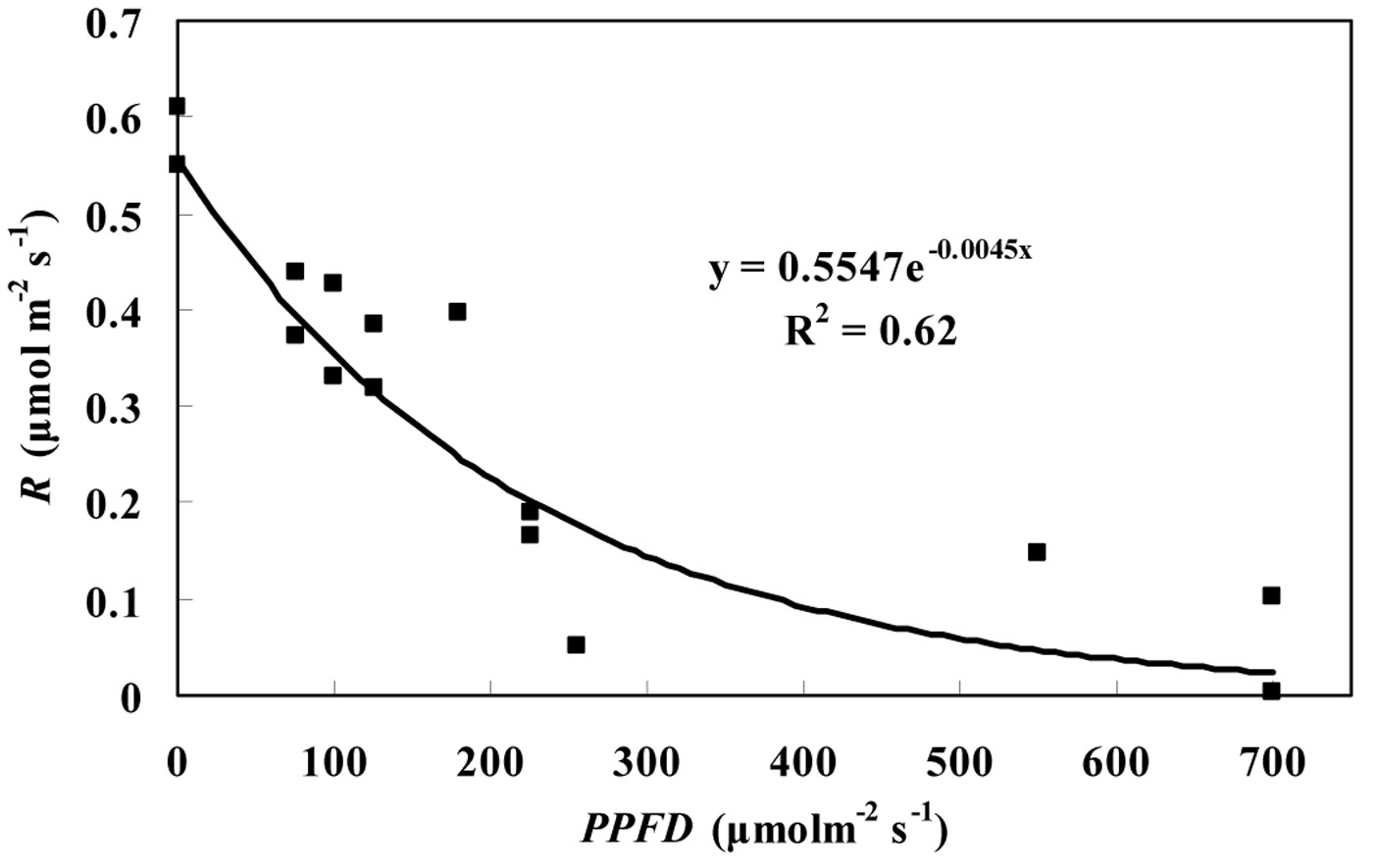
The average RL inhibition of the three tree species was found to be 55.9% and 52.6% for the Kok and Laisk methods, respectively. No significant differences were observed among the three tree species in the light inhibition on RL. The degree of light inhibition on RL was different under different PPFD values. Based on our data, the relationship between R and PPFD can be described by the following exponential equation (eqn. 5):
(R2 = 0.62, P < 0.05) where R is the leaf dark respiration, including both leaf dark respiration in light and darkness. As shown in Fig. 6, the value of R for P. koraiensis was the highest in darkness, and then R decreased rapidly following an increase in the incident PPFD. The decrease rate of R gradually declined with PPFD increase until it remained constant.
Fig. 6 - The effects of light on leaf dark respiration. Leaf dark respiration (R), including day respiration estimated by the Laisk method and night respiration, of P. koraiensis at different PPFD values in August and September.
Discussion
Uncertainties in day respiration estimate
The corrected day respiration was 18% higher and 22% lower than the apparent values obtained by the Kok and the Laisk methods, respectively. Kirschbaum & Farquhar ([21]) showed that RL measured by the Kok method is underestimated because it is assumes that the Ci values remain constant with changing PPFD. However, it has been shown that when PPFD decreases, stomatal closure is altered in response to environmental changes ([47]), and Ci gradually increases because of the decreased stomatal conductance. Consequently, a fraction of the respired CO2 is not promptly released and remains involved in the photosynthesis process. This results in an increase in An values and a decline in the slope of regression of An vs. PPFD ([21], [51]), which accounts for the underestimation of RL in the Kok method. RLL was overestimated in the Laisk method as the measurement must be conducted at low CO2 concentration. Some studies have proposed that the overestimation of RLL is caused by the CO2 acting as an inhibitor of certain enzymes ([7], [21], [49]). However, a large number of studies suggest that leaf respiration changes with CO2 possibly due to small leaks or diffusion in the gas exchange measurement systems ([3], [33], [43], [14], [40]). Therefore, in this study RLL was corrected based on the method suggested by Li-Cor Inc ([26]) to compensate the CO2 leakage in the Laisk method.
Differences between dark respiration of broadleaved and conifer species
Consistent with several previous studies ([52], [5]), RL was found in this investigation to be positively correlated with RD. Both RL and RD of the deciduous broadleaved species were higher than those of the conifer species. In addition, Cornelissen et al. ([11]) reported a growth rate of deciduous broadleaved species higher than that of the conifer species. RL and RD were higher in the rapidly growing tree species, likely because rapid-growth species generally require more carbon skeletons, energy and reductant-producing organelles, which were derived from the respiration process in tree species ([1], [52], [25], [5]). Atkin et al. ([5]) also reported that RL and RD were positively correlated with An and the relative growth rate in Poa species. Hence, greater values of RL and RD can be hypothesized to occur in fast-growing plants. Wang et al. ([54]) also has suggested that higher biomass production in fast-growing species demand greater respiration production, and this requirement is fulfilled by higher RL and RD values. A positive correlation between RL and leaf N concentration was also found in our study, which is consistent with several previous reports on a positive correlation between plant respiration and the leaf N concentration ([1], [41]). Furthermore, we found that leaf N concentration in P. koraiensis was lower than in F. mandshurica and T. amurensis. This is consistent with previous reports by Reich et al. ([38]) and Villar et al. ([52]) showing that the leaf N concentration is lower in evergreen species than in deciduous species.
Light inhibition of day respiration
In this study, RL was significantly lower than RD for the three tree species, indicating that RL was inhibited by light. This result is in agreement with those of several previous studies ([44], [7], [28], [16], [46]). Some studies have suggested that inhibition of leaf dark respiration in light is only apparent and possibly results from internal CO2 re-fixation. Loreto et al. ([27]) and Pinelli & Loreto ([32]) found that RL and RL/RD were inversely correlated with photosynthesis, and suggested that most of the respiratory CO2 was re-fixed by photosynthesis. Pinelli & Loreto ([32]) also reported that the sum of re-fixed and emitted 12CO2 was close to mitochondrial respiration in the dark. However, the authors also suggested that mitochondrial respiration may be inhibited in light when the leaf is in salt- and water-stressed condition, because the sum of re-fixed and emitted 12CO2 under the above conditions was lower than that in the dark. Furthermore, Pärnik & Keerberg ([30]) stated that leaf RL is lower than RD in light even when the re-fixed respiratory CO2 is taken into consideration. Additionally, many studies have suggested that the inhibition in light may be due to inhibition of respiratory enzymes by co-factors such as ATP and NADPH ([15], [28]). Tcherkez et al. ([46]) showed that the inhibition mainly occurred during the Krebs cycle and the inhibition in the Krebs cycle was reduced by 95% in light, while the pyruvate dehydrogenase was reduced by only 27%. The reduction in the Krebs cycle, which is one of the most important processes of leaf respiration, occurs mainly because some enzymes are inhibited by the high ratio of NADPH/NADP+ in light ([45]). Likewise, it has been suggested that high NADPH/ NADP+ ratios could also inhibit isocitrate dehydrogenase in illuminated mitochondria ([19]). Thus, there is considerable evidence supporting that day respiration is indeed inhibited in light. In this study, no significant differences were found in the light inhibition of RL between broadleaved and conifer trees, in spite of their different photosynthetic capacities and relative growth rates. Therefore, light inhibition on RL may not be correlated with An or relative growth rate. This conclusion is consistent with results reported by Atkin et al. ([5]).
Our results showed that leaf dark respiration varied under different PPFD levels. The relationship between RL and PPFD showed in Fig. 6 is consistent with that obtained in previous studies ([7], [51], [52]) by the Laisk method. The photosynthetic rate generally increases with increasing PPFD, but the rate of increase gradually declines. In this study, RL was found to decrease with increasing PPFD, likely because more ATP and NADPH were available for increasing the photosynthetic rate ([50]), causing the energy demand to step up gradually from RL to plateau ([52]).
Both the comparison of RL and RD of the three tree species and the analysis of the relationship between R and PPFD demonstrated a remarkable inhibition of the day respiration. Peisker & Apel ([31]) reported that the ratio of RL to RD ranges from 30 to 100%. However, Hurry et al. ([18]) concluded that RL is higher in light than in darkness in rye using the 14C-labeling measurement technique. Additionally, Zaragoza-Castells et al. ([57]) found that RL is inhibited in light only under certain conditions. Thus, RL inhibition in light may not occur in all species and under all conditions. Light inhibition of RL and the degree of inhibition of RL may depend on the species and growth conditions. Hence, additional studies on various species and under different environmental conditions are needed to obtain a deeper understanding of the above physiological processes. Variations in RL with change in PPFD and CO2 concentration were analyzed in this investigation. However, many other factors affecting RL may exist, so further studies should be carried out to fully understand the mechanism by which RL is inhibited in light.
Conclusions
In summary, our findings revealed that RLK and RLL in conifer trees were lower by 31.7% and 36.8%, respectively, than those in broadleaved trees considered in this study. We detected no significant difference in light inhibition of respiration between the coniferous and the broadleaved tree species. The average RL inhibition in the three tree species was 55.9% and 52.6% for RLK and RLL values, respectively, relative to RD. RL was found to be positively correlated with RD in the three tree species. RL also decreased with increasing PPFD, but the decrease rate of RL gradually declined. These findings may be useful in estimating the component of carbon exchange and carbon budget (e.g., Re, NEE, GPP) of the forest ecosystem by evaluating the light inhibition of respiration in the representative tree species.
Acknowledgments
We are deeply grateful to all the staff of the National Forest Ecosystem Research Station of Changbai Mountain for their assistance in the maintenance of instruments and collection of field data. We sincerely thank Dr. M. Kirschbaum for providing the program for correcting the RL values estimated by the Kok method. This study was supported by the National Science Foundation of China (no. 41375119), the State Key Laboratory of Forest and Soil Ecology (no. LFSE2013-11) and the Youth Fund for Creative Research Groups, Institute of Applied Ecology, CAS.
References
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Dexin Guan
Jiabing Wu
Yanli Jing
Fenghui Yuan
Anzhi Wang
Changjie Jin
State Key Laboratory of Forest and Soil Ecology, Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 110016 (China)
Yanli Jing
Fenghui Yuan
Graduate University of Chinese Academy of Sciences, Beijing 100049 (China)
Corresponding author
Paper Info
Citation
Sun J, Guan D, Wu J, Jing Y, Yuan F, Wang A, Jin C (2015). Day and night respiration of three tree species in a temperate forest of northeastern China. iForest 8: 25-32. - doi: 10.3832/ifor0982-007
Academic Editor
Giustino Tonon
Paper history
Received: Feb 28, 2013
Accepted: Mar 31, 2014
First online: May 26, 2014
Publication Date: Feb 02, 2015
Publication Time: 1.87 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 57172
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 47576
Abstract Page Views: 3476
PDF Downloads: 4632
Citation/Reference Downloads: 34
XML Downloads: 1454
Web Metrics
Days since publication: 4258
Overall contacts: 57172
Avg. contacts per week: 93.99
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 12
Average cites per year: 1.09
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Evergreen species response to Mediterranean climate stress factors
vol. 9, pp. 946-953 (online: 07 July 2016)
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Research Articles
Photosynthesis of three evergreen broad-leaved tree species, Castanopsis sieboldii, Quercus glauca, and Q. myrsinaefolia, under elevated ozone
vol. 11, pp. 360-366 (online: 04 May 2018)
Research Articles
Seasonal dynamics of soil respiration and nitrification in three subtropical plantations in southern China
vol. 9, pp. 813-821 (online: 29 May 2016)
Research Articles
Evergreen Quercus aquifolioides remobilizes more soluble carbon components but less N and P from leaves to shoots than deciduous Betula ermanii at the end-season
vol. 11, pp. 517-525 (online: 01 August 2018)
Research Articles
Carbon and water vapor balance in a subtropical pine plantation
vol. 9, pp. 736-742 (online: 25 May 2016)
Research Articles
Extreme climatic events, biotic interactions and species-specific responses drive tree crown defoliation and mortality in Italian forests
vol. 17, pp. 300-308 (online: 30 September 2024)
Research Articles
Wintertime photosynthesis and spring recovery of Ilex aquifolium L.
vol. 12, pp. 389-396 (online: 31 July 2019)
Research Articles
Multi-temporal influence of vegetation on soil respiration in a drought-affected forest
vol. 11, pp. 189-198 (online: 01 March 2018)
Short Communications
Variation in growth, photosynthesis and water-soluble polysaccharide of Cyclocarya paliurus under different light regimes
vol. 10, pp. 468-474 (online: 04 April 2017)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword