Evergreen Quercus aquifolioides remobilizes more soluble carbon components but less N and P from leaves to shoots than deciduous Betula ermanii at the end-season
iForest - Biogeosciences and Forestry, Volume 11, Issue 4, Pages 517-525 (2018)
doi: https://doi.org/10.3832/ifor2633-011
Published: Aug 01, 2018 - Copyright © 2018 SISEF
Research Articles
Abstract
Remobilization is an important mechanism of resource conservation in plants. However, our understanding of whether the responses of resource remobilization to global warming differ between deciduous and evergreen trees remains unclear. We assessed resource remobilization from leaves to 1-year-old shoots in a deciduous (Betula ermanii) and an evergreen (Quercus aquifolioides) species along elevational gradients (i.e., temperature gradient) at the end of growing season. We aimed to test the hypotheses that the reallocation rate increased with increasing elevation and that more resources were reallocated from leaves to storage tissues in deciduous species than in evergreen species. We analyzed the concentrations of non-structural carbohydrates (NSC), nitrogen (N) and phosphorus (P), and compared the differences in remobilization efficiency of NSC, N, and P between leaves and shoots within each species and between the two species along the elevational gradients. Due to the different strategies of evergreen and deciduous species in nutrients use, the deciduous species had higher N and P remobilization rate, but lower remobilization rate of sugars, starch, and NSC than the evergreen species at the end of growing season. The remobilization rate of NSC, N, and P was significantly higher in trees at their upper limits compared to lower elevations. Our results suggest that trees reallocate resources from leaves to storage tissues before leaf senescence or at the end of growing season, to increase the resource use efficiency and to adapt to the harsh alpine environments. These results contribute to better understanding of the alpine treeline phenomenon in a changing world.
Keywords
Altitudinal Gradient, Non-structural Carbohydrates, Sugars, Starch, Nutrients, Reallocation
Introduction
Plants reallocate resources from leaves to storage tissues prior to senescence, to support growth after dormancy ([1], [7]), but also for defense and reproduction ([6]). To increase the resource use efficiency and to reduce the costs, leaf carbon components, nitrogen (N), and phosphorus (P) are recycled through remobilization from leaves to storage organs with a major pulse shortly before senescence ([6]). The main reallocatable carbon components in trees are non-structural carbohydrates (NSC), and the NSC concentration is always higher in leaves (carbon source) than in shoots, stem, and roots (carbon sink) of trees ([16], [24], [13]). Similarly, leaf nutrient (e.g., N and P) concentration is also always higher in leaves than in shoots (e.g., [17], [25]). At the end of the growing season, resources can be reallocated from leaves to shoots to increase the resource use efficiency. Resource remobilization efficiency can be higher at low nutrient availability or in low-temperature environment ([38], [2]), representing an adaptation to the less suitable habitats ([4]). A recent review ([12]) and a research paper ([28]) indicated that, during leaf senescence, N and P were always remobilized from leaves to shoots, irrespective of plant species, and environmental factors, such as drought and nutrient deficiency, modified the remobilization efficiency. Despite the importance of such processes, it is still unclear whether elevation affects remobilization of nutrients and carbohydrates, and whether this elevation-remobilization relation differs between deciduous and evergreen species.
The patterns of resource remobilization differ between deciduous and evergreen species ([6], [1]). Larger allocation to storage in deciduous than in evergreen species reflects the more pronounced asynchrony of supply and demand undergone by deciduous species ([6]), since storage of carbohydrates is particularly important for deciduous trees that enter early spring without photosynthesizing tissue, with later sink to source transition in comparison with evergreen trees ([29], [30]). In early spring, deciduous species make new leaves at low carbon cost per unit leaf area, but at high cost in terms of carbohydrate reserves ([8]). In evergreen species, carbohydrates can be supplemented through photosynthesis of leaves from previous-years leaf cohorts ([11]). Carbon construction cost of evergreen leaves, being rich in carbon-based compounds, is relatively high ([37]), and only a portion of leaves is renewed annually.
Remobilization of plant’s N also differs between leaf habits of the species ([7]). Nitrogen resorption efficiency is much higher in deciduous than in evergreen shrubs and trees, while P resorption from senescing leaves does not significantly differ between evergreen and deciduous species ([1]). In contrast to deciduous species that recycle N back into storage organs before leaf abscission, evergreen species can directly retain resources in over-wintering leaves ([6], [46]). Indeed, N is stored mainly in the youngest age class of foliage in evergreen species ([32]). However, exceptions seem to exist, for example, no major differences in patterns of N and P distribution have been shown between deciduous and evergreen Alaskan tree species ([5]).
We investigated differences in resource remobilization between deciduous and evergreen species. Evergreen trees may maintain the resources directly in over-wintering leaves to reduce the costs of resource transport in the fall and next early spring, whereas deciduous trees need to reallocate resources from leaves to storage tissues in the fall to increase the resource use efficiency. In particular, we tested the hypotheses that: (1) both deciduous and evergreen trees reallocate resources from leaves to shoots at the end of growing season, but the former have higher resource remobilization than the latter; and (2) regardless of foliar habit, resource remobilization increases with increasing elevation (e.g., harsher physical environments and earlier leaf senescence). We used elevational gradients as proxy of changed environmental conditions and of shift in leaf phenology ([18]). Two broad-leaved tree species, a deciduous (Betula ermanii Cham.) and an evergreen (Quercus aquifolioides Rehder & E.H. Wilson) species, growing along elevational gradients up to their upper limits were chosen for the present study. We analyzed: (1) the concentrations of NSC, N and P in 1-year-old leaves and 1-year-old shoots; (2) the differences in NSC, N and P between leaves and shoots; and (3) the remobilization efficiency from leaves to shoots in both tree species along two elevational gradients in China.
Materials and methods
Sites description and species
The study was conducted in west Changbai Mountain, which is located in the Changbai Mountain Natural Reserve (41° 59′ N, 127° 59′ - 128° E) in Northeast China, and south Balang Mountain, which is located in the Wolong Natural Reserve (30° 53′ N, 102° 57′ E), at the eastern edge of Qinghai-Tibetan Plateau in Southwestern China.
At the Changbai Mountain site, the treeline elevation ranges from 2000 to 2030 m a.s.l., where groups of trees with height greater than 3 m have the deciduous broad-leaved Betula ermanii as the dominant species. Above 2030 m, the distribution of B. ermanii is scattered and can reach up to about 2200 m (upper limit - Tab. 1). The growing season at the treeline is generally from late May to late September (the first severe frost). According to long-term climate data collected in Tian-Chi Meteorological Station located at 2623 m a.s.l., the climate varies greatly from year to year. For instance, mean growing season temperature ranges from 3.37 to 8.82 °C (mean temperature is 5.87 °C), and average precipitation ranges from 700 to 1400 mm, the annual frost-free period varies from 65 to 70 days, and the snow cover period is about 199-219 days at 2623 m a.s.l. Soils are classified as mountain soddy forest soil.
Tab. 1 - Characteristics of the plots and the sampling trees Betula ermanii (mean ± standard deviation; n = 5 trees), Quercus aquifolioides (mean ± standard deviation; n = 6 trees) located in the Changbai Mountain (Jilin, NE China) and Balang Mountain (Sichuan, SW China), respectively. (a): Basal diameter (about 1 cm above the ground surface).
| Species and Site No. |
Elevation (m) |
Average | Slope exposure |
|
|---|---|---|---|---|
| DBH (cm) | Height (m) | |||
| Betula ermanii Site 1 |
2187 | 1.1 ± 0.5a | 0.4 ± 0.1 | West |
| 2137 | 1.6 ± 0.5a | 0.6 ± 0.1 | West | |
| 2097 | 1.5 ± 0.3a | 0.9 ± 0.1 | West | |
| 2027 | 4.9 ± 0.7 | 4.7 ± 0.3 | West | |
| 1977 | 28.4 ± 2.8 | 14.9 ± 0.9 | West | |
| Quercus aquifolioides Site 2 |
3589 | 5.5 ± 1.0 | 1.8 ± 0.6 | South |
| 3441 | 7.4 ± 1.0 | 2.8 ± 0.5 | South | |
| 3327 | 5.1 ± 1.7 | 2.8 ± 0.9 | South | |
| 3159 | 5.6 ± 2.0 | 2.8 ± 0.9 | South | |
| 2978 | 7.1 ± 2.8 | 3.1 ± 0.7 | South | |
| 2843 | 8.2 ± 2.0 | 3.7 ± 0.3 | South | |
At the Balang Mountain site, pure evergreen broad-leaved Quercus aquifolioides trees range from 2800 to 3600 m a.s.l. (upper limit - Tab. 1). This naturally generated tree stand is 30-35 years old and comprises multi-stemmed clumps. A nature reserve was established in 1976, and the Q. aquifolioides stands have not been disturbed since then. The study area has a subtropical inland mountain climate with sunny, dry winter (November to April) and warm, humid summer (June to September). According to long-term climate data collected at 1920 m a.s.l. (Wolong Nature Reserve Authority), the annual mean precipitation is 995 mm, with rainfall mainly occurring from May to September, and the annual mean temperature is 12.8 °C, with the monthly mean temperature of 17.0 °C in July and -1.7 °C in January. Soils are classified as mountain brown soil.
Field sampling
To compare the differences in concentrations of NSC and nutrients between leaves and shoots, and then to calculate the end-season remobilization rate between tissues, samples were taken at the end of growing season (September for Changbai Mt. in northeastern China and November for Balang Mt. in southwestern China), because end-season tissue NSC and nutrient concentrations are relatively stable ([15]). We sampled 1-year-old leaves and 1-year-old shoots from six plots (n = 6) for Q. aquifolioides at each of six elevations along an elevational gradient, i.e., 2843, 2978, 3159, 3327, 3441, and 3589 (± 5) m a.s.l., and from five plots (n = 5) for B. ermanii at each of five elevations along an elevational gradient, i.e., 1977, 2027, 2097, 2137, and 2187 m a.s.l. Several healthy trees of similar age, as revealed by their uniformity in height and diameter at breast height (DBH) were selected for tissue collection in each plot. From each selected tree, we collected 4-8 pieces of 1-year-old leaves and 1-year-old shoots from 4 crown directions. Leaves and shoots collected in a plot were pooled for a mixed leaf sample and shoot sample, respectively. To diminish the influence of diurnal temperature range and light differences, all samples were collected in the period between noon and 2 p.m. and immediately stored in a cool box. The samples were heated in a microwave oven at 600 W for 40 s, dried to a constant mass at 65-75 °C ([25]), ground to powder, and stored after being sealed with silica gel at 4 °C for analysis. In each plot, we measured the mean height and DBH of each tree (Tab. 1). For juvenile trees with small DBH, basal diameter (about 1 cm above ground surface) was measured.
Analysis of non-structural carbohydrates (NSC)
To compare NSC and their composition (sugar:starch ratio) at different elevations, we measured the concentration of NSC using the anthrone colorimetric method, because few samples are required for microanalysis ([9]). Dried 1-year-old leaves and 1-year-old shoots were respectively ground to pass a 1 mm sieve. For each sample, 0.1 g of the powdered material was placed into a 10 ml centrifuge tube and mixed with 5 ml of 80 % ethanol. The mixture was incubated in a water shaker at 80 °C for 30 min, cooled to ambient temperature, and then centrifuged at 4000 rpm for 10 min. The sediments were re-extracted twice with 80 % ethanol to extract the soluble sugars. The ethanol-insoluble pellet was used for starch extraction, and the combined supernatants retained for soluble sugar determinations by the anthrone method. Glucose was used as a standard. Starch was extracted from the solid residues after placing in water at 80 °C to remove the ethanol by evaporation. The ethanol-insoluble residues were boiled with 2 ml of distilled water for 15 min. After cooling to room temperature, 2 ml of 9.2M HClO4 was added for 15 min to hydrolyze the starch, 4 ml distilled water was added and mixed, and then the mixture was centrifuged at 4000 rpm for 10 min. Subsequently, the solid residues were added with 2 ml of 4.6M HClO4 for a final extraction. Soluble sugars and starch concentrations were determined at 620 nm using a 721 spectrophotometer. The concentrations of soluble sugars, starch, and NSC were calculated on a dry matter basis (% d.m.).
Analysis of nitrogen and phosphorus
Oven-dried samples were ground to pass a 1 mm sieve. For each sample, 0.1 g of plant tissue was digested in 5 ml of H2SO4 and heated to boiling for 15 min. After cooling to room temperature, the digestion solution was added 2 ml H2O2 and then boiled for 15 min. The procedure described earlier was repeated until a colorless transparent liquid was produced. The total N concentration (% d.m.) was determined on a semi-automatic azotometer, and phosphorus was determined at 450 nm using a 721 spectrophotometer ([45]).
Methods for evaluating resource remobilization
To clarify the remobilization of mobile carbohydrates and nutrients from leaves to shoots differences between deciduous and evergreen species at the end of growing season concentrations were expressed relatively to a constant parameter. Therefore, mobile carbohydrates, sugars, starch, N, and P remobilization efficiency (%) was calculated as (eqn. 1):
where Cl and Cs represent concentrations of NSC, N, or P in leaves and shoots at the end of growing season, respectively. A negative R-value (Cl < Cs) indicates accumulation of NSC, N, or P in shoots, whereas a positive R-value (Cl > Cs) reflects the direction of resource fluxes. The larger the R-value, the lower remobilization efficiency is.
Data analysis
Given the large difference in elevation of the upper limit of the studied species, irrespective of the length of the growing season, the growing season soil (-10 cm) temperatures at their upper limit are the same being 6.5 ± 0.8 °C ([20]), which makes the present north-south comparison reasonable. NSC, soluble sugars, starch, N and P data were confirmed for normality (Kolmogorov-Smirnov test) before statistic analysis. Three-way analyses of variance (ANOVAs) were used to test the effects of elevation, species, and type of tissue, and their two-way and three-way interactions on the concentrations of NSC, sugars, starch, N, and P, as well as on sugars/starch ratio and N/P ratio. At the species level, two-way ANOVAs were repeatedly performed with elevation and tissue type as factors to test the elevational trends in parameters mentioned above. Two-way ANOVAs were performed with elevation and species as factors to identify the trends in the remobilization efficiency of NSC, sugars, starch, N and P. Differences in the studied parameters were tested for significance using Duncan’s test or Tukey’s HSD test at α = 0.05. Since we were interested in the effects of species and elevation on NSC, sugars, starch, N and P, other effects such as the interaction of elevation × tissue type and the interaction of elevation × tissue type × species are not systematically discussed.
Results
Species-specific responses
Elevation significantly affected tissue concentrations of soluble sugars, starch, NSC, N, and P, but it did not influence tissue sugars/starch (p = 0.463) and N/P ratios (p = 0.232 - Tab. 2). All parameters differed significantly between deciduous and evergreen tree species (Tab. 2). Tissue type had a significant effect on the concentrations of soluble sugars, NSC, and N, as well as on sugars/starch ratio, whereas it had no effects on starch, P concentrations, and N/P ratio (Tab. 2). The elevation × species interaction was significant for soluble sugars, starch, NSC, and P concentrations, but not for N concentration, and sugars/starch and N/P ratios (Tab. 2). The interaction between tissue type and species was significant for all parameters, except for P (p = 0.763 - Tab. 2).
Tab. 2 - Results of three-way nested ANOVAs with elevation, species, tissue type as factors. F and Prob values are given.
| Factors | Soluble sugars | Starch | NSC | Nitrogen (N) |
Phosphorus (P) |
Sugars/ Starch |
N / P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Prob | F | Prob | F | Prob | F | Prob | F | Prob | F | Prob | F | Prob | |
| Elevation (E) | 2.98 | 0.015 | 51.78 | <0.001 | 19.91 | <0.001 | 991.69 | <0.001 | 186.12 | <0.001 | 0.93 | 0.463 | 1.40 | 0.232 |
| Species (S) | 494.79 | <0.001 | 64.23 | <0.001 | 205.03 | <0.001 | 258.08 | <0.001 | 10.30 | 0.002 | 652.27 | <0.001 | 19.20 | <0.001 |
| Tissue type (T) | 444.99 | <0.001 | 1.81 | 0.182 | 286.78 | <0.001 | 3.11 | 0.012 | 0.34 | 0.886 | 331.66 | <0.001 | 2.07 | 0.154 |
| E × S | 18.88 | <0.001 | 19.90 | <0.001 | 5.00 | 0.001 | 3.04 | 0.085 | 30.83 | <0.001 | 1.53 | 0.199 | 0.81 | 0.519 |
| T × S | 804.41 | <0.001 | 5.71 | 0.019 | 517.92 | <0.001 | 3.28 | 0.009 | 0.52 | 0.763 | 315.12 | <0.001 | 16.45 | <0.001 |
| E × T | 17.99 | <0.001 | 9.87 | <0.001 | 20.23 | <0.001 | 5.35 | 0.001 | 2.19 | 0.076 | 0.82 | 0.54 | 0.42 | 0.832 |
| E × T × S | 4.07 | 0.004 | 4.34 | 0.003 | 5.09 | 0.001 | 2.58 | 0.042 | 0.89 | 0.475 | 0.91 | 0.46 | 0.53 | 0.716 |
Elevation significantly affected the remobilization efficiency of sugars, starch, NSC and N, but not P (Tab. 3). The remobilization efficiency of sugars, NSC, N and P differed significantly between deciduous and evergreen tree species (Tab. 3). The elevation × species interaction was significant for all parameters, except for P (p = 0.528) (Tab. 3).
Tab. 3 - Results of two-way nested ANOVAs with elevation and species as factors. The F and Prob values are given. R refers to remobilization efficiency.
| Factors | R sugars | R starch | R NSC | R Nitrogen | R Phosphorus | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | Prob | F | Prob | F | Prob | F | Prob | F | Prob | |
| Elevations (E) | 18.69 | <0.001 | 9.20 | <0.001 | 50.98 | <0.001 | 4.78 | 0.001 | 0.74 | 0.600 |
| Species (S) | 239.56 | <0.001 | 0.11 | 0.742 | 430.02 | <0.001 | 16.21 | <0.001 | 69.29 | <0.001 |
| E × S | 20.31 | <0.001 | 8.16 | <0.001 | 46.16 | <0.001 | 2.93 | 0.030 | 0.81 | 0.528 |
Betula ermanii on Changbai Mountain
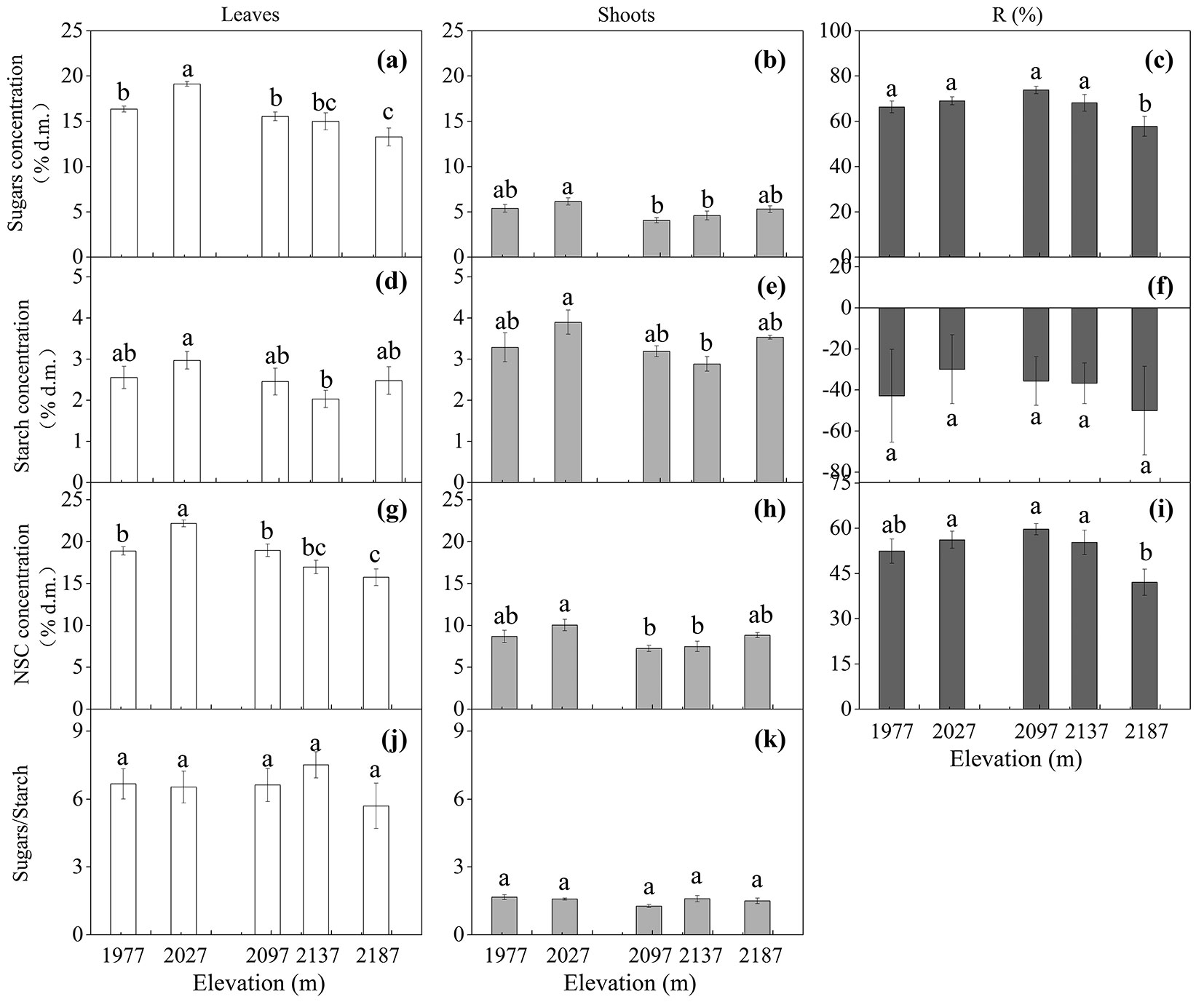
Tissue NSC concentration of B. ermanii was significantly affected by both elevation and tissue type (both p < 0.001 - Tab. 4). Leaf sugars (Fig. 1a) and NSC (Fig. 1g) decreased significantly with increasing elevation (both p < 0.05), except for trees at 1977 m a.s.l. However, the concentrations of sugars (Fig. 1b) and NSC in shoots (Fig. 1h) tended to have lower levels at the mid-higher elevations (2097 and 2137 m a.s.l.). In particular, the sugar and NSC concentrations were always significantly higher in leaves (Fig. 1a, Fig. 1g) than in shoots (p < 0.001 - Fig. 1b, Fig. 1h, Tab. 4).
Tab. 4 - Results of two-way nested ANOVAs with elevation and tissue type as factors. The F and Prob values are given.
| Species | Factor | Soluble sugars |
Starch | NSC | Nitrogen (N) |
Phosphorus (P) |
Sugars/ Starch |
N / P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Prob | F | Prob | F | Prob | F | Prob | F | Prob | F | Prob | F | Prob | ||
| Betula ermanii | Elevations (E) | 11.93 | <0.001 | 3.47 | 0.018 | 12.74 | <0.001 | 3.71 | 0.013 | 2.28 | 0.080 | 0.60 | 0.670 | 4.38 | 0.006 |
| Tissue types (T) | 925.90 | <0.001 | 23.95 | <0.001 | 557.60 | <0.001 | 59.85 | <0.001 | 6.19 | 0.018 | 184.12 | <0.001 | 53.52 | <0.001 | |
| E × T | 4.54 | 0.005 | 0.11 | 0.979 | 3.43 | 0.019 | 2.31 | 0.078 | 0.32 | 0.861 | 0.51 | 0.731 | 1.92 | 0.129 | |
| Quercus aquifolioides | Elevations (E) | 7.91 | <0.001 | 62.72 | <0.001 | 13.48 | <0.001 | 1.52 | 0.198 | 1.07 | 0.384 | 45.96 | <0.001 | 1.27 | 0.288 |
| Tissue types (T) | 40.33 | <0.001 | 1.79 | 0.186 | 23.37 | <0.001 | 325.37 | <0.001 | 37.67 | <0.001 | 6.25 | 0.015 | 13.17 | 0.001 | |
| E × T | 21.36 | <0.001 | 12.00 | <0.001 | 26.79 | <0.001 | 2.57 | 0.036 | 0.96 | 0.448 | 7.40 | <0.001 | 0.58 | 0.716 | |
Fig. 1 - Tissue mean concentration (mean ± SE; % of dry matter) of soluble sugars, starch, non-structural carbohydrates (NSC), sugars/starch ratio, and remobilization efficiency (R %) in Betula ermanii trees grown along the altitudinal gradients on Changbai Mountain (n = 5 for each elevational site and tissue type). Different letters indicate significant differences (p<0.05) among elevations as determined by Duncan test single-factor ANOVAs.
Both elevation and tissue type had significant effect on starch concentration (Tab. 4). Both leaf and shoot starch concentration showed the lowest level in trees at 2137 m a.s.l. (Fig. 1d, Fig. 1e). In contrast to sugars (Fig. 1a, Fig. 1b) and NSC (Fig. 1g, Fig. 1h), the starch concentration was always significantly lower in leaves than in shoots (p < 0.001 - Tab. 4, Fig. 1d, Fig. 1e), and thus, leaves had significantly higher sugars/ starch ratio than shoots (Fig. 1j, Fig. 1k), and the ratio did not change with elevation within a tissue type (Fig. 1j, Fig. 1k).
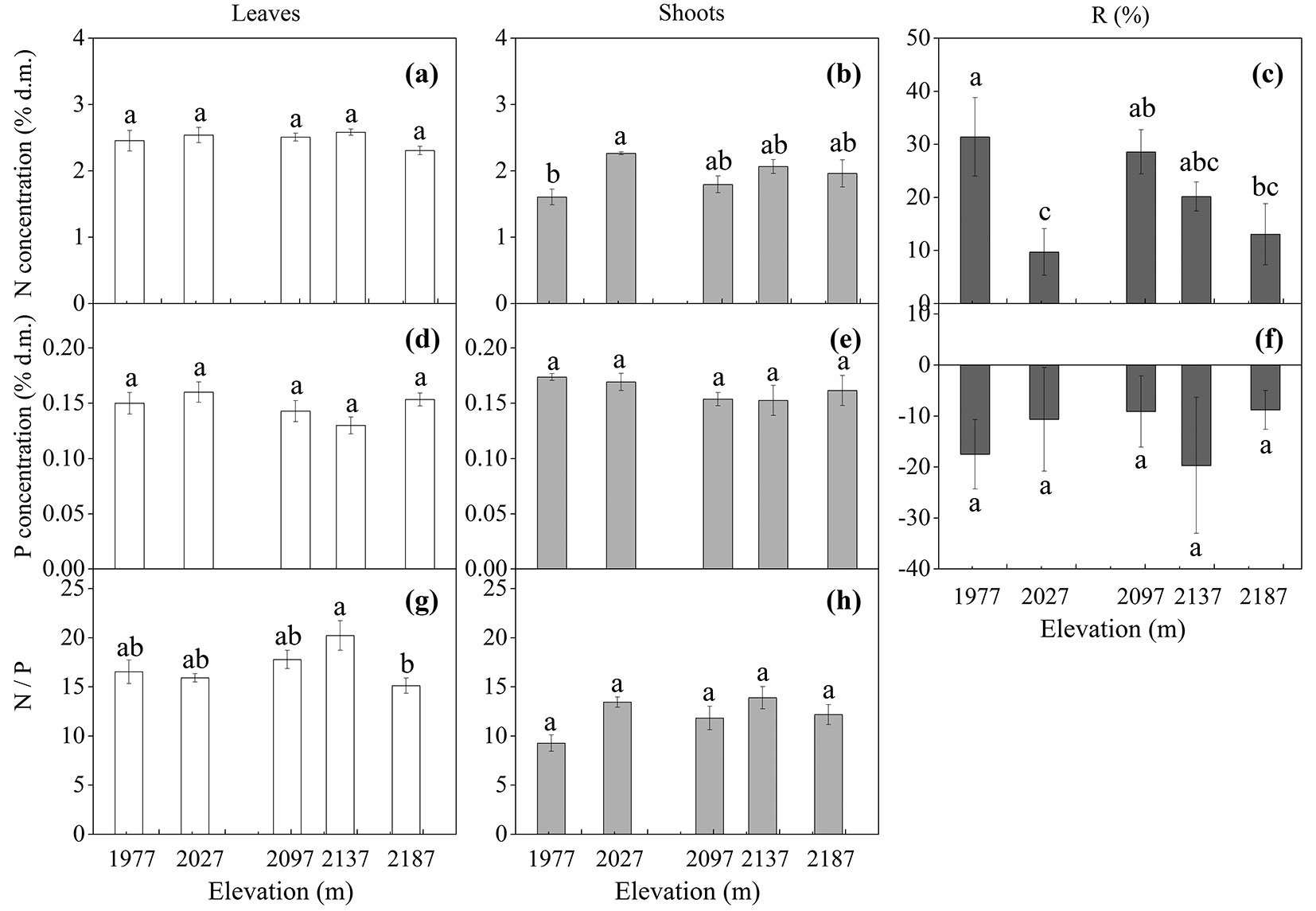
Elevation had no effect on tissue N (Fig. 2a, Fig. 2b) and P concentrations (Fig. 2d, Fig. 2e), except for a lower shoot N concentration in trees at 1977 m a.s.l. (Fig. 2b). Similarly, tissue N/P ratio also did not change with elevation (Fig. 2g, Fig. 2h), except for a lower leaf N/P ratio in trees at 2187 m a.s.l. (Fig. 2g). The N/P ratio was higher in leaves than in shoots (Fig. 2g, Fig. 2h). The mean N concentration was significantly higher in leaves than in shoots (p < 0.001 - Tab. 4, Fig. 2a, Fig. 2b). In contrast to N, the mean P concentration was significantly higher in shoots than in leaves (p = 0.018 - Tab. 4, Fig. 2d, Fig. 2e).
Fig. 2 - Tissue mean concentration (mean ± SE; % of dry matter) of total nitrogen (N) and phosphorus (P), N/P ratio, and remobilization efficiency (R %) in Betula ermanii trees grown along the altitudinal gradients on Chanbai Mountain (n = 5 for each elevational site and tissue type). Different letters indicate significant differences (p<0.05) among elevations as determined by Tukey’s HSD test single-factor ANOVAs.
Trees at the highest elevation (2187 m a.s.l.) remobilized the highest quantities of sugars (Fig. 1c) and NSC (Fig. 1i) from leaves to shoots (both p < 0.05), compared to trees at other elevations (Fig. 1c, Fig. 1i). The remobilization efficiency of starch from leaves to shoots did not change with elevation (Fig. 1f). The N remobilization from leaves to shoots tended to increase with increasing elevation (Fig. 2c), whereas the P remobilization did not change with elevation (Fig. 2f).
Quercus aquifolioides on Balang Mountain
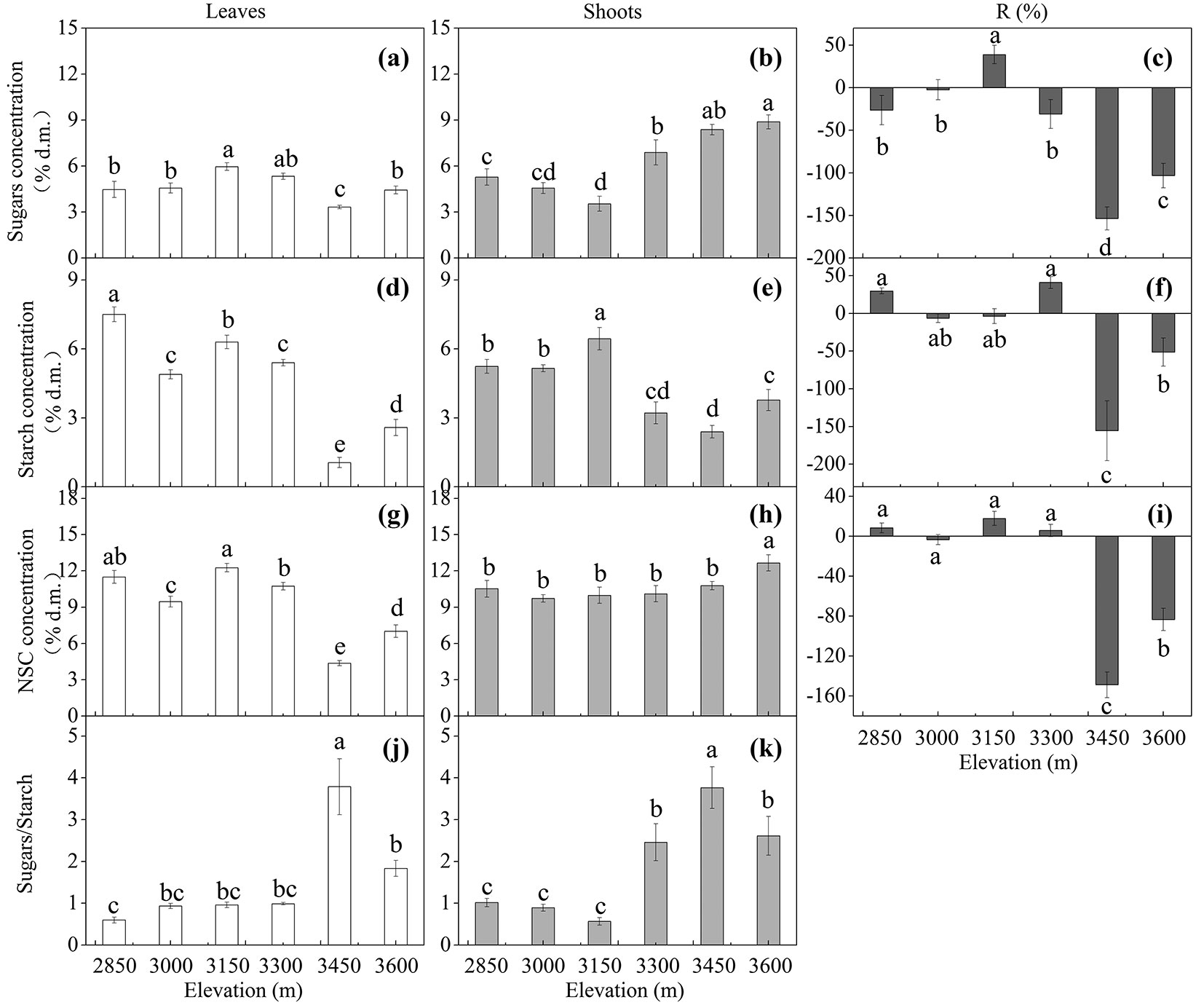
The concentration of leaf sugars had a relatively stable level across the elevational gradient, except for a higher level in trees at 3150 m a.s.l. and a lower level at 3450 m a.s.l. (Fig. 3a), whereas the concentration of shoot sugars tended to increase with increasing elevation (Fig. 3b). Leaf NSC concentration decreased with increasing elevation, with a 39 % decrease between the lowest and highest elevation (Fig. 3g). Shoot NSC concentration was relatively stable across the elevational gradient, with an exceptional higher shoot NSC level in trees at the upper limit (Fig. 3h). Mean NSC concentrations were significantly higher in shoots than in leaves (p < 0.001 - Tab. 4, Fig. 3g, Fig. 3h). The starch concentration was significantly affected only by elevation (p < 0.001 - Tab. 4). The starch concentration in both leaves and shoots tended to decrease with increasing elevation (Fig. 3d, Fig. 3e). The sugars/starch ratio increased significantly with elevation for the two tissue types (p < 0.001), and mean sugars/ starch ratio was significantly higher in shoots than in leaves (p = 0.015 - Tab. 4, Fig. 3j, Fig. 3k).
Fig. 3 - Tissue mean concentration (mean ± SE; % of dry matter) of soluble sugars, starch, non-structural carbohydrates (NSC), sugars/starch ratio, and remobilization efficiency (R %) in Quercus aquifolioides trees grown along the altitudinal gradients on Balang Mountains (n = 6 for each elevational site and tissue type). Different letters indicate significant differences (p<0.05) among elevations as determined by Duncan test single-factor ANOVAs.
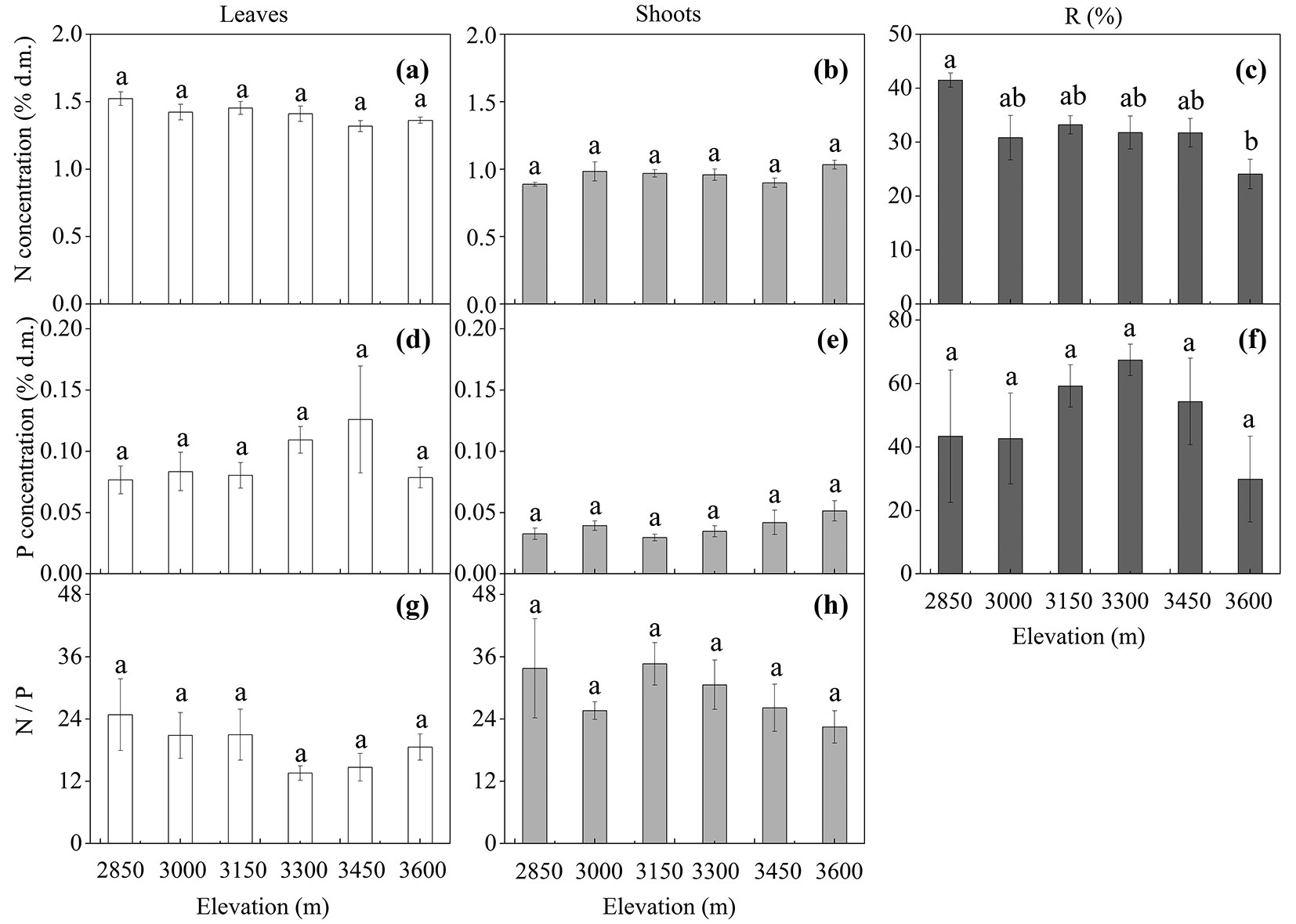
The N (Fig. 4a, Fig. 4b) and P concentrations (Fig. 4d, Fig. 4e), as well as the tissue N/P ratio (Fig. 4g, Fig. 4h), in both leaves and shoots were not affected by elevation. Only tissue type significantly affected N (p < 0.001), P (p < 0.001), and N/P ratio (p = 0.001 - Tab. 4). The N (Fig. 4a, Fig. 4b) and P concentration (Fig. 4d, Fig. 4e) was significantly higher in leaves than in shoots, whereas the mean N/P ratio was higher in shoots than in leaves (Fig. 4g, Fig. 4h).
Fig. 4 - Tissue mean concentration (mean ± SE; % of dry matter) of total nitrogen (N) and phosphorus (P), N/P ratio, and remobilization efficiency (R %) in Quercus aquifolioides trees grown along the altitudinal gradients on Balang Mountains (n = 6 for each elevational site and tissue type). Different letters indicate significant differences (p<0.05) among elevations as determined by Tukey’s HSD test single-factor ANOVAs.
The remobilization of sugars, starch, and NSC from leaves to shoots showed similar elevational pattern (Fig. 3c, Fig. 3f, Fig. 3i). Trees at higher elevations (3450 and 3600 m a.s.l.) remobilized more soluble carbon components from leaves to shoots, compared to trees at lower elevations (Fig. 3c, Fig. 3f, Fig. 3i). The N and P remobilization efficiency was relatively stable across the elevational gradient, with an exception for higher N remobilization in trees at the upper limit (3600 m a.s.l.) than in trees at lower elevations (Fig. 4c, Fig. 4f).
Discussion
End-season remobilization of soluble carbon components
Regardless of leaf habit, the concentration of NSC in leaves tended to decrease with increasing elevation (Fig. 1g, Fig. 3g), while being relatively stable across the elevational gradients in shoots (Fig. 1h, Fig. 3h). Richardson ([39]) and Li et al. ([24]) showed that plants suffering from environmental stress had lower tissue NSC concentrations. However, Li et al. ([26]) found that plants grown with low water availability had higher tissue NSC concentrations, because drought may restrict plant growth rather than reduce the rate of photosynthesis, leading to an accumulation of NSC ([41]). Contradicting results on the variation of NSC, sugars, and starch concentrations with elevation are reported in the literature (increase, steady, decrease - [16], [24], [13]).
Our results indicated that mobile carbohydrates for both deciduous and evergreen tree species were remobilized from leaves to shoots at the end of season, especially in the evergreen species (Fig. 1c, Fig. 1f, Fig. 1i, Fig. 3c, Fig. 3f, Fig. 3i). This finding partly confirmed our first hypothesis that both deciduous and evergreen trees reallocate carbon resources from leaves to shoots at the end of growing season. Leaf carbon components decreased at the end of growing season in order to maximize resource allocation, i.e., during leaf senescence, recycling nutrients from assimilating leaves back to storage organs ([6]). Stored resources may support new leaf and shoot growth after dormancy ([47]), as well as defense and reproduction ([6]). Many studies have shown that leaves play a major role in resources remobilization ([33], [10], [7]). However, few studies have revealed that woody tissues of evergreen trees could be potential carbon ([22]) or N pools ([40]). The availability of carbohydrates is strictly dependent on and connected to the growth and development of leaves and shoots, the partitioning of carbohydrates being determined by resource availability, growth capacity and maintenance requirement ([31]). Several studies have highlighted the function of leaves as storage of nutrients, and resources remobilized from leaves to support new growth ([1], [7]). The evidence showing that leaves have a primary role in resource remobilization in comparison with woody tissues seems to be overwhelming.
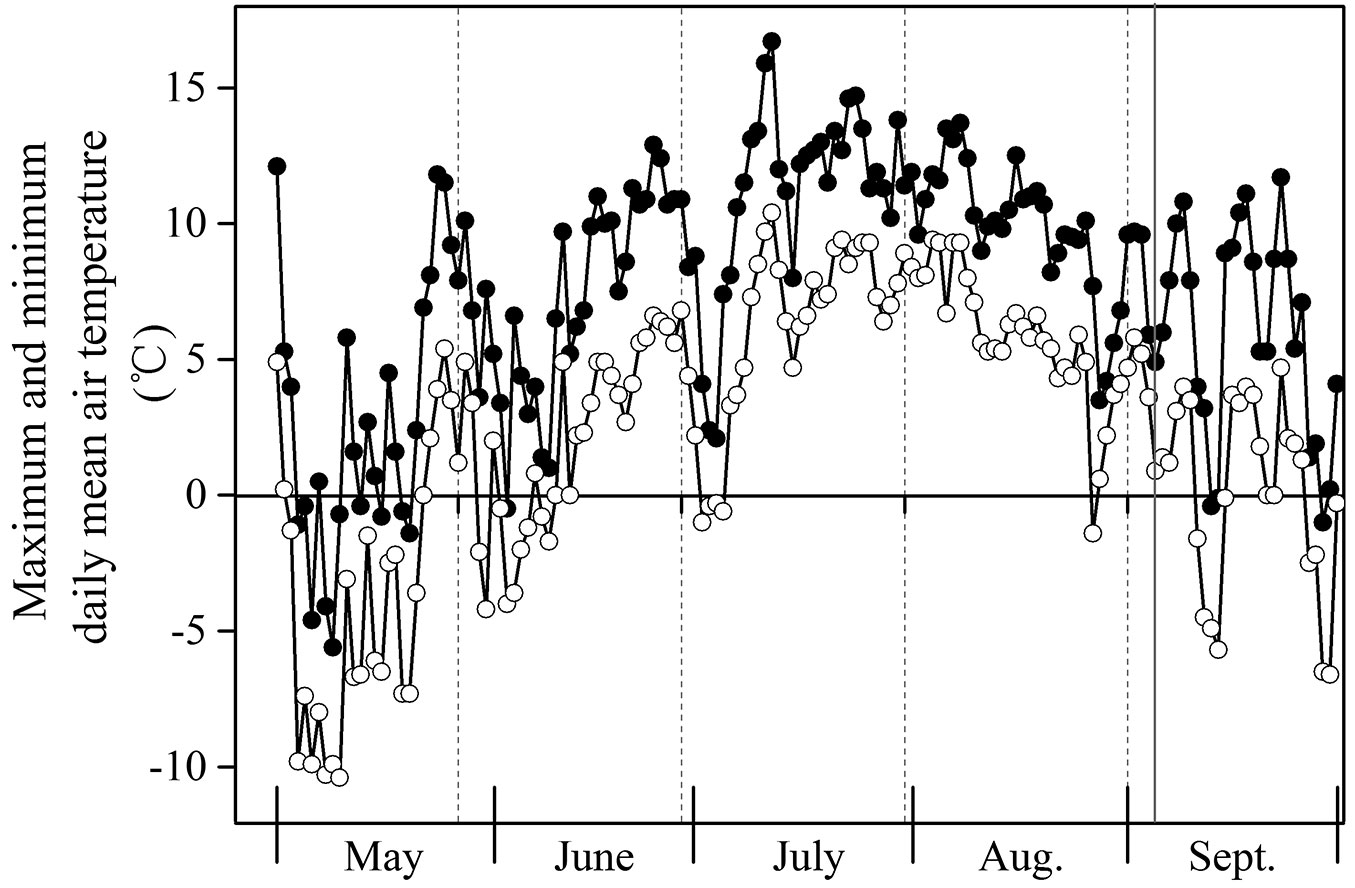
We expected that the deciduous species had higher resource remobilization efficiency from leaves to shoots at the end of the growing season than the evergreen species. However, our results revealed a significantly lower mean remobilization efficiency of mobile carbohydrates from leaves to shoots in B. ermanii than in Q. aquifolioides at the end of the growing season (p <0.001 - Fig. 1c, Fig. 1f, Fig. 1i vs. Fig. 3c, Fig. 3f, Fig. 3i). In early season, deciduous trees have no photosynthesizing tissues and rely on storage, whereas in evergreen species carbohydrates can be supplied by photosynthesis of previous-years leaf cohorts ([11]). We observed a tendency for pronounced lower remobilization efficiency of soluble sugars in deciduous trees (p <0.001 - Fig. 1a). No significant differences in starch remobilization efficiency were observed between these tree species (p = 0.742 - Tab. 3). Contrary to our results, lower carbohydrate contents allocated to storage were more frequently found in evergreen than in deciduous species ([46]). A possible reason explaining results of the present study was the failure of the deciduous trees to efficiently remobilize carbohydrates reserves to storage. Previously, Ziegler ([50]) found that carbohydrates reserves can be inaccessible to storage organs, since they are in dead cells and, therefore, impossible to be retrieved. Pattern of air temperature at Changbai Mt. confirmed this view (Fig. 5). There was a sudden change in temperature, with minimum daily air temperature below zero on August 26, before our sampling date. Due to the effect of flash freezing in cold temperate mountains, carbohydrates in leaves were difficult to remobilize, and were probably forced to storage. In addition, we found that sugars accounted for a larger proportion of NSC than starch did, in both leaves and shoots of deciduous and evergreen trees (Fig. 1 and Fig. 3). Similar results have been reported for other temperate deciduous and evergreen species ([43], [49]). It must be bear in mind, however, that concentration of carbohydrates alone does not allow plant allocation patterns to be completely understood, storage tissues being distributed in roots as well as in stems. Yet, it is possible that allocation patterns vary during the season.
Fig. 5 - Maximum and minimum daily mean air temperature (°C) at Changbai Mountain from late May to late September. Vertical grey line indicates the sampling date.
The remobilization efficiency of sugars, starch and NSC was significantly higher in trees at their upper limit compared to lower elevations (Fig. 1c, Fig. 1i, Fig. 3c, Fig. 3f, Fig. 3i), except for starch in B. ermanii (Fig. 1f). This finding partly confirmed our second hypothesis. The remobilization of soluble carbon components from leaves to shoots increased at the upper limit, which may help trees to withstand low temperature at high elevations. Soluble carbon components may act as a signal molecule to adapt to environmental changes ([42]), because they participate in cell osmotic regulation and prevent intracellular ice formation through lowering cytoplasm freezing point ([34]), to ensure tree survival in winter. Therefore, trees growing at the treeline depend not only on mobile carbohydrates to maintain respiration and growth ([19]), but also on accumulated soluble sugars to prevent intracellular ice formation in winter, which can severely injure trees ([34]). At the end of season, sugars (and starch to sugar conversion) can be unloaded in the direction of sites of storage within the xylem ([3]), to be used as osmoticum for refilling of freeze-thaw induced embolism when the growth season starts. Accumulation of sugars would prime stems for recovery from winter embolism, when stress is relieved, thus maintaining long-distance and long-lasting water transport tissues, i.e., requiring high carbon investments.
Deciduous trees showed a significantly higher mean sugars:starch ratio in tissues (4.33) than evergreen ones (1.70 - Tab. 2). Moreover, we found that sugars:starch ratio in leaves and shoots of Q. aquifolioides increased significantly with elevation. A sugar-starch system that adjusts this ratio in response to low temperature environment can be hypothesized in these trees ([24]). Patton et al. ([36]) revealed that the sugar:starch ratio in Zoysia spp. was positively correlated with cold hardiness, indicating that sugar:starch ratio actively adapted to environmental conditions.
End-season remobilization of nitrogen and phosphorus
There were no elevational effects on tissue N and P concentrations in both tree species (Fig. 2a, Fig. 2b, Fig. 2d, Fig. 2e, and Fig. 4a, Fig. 4b, Fig. 4d, Fig. 4e), except for a lower shoot N concentration in B. ermanii at 1977 m a.s.l., suggesting similar supply and demand ratio. Previous studies have proposed that nutrient accumulation and conservation are adaptive responses that enhance metabolic activity and growth rates in cold climates ([35], [18]). Kutbay & Ok ([21]) reported, however, that absolute and proportion N and P resorption rates did not change significantly along elevational gradients ([17], [25], [27]), which is consistent with our results.
Nutritional elements are crucial to growth, particularly N and P, which are the most important growth limiting nutrients ([4]). N and P that support reproduction come largely (about 50 %) from recycling of nutrients from senescing leaves in deciduous as well as evergreen trees ([5], [6], [1]). However, Vergutz et al. ([44]) showed, after a leaf mass loss correction, general N and P average resorption of 62 % and 65 %, respectively. We observed a significant difference in N remobilization from senescing leaves to shoots between deciduous and evergreen trees; the N remobilization efficiency was significantly higher in deciduous than in evergreen trees (p <0.001 - Fig. 2c vs. Fig. 4c). In line with this result, Aerts ([1]) reported that N resorption efficiency in deciduous shrubs and trees was higher than in evergreen ones. The patterns of N remobilization seem to differ between deciduous and evergreen species ([33]). In contrast to deciduous species that recycle N back into storage organs before leaf abscission, evergreen species retain leaf N for newly developing shoots ([6], [46]). The tendency for significantly higher mean P remobilization efficiency in deciduous species was also shown in our study (p <0.001 - Fig. 2f vs. Fig. 4f). The patterns of N and P remobilization supported our first hypothesis. Other authors, however, have observed that resorption efficiency does not differ significantly between evergreen and deciduous shrubs and trees ([10]).
N/P ratio has been applied to identify thresholds of nutrient limitation ([14]). Based on studies of European wetland plants, thresholds of foliar N/P ratios are < 14 for N limitation and > 16 for P limitation ([14]). According to this view, both B. ermanii and Q. aquifolioides canopies were relatively P-limited (Fig. 2g, Fig. 4g), although these N/P thresholds may indicate nutrient limitations when leaf N or P content is sufficient, but not when their content is deficient ([48]). Leaf N/P ratio did not decrease significantly with increasing elevation, suggesting that N and P limitation did not markedly vary within the present altitudinal range at the end of season. Due to the different remobilization strategy between evergreen and deciduous tree species, deciduous trees may remobilize more P from leaves to shoots under P-limited conditions than evergreen ones, which can be ecologically advantageous in soils that are naturally low in bioavailable P.
Conclusions
In line with our hypotheses, we found that mobile carbohydrates and nutrients (N and P), in both the deciduous and evergreen tree species, were remobilized from leaves to shoots at the end of season, and the remobilization efficiency was significantly higher in trees at the upper limit, helping trees to adapt to low temperature at high elevations. We also found that mobile carbohydrates, N and P had different patterns of leaf-to-shoot remobilization between the two species at the end of growing season. Compared to the deciduous B. ermanii, the evergreen species Q. aquifolioides remobilized more carbon components, but less N and P from leaves to shoots. Differences in resource remobilization between deciduous and evergreen woody species seem to be related to different strategies in using resources ([5]), though they can be over-ridden by interspecies variability. Comparative studies on resource remobilization carried out under similar growth conditions warrant to draw general ecological patterns on species-specific storage processes.
The present study contributes to better understand how trees adapt to growth-limiting temperatures at the alpine treeline, showing that the trees of mountain environments differ in their storage physiology, which may implicate heterogeneous distributional responses to climate warming. A limitation of the present study is that we were not able to quantitatively estimate the pool size of resource remobilization (concentration × biomass) from leaves to shoots ([23]), because destructive biomass sampling was not allowed for both tree species in those nature reserves. Further studies may take advantage of stable isotope labeling (e.g., 13C, 15N) to accurately assess carbon and N remobilization from senescing leaves to shoots, and to determine whether other organs (e.g., roots, seeds) contribute to internal resource remobilization ([32]).
Acknowledgements
This study was supported by the China Global Expert Recruitment Program (the Thousand Talents Plan), the National Key Research and Development Project (2016Y FA0602301), the National Natural Science Foundation of China (41371076; 41601052; 41501089), the China Postdoctoral Science Foundation (2015M580241), and the Fundamental Research Funds for the Central Universities (2412016KJ010). We thank Haibo Du, Kai Liu and Xiangyu Guo (School of Geographical Sciences, Northeast Normal University, Changchun, China) for their assistance in the field.
YC and AW contributed equally to this work.
References
Gscholar
Authors’ Info
Authors’ Affiliation
Fei-Hai Yu
Xue Wang
Zhejiang Provincial Key Laboratory of Plant Evolutionary Ecology and Conservation, Taizhou University, Taizhou 318000, Zhejiang (China)
School of Natural Resources, University of Missouri, Columbia, MO 65211 (USA)
School of Nature Conservation, Beijing Forestry University, Beijing 100083 (China)
Dipartimento di Agricoltura, Ambiente e Alimenti, Università degli Studi del Molise, v. de Sanctis, 86100 Campobasso (Italy)
European Forest Institute (EFI) Project Centre on Mountain Forests (MOUNTFOR), v. E. Mach 1, I-38010 San Michele all’Adige (Italy)
Mai-He Li
Swiss Federal Institute for Forest, Snow and Landscape Research WSL, Zuercherstrasse 111 CH-8903 Birmensdorf (Switzerland)
Corresponding author
Paper Info
Citation
Cong Y, Wang A, He HS, Yu F-H, Tognetti R, Cherubini P, Wang X, Li M-H (2018). Evergreen Quercus aquifolioides remobilizes more soluble carbon components but less N and P from leaves to shoots than deciduous Betula ermanii at the end-season. iForest 11: 517-525. - doi: 10.3832/ifor2633-011
Academic Editor
Silvano Fares
Paper history
Received: Sep 18, 2017
Accepted: May 16, 2018
First online: Aug 01, 2018
Publication Date: Aug 31, 2018
Publication Time: 2.57 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 47897
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 40374
Abstract Page Views: 3372
PDF Downloads: 3300
Citation/Reference Downloads: 19
XML Downloads: 832
Web Metrics
Days since publication: 2772
Overall contacts: 47897
Avg. contacts per week: 120.95
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2018): 13
Average cites per year: 1.63
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Carbohydrate metabolism during new root growth in transplanted Larix olgensis seedlings: post-transplant response to nursery-applied inorganic fertilizer and organic amendment
vol. 10, pp. 15-22 (online: 22 September 2016)
Research Articles
Influences of mature Pinus nigra plantations on the floristic-vegetational composition along an altitudinal gradient in the central Apennines, Italy
vol. 13, pp. 279-285 (online: 03 July 2020)
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Research Articles
Dynamics of humus forms and soil characteristics along a forest altitudinal gradient in Hyrcanian forest
vol. 14, pp. 26-33 (online: 10 January 2021)
Research Articles
Soil respiration along an altitudinal gradient in a subalpine secondary forest in China
vol. 8, pp. 526-532 (online: 01 December 2014)
Research Articles
How environmental factors condition natural regeneration in the altitudinal gradient of a montane rainforest
vol. 17, pp. 132-139 (online: 04 May 2024)
Research Articles
Vertical position of dry mass and elemental concentrations in Pinus sylvestris L. canopy under the different ash-nitrogen treatments
vol. 8, pp. 838-845 (online: 25 March 2015)
Research Articles
Do different indices of forest structural heterogeneity yield consistent results?
vol. 15, pp. 424-432 (online: 20 October 2022)
Research Articles
Auxin (IAA) and soluble carbohydrate seasonal dynamics monitored during xylogenesis and phloemogenesis in Scots pine
vol. 11, pp. 553-562 (online: 01 September 2018)
Research Articles
Functional turnover from lowland to montane forests: evidence from the Hyrcanian forest in northern Iran
vol. 8, pp. 359-367 (online: 16 September 2014)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword