Addressing post-transplant summer water stress in Pinus pinea and Quercus ilex seedlings
iForest - Biogeosciences and Forestry, Volume 8, Issue 3, Pages 348-358 (2015)
doi: https://doi.org/10.3832/ifor1256-007
Published: Sep 16, 2014 - Copyright © 2015 SISEF
Research Articles
Abstract
In central Spain, post-transplant water stress produces high seedling mortality after the first summer following outplanting. Our study was designed to determine whether survival and performance of outplanted stone pine (Pinus pinea) and holm oak (Quercus ilex) seedlings in a burned area could be improved by summer irrigation and mulching and to identify whether there is a species-specific adaptive capacity to respond to treatment and environment. Seedlings were outplanted in March 2011 in 200 planting holes in an area of 1.1 ha. Mulch was added in June; irrigation started in July and was repeated every week until mid-September. The severity of the 2011 summer drought constrained growth rates and photosynthetic characteristics, mainly in the non-irrigated seedlings, whose survival at the end of the year after planting was approximately 2.5%. Stone pine and holm oak seedlings responded more to irrigation than to mulching in terms of shoot growth, biomass and survival. Furthermore, stone pine seedlings were found to be more responsive to the partial alleviation of summer drought than holm oak seedlings. Irrigation alone produced similar results to those obtained when both irrigation and mulching were employed. In conclusion, first year summer irrigation should be considered as a planned adaptation measure in the management of outplanted Mediterranean ecosystems, because once a gravimetrically measured soil moisture level as low as 2% is achieved seedling survival and physiological performance can be guaranteed. However, the repercussions for the potential persistence of both species in the area will not only be related to the recurrence and intensity of summer droughts but also to drought duration.
Keywords
Stone Pine, Holm Oak, Irrigation, Drought, Seedling Survival, Physiological Traits
Introduction
Successful seedling establishment is the greatest challenge to new plantings in the Mediterranean environment ([9]), where seedling mortality rates are generally high after the first summer following outplanting ([11]). Newly established seedlings must be capable of “coupling” to the forest ecosystem in order to restore their physiological functioning and initiate growth ([24]). Inadequate contact between roots and soil leads to increased water stress, which in turn can result in carbon starvation, hydraulic failure, and consequent seedling death ([25]). A delay in such “coupling” can be particularly detrimental in Mediterranean ecosystems, which are characterized by high seasonality and where soil water availability is the primary factor affecting a successful establishment. Factors determining low survival rates and slow growth during the first summer of outplanted Mediterranean species can be highly variable and complex, often involving interaction and mutual influence between factors ([31]). These factors include summer drought, excessive solar radiation, nutrient-poor soils, herbivore pressure, and competition with shrubs and herbaceous vegetation. Summer drought, which is characterized by declining soil water content and increasing vapor pressure deficit, induces stomatal closure, reduces photosynthetic capacity, stomatal conductance and electron transport rates ([14]). According to their drought resistance, plants can be classified as either drought tolerant (achieved by turgor maintenance and/ or desiccation tolerance) or drought avoidant (related to stomatal closure and increased root growth relative to shoots - [34]).
Different post-planting treatments have been used to improve seedling establishment under Mediterranean conditions: soil preparation ([19]), irrigation, mulching, use of nurse plants [21]), tree-shelters ([46]), runoff harvesting ([64]), neighboring vegetation control [9]), fertilization ([66], [67]). The combination of summer irrigation and the use of organic mulch around the newly planted seedlings can potentially increase first year survival and early growth of planted seedlings. Results with regard to the effectiveness of organic mulch in forest plantations have been variable, but in those cases where mulch application positively affected growth, this was attributed to reduced soil water loss ([1], [15]) and increased water infiltration ([57]). The use of irrigation is common in reforestation in Spain, increasing first summer survival and growth under harsh environmental conditions ([48], [49], [56]), although due to the increasing economic cost and the concern that watered seedlings display decreased drought tolerance and increased demand for water, this cultural practice is unadvisable. Previous studies have addressed the potential growth response of plantations to irrigation, as well as the physiological mechanisms related to the growth response ([31], [12], [15]).
Stone pine (Pinus pinea L.) typically forms mixed stands with holm oak (Quercus ilex L.) in the Tiétar and Alberche valleys in central Spain. Most of these stands are located on steep slopes, with sandy soils, under a genuine continental Mediterranean climate, where summer drought is the primary determining factor in the success of vegetation establishment. The traditional, multifunctional management of these stands was based on the production of pine nuts, livestock and holm oak firewood production. However, the dramatic decrease in the use of firewood as a fuel source, the increased risk of wildfire as well as overgrazing and the maintenance of large nut producing trees has led to open pine stands with a dense holm oak coppice understory. Burned areas are commonly occupied primarily by holm oak due to its early resprouting capacity, which gives it an advantage over stone pine regeneration, which relies solely on early seed germination from its canopy seedbank ([46]). Hence, the prevalence of stone pine is declining in these burned areas, although the failure of stone pine regeneration to recruit is not fully understood, particularly since residual large parent producer trees (“green islands”) can be found ([37]). Moreover, individual stone pine trees or groups of trees usually survive in burned areas because of their thick bark and their umbrella-like shape ([50]). In summer 2003, 600 ha of these mixed stands were burned. Since then, the Regional Forest Service has repeatedly tried to reforest the area with stone pine seedlings. However, even where summer irrigation has been used, none of these efforts have been successful to date. Heavy losses of plants have occurred through desiccation in the dry season as transplants were carried out in open, fully sun-exposed sites.
To determine the potential of summer irrigation and the application of pine bark mulch as treatments for improving first-year planting success in a burned area, we established a stone pine-holm oak plantation trial study in the western part of Madrid. Both species are particularly susceptible in summer to the high irradiances and drought conditions, which are quite different from the shaded and well-watered conditions in the nursery where they were grown. Under frequent water-limiting conditions, Mediterranean plants have adapted to optimize water use, allowing them to survive and grow ([10]). In the open conditions of plantations, there is scarce shade to mitigate the impacts of summer drought stress on seedling survival and growth ([29], [54], [47]). Interspecific differences in morphological and physiological responses to summer drought stress will likely modulate seedling growth ([14], [25]). The ability of both species to adjust physiologically to field conditions will indicate their different degree of acclimation and this may serve to enhance the seedling establishment success rate in reforestation ([27], [64]). Both species need a deep, soft soil, with some plant cover to retain soil moisture ([13], [17]). If the water table is absent and precipitation is scarce, as occurs in the study area, irrigation will partially compensate such water deficit.
The aim of this experiment was to define a reforestation strategy for this burned area. Prior to setting up the experiment, the area was occupied by approximately 10-15% of the originally planted pine seedlings and a sprouted holm oak and rockrose (Cistus ladanifer) understory that is slowly colonizing the whole burned area. The objective was to determine how summer irrigation and pine bark mulch can ameliorate the impact of summer drought on first-year survival and to identify the morphological and physiological responses of the plants, which in turn might explain the underlying mechanisms that enable stone pine and holm oak to withstand transplant. The specific objectives were: (1) to compare the effect of post-planting treatments on seedling survival and performance; (2) to determine the different physiological ability of each species to recover once water stress is alleviated; (3) to compare stone pine and holm oak with respect to their acclimation to summer drought; and (4) to identify their strategies to withstand summer drought. Thus, we attempt to determine whether one species outperforms the other in terms of seedling survival, growth or physiology.
Materials and Methods
Study site
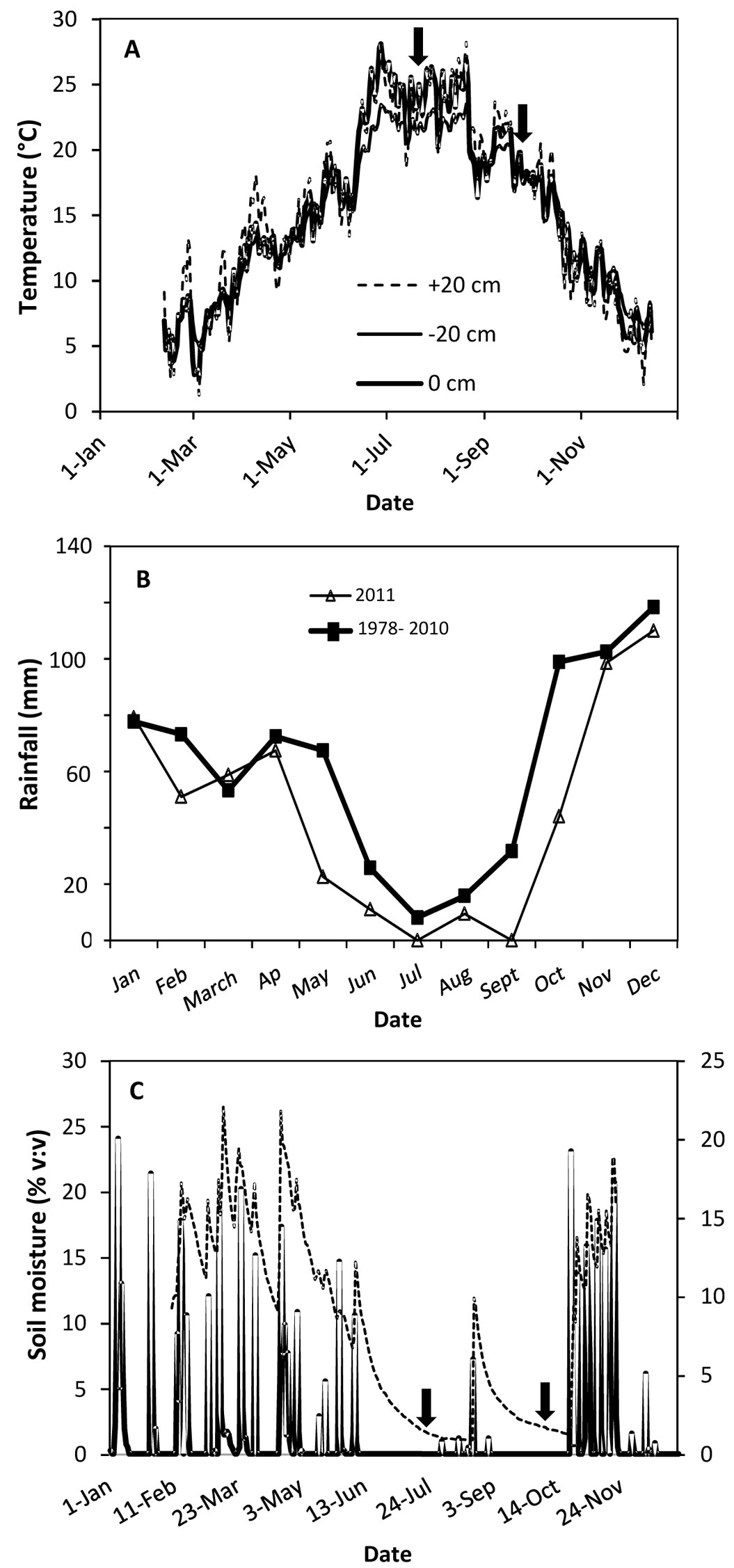
The study site is located west of Madrid (40° 23′ 25″ N, 4° 18′ 7″ W) at 650 m a.s.l. Mixed stands of stone pine (Pinus pinea L.) and holm oak (Quercus ilex L.) occupy approx. 19 000 ha on the rocky granitic slopes that define the valleys of the Alberche and Tiétar rivers. The orography is characterized by a succession of hills and small valleys, with steep slopes defining an abrupt landscape ([6]). Soils are poor, mostly sandy, with high presence of granite rocks in the surface layers. The long history of human-induced disturbances (mainly overgrazing and wildfires) has led to a relatively low vegetation cover composed of an understory of resprouted holm oak and rockrose and large areas of bare soil exposed to full sunlight. The climate is continental Mediterranean (annual precipitation is 745 mm and mean annual temperature is 12.5 °C), defined by long, hot and dry summers (mean monthly precipitation from June to September is around 20 mm) along with irregular and scarce autumn and spring precipitations. Mean monthly temperatures during the study period (March to December 2011) were similar to historical means, but precipitation was 49% lower (Fig. 1a, Fig. 1b).
Fig. 1 - (A) Air temperature (+20 cm), soil temperature at the surface (0 cm) and soil temperature at 20 cm deep (-20 cm) in 2011; (B) monthly rainfall in 2011 vs. mean monthly rainfall in the 1978-2010 period; and (C) daily soil moisture (thick line) and daily rainfall (dashed line) during 2011 in the area of study. Arrows indicate when the physiological measurements were done (July 22 and September 23)
Treatments
Stone pine and holm oak seeds were collected at the study site and sown in autumn 2010 in FP-300 containers. Seedlings were grown under standard operational conditions (peat substrate, slow releasing fertilizer, irrigation to field capacity). In March 2011, pairs of these stone pine and holm oak containerized seedlings were hand planted into the same hole (200 holes in total, 0.5 m2, 200 stone pine seedlings and 200 holm oak seedlings) in a steeply sloping (62.2%) area of 1.1 ha. Mean height of seedlings when planted was 12.4 ± 1.6 cm (stone pine) and 14.6 ± 3.3 cm (holm oak). The trial was established within the perimeter of the 600 ha of mixed stands burned in 2003. We used the same planting holes that had been repeatedly used in previous years by the Regional Forest Service for unsuccessful stone pine reforestations. Hand-made planting holes (0.8×0.7×0.5 m) were arranged in ten rows following contour lines, 20 holes per row. The holes were initially cleared of competing vegetation and no further vegetation control was necessary during the study period. The experiment was setup as a completely randomized design with two factors (irrigation and organic mulching), with two levels per factor (irrigation, W+ vs non-irrigation, W0; mulching, M+ vs non-mulching M0), for a total of four treatment combinations: W0M0 (no irrigation, no mulching), W0M+ (no irrigation, mulching), W+M0 (irrigation, no mulching) and W+M+ (irrigation and mulching).
Pine bark was used as organic mulching and was applied on June 7 in alternate planting holes (that is, in 100 planting holes: 100 stone pine seedlings and 100 holm oak seedlings). The area covered by the mulching was approximately 0.7 m diameter and 20 cm deep, around the seedlings. Mean length and diameter of pine bark granules were 2.0 cm and 0.8 cm, respectively. Irrigation started on July 20 because due to severe summer water restrictions in the region, irrigation of afforested stands is only permitted after a month without rainfall and a mean temperature above 20 °C. Seedlings still alive by July 20 (138 stone pine seedlings and 99 holm oak seedlings) were randomly divided into two groups, one of which was irrigated every 7 days until September 15 (W+, 69 stone pines seedlings and 49 holm oak seedlings) and the other group was treated under natural drought conditions (W0, 69 stone pines seedlings and 50 holm oak seedlings). Forty l m-2 of water was added on every irrigation date.
Measurements and harvests
Hemispherical photographs were taken to characterize the light environment at each planting hole. A global site factor (GSF, %) was calculated at each planting hole based on a solar transmissivity of 0.8 and 0.1 diffuse:direct radiation (Hemiview 2.1, Canopy Analysis Software, Delta-T Devices Ltd., Burwell, Cambridge, UK). On July 22 and September 23, coinciding with the physiological measurements, soil samples were taken in each planting hole from the first 20 cm, and surface soil moisture was calculated gravimetrically. This soil moisture calculation was used as a proxy of plant water status, which reflects the extent of water stress ([5]) because seedlings had no lateral branches to carry out destructive measurements of leaf water potential. To characterize the environmental conditions in the study area, air temperature and relative humidity at a height of 20 cm above the ground and soil water content at a depth of 20 cm were continuously monitored using a HOBO micro-weather station (Micro-HWS, ONSET, MS, USA).
Survival was monitored every 25-30 days from May 9 to July 20, every 7 days during the irrigation period irrigation, and every 15 days once irrigation ceased and until the end of the experiment on December 12. Initial seedling height (H) and root collar diameter (d) were measured one week after planting. In addition, seedling height and root collar diameter were measured after the mulching application (June 7), at the beginning and end of the irrigation period (July 20 and September 15), and at the end of the experiment (December 12).
Chlorophyll a fluorescence and gas exchange parameters were measured at mid-morning (10 a.m.-12 p.m.) on the main shoot of seedlings on two occasions: at the beginning and end of the irrigation period (July 22 and September 23). On July 22, eight stone pine seedlings and 3-4 holm oak seedlings per treatment combination were measured. On September 23 all living seedlings of both species were measured. However, by this date, there were only 1-2 surviving seedlings in W0M0 and W0M+, hence, these two treatment combinations were discarded from the statistical analysis. A portable infrared gas analyzer (IRGA LCA 4 Analytical Development Corporation, UK) was used to measure photosynthesis (An, μmol CO2 m-2 s-1) and stomatal conductance (gs, mol H2O m-2 s-1). Photosynthesis (An) and gs were expressed on a projected leaf area basis that was determined after harvest. Vapor pressure deficit (VPD, KPa) was calculated from air temperature and relative humidity. Chlorophyll a fluorescence was measured with a portable pulse-modulated fluorometer (FMS 2, Hansatech Instruments Ltd., UK). The yield of photochemistry in PSII (ΦPSII) was calculated according to Kramer et al. ([32] - eqn. 1):
where F´m is the maximal fluorescence in light and Fs is the steady-state light-adapted fluorescence.
Apparent photosynthetic electron transport rate (ETR) at midday was calculated following Rosenqvist & Van Kooten ([52] - eqn. 2):
where PPFD is the photosynthetic photon flux density.
Whole-seedling harvests were conducted on June 7 (to obtain initial dry mass before mulching application) and December 12 (end of the experiment). Six to eight seedlings were harvested on June 7, and almost all living seedlings (18 stone pine seedlings and 9 holm oak seedlings, corresponding to W+M+ and W+M0) were harvested on December 12. At both harvests, seedlings were excavated and stored on ice until they were processed. Seedlings were dried in a forced-air oven at 70 °C until constant mass and weighed (total, TDW and root, RDW). Relative root growth rate (RGR) was calculated between June 7 and December 12, as described in Hunt et al. ([30]). The ratio between root dry weight and total dry weight (RMR = RDW/TDW) was also calculated.
Statistical analysis
Total biomass (TDW) and root biomass (RDW) were related to height (H, in mm) and root collar diameter (d, in mm) through a linear function adjusted for our data, according to Ruiz-Peinado et al. ([53]), in order to estimate biomass weight over time (eqn. 3, eqn. 4):
for Pinus pinea and (eqn. 5, eqn. 6):
for Quercus ilex.
Shoot height, root collar diameter, total biomass growth and root biomass over time were analyzed through a general mixed model for repeated measurements ([65]). A logarithm model was fitted to the data (eqn. 7):
where Yij is height, or root collar diameter, or total biomass, or root biomass for the i-th individual measured at time tj (i=1, …, N; j=1, …, ni); tj indicates the logarithm of the number of days from the start of the mulching treatment (92 days from plantation, tj = log (measuring date-91); intercepts β0i and time effect slopes β1i are divided into a common population fixed component β0 and β1, defining population marginal response; and random plant specific parameters ui and vi, characterize subject-specific individual response ([65]). Contrast for homogeneity of slopes between the different levels of treatments (irrigation: W, mulching: M, and their interaction: W×M) was also carried out. Preliminary analysis revealed no differences between intercepts associated with treatment effects.
Seedling physiology was tested separately for each species and measuring date. For the July 22 measurement, a two-way analysis of variance was tested, while on September 23 it was only possible to test the effect of mulching on the irrigated seedlings because of the lack of sufficient replicates in the non- irrigated treatment. Significant differences between treatments within the same species and day of measurement were evaluated by a multiple means comparison test. In addition, we defined the vigor status as the quotient (rate between 0 and 1) between the percentages of green leaves within a seedling in two successive survival measurements. This quotient is an indicator of the loss of seedling vigor and was calculated over the periods July 20 - August 4 and September 15 - 29. The relationship between the vigor status on these dates and both the net assimilation rate measured on July 22 and September 23 for each plant and soil moisture in the planting hole was evaluated by fitting a logistic function and comparing fitted parameters and pattern of survival between species.
In the analysis of seedling survival, we must consider that our data included two kinds of censoring. Some seedlings (n = 12 holm oak; n = 37 stone pine) survived the entire period (i.e., right censored), while for the rest of seedlings (n = 178 holm oak; n = 162 stone pine) we only know the interval in which they died (i.e., interval censored) and the length of the interval (between 6 and 42 days). Since our aim is to determine the effect of irrigation and mulching on seedling survival, we consider survival time to be the period between the mulching application and seedling death or until the end of experiment. The survival analysis was then performed in three steps. In a first step, we estimated and represented the survival distribution of stone pine and holm oak seedlings in each treatment using a nonparametric maximum likelihood estimator (NPMLE), which is computed through the algorithm developed by Wellner & Zhan ([68]). In a second step, we used two generalized log-rank tests developed for interval-censored failure time data to test the hypothesis of whether there was a significant treatment effect or not ([70], [62]). These two firsts steps of the survival analysis were implemented in SAS using the macros developed by So & Johnston ([58]). Finally, to get an estimate of the treatment effect and to adjust the analysis for other covariates, we fitted a parametric model for interval-censored survival data with time-dependent covariates ([60]) using a regression approach based on the Weibull distribution as implemented in the SAS macro written by Bautista & Sparling ([3]). The regression model used (i) treatments: irrigation (1 when irrigated, 0 otherwise), mulching (1 if mulching, 0 otherwise), (ii) seedling traits: initial seedling height and root collar diameter measured at mulching treatment, and (iii) environmental covariates: global site factor, soil water content, and daily values of relative humidity, maximum air temperature and vapor pressure deficit.
All computations were performed in SAS version 9.2 software (SAS Institute, Cary, NC, USA).
Results
Environmental data
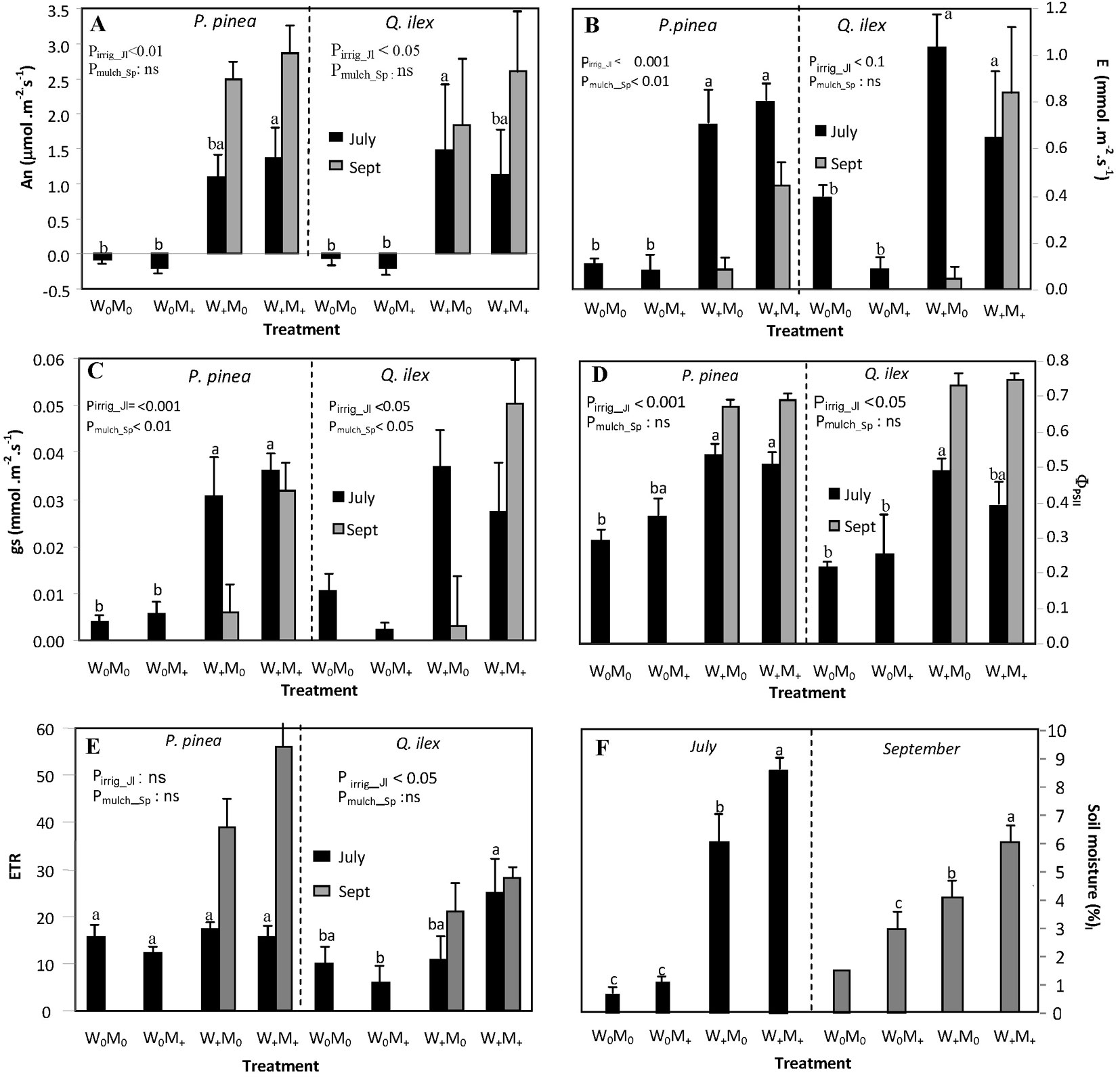
The year 2011 was particularly dry during late spring, summer, and early autumn in comparison with the previous 12 years (Fig. 1b). The last rainfall event (>0.5 mm) in late spring occurred on June 7 followed by a period of 54 days with no rain. Four rainfall events during August (9.4 mm in total) did not alleviate dry soil conditions, which persisted until October 23 (Fig. 1c). Total rainfall between June 7 and October 22 was 10.2 mm (2.3 % of total annual rainfall in 2011). Relative air humidity during this period was 44.7 ± 0.96 % (data not shown), which corresponded to a daytime VPD of 2.9 ± 0.09 KPa (data not shown). Soil water content at a depth of 20 cm fell by 85.3 % between March and almost the end of October (Fig. 1c). The irrigation treatments during the summer months successfully raised the soil water moisture content relative to non-irrigated planting holes. Surface soil water content calculated on the dates of the physiological measurements was positively affected by both irrigation (p<0.001) and mulch (p<0.01 - Fig. 2F). Although irrigation reduced soil temperature slightly, differences between treatments were not significant (data not shown). Mean GSF in the plot (used to characterize the light environment) was 0.80±0.01, i.e., plants grew under full sunlight.
Fig. 2 - Mean physiological parameters and soil moisture measured on July 22 and September 23 for each treatment combination: (A) net assimilation rate (An, µmol m-2 s-1); (B) transpiration rate (E, mmol m-2 s-1); (C) stomatal conductance (gs, mol m-2 s-1); (D) yield of photochemistry in PSII (ΦPSII); (E) apparent photosynthetic electron transport rate (ETR); and (F) mean soil moisture per treatment (%). Physiological parameters were measured separately in Pinus pinea and Quercus ilex seedlings, while soil moisture represents the mean value for each planting hole where a pair of Pinus pinea and Quercus ilex seedlings was planted. For the physiological parameters, different letters show significant differences in July between treatment combinations for each species. For soil moisture, different letters show significant differences between treatment combinations and measurement day (no letter is shown for W0M0 in September, because the value refers only to one planting hole). P-values show the effect of irrigation in July and the effect of mulch for irrigated seedlings in September. No letter separation is included in September as only two treatments are shown.
Shoot height, root collar diameter and biomass over time
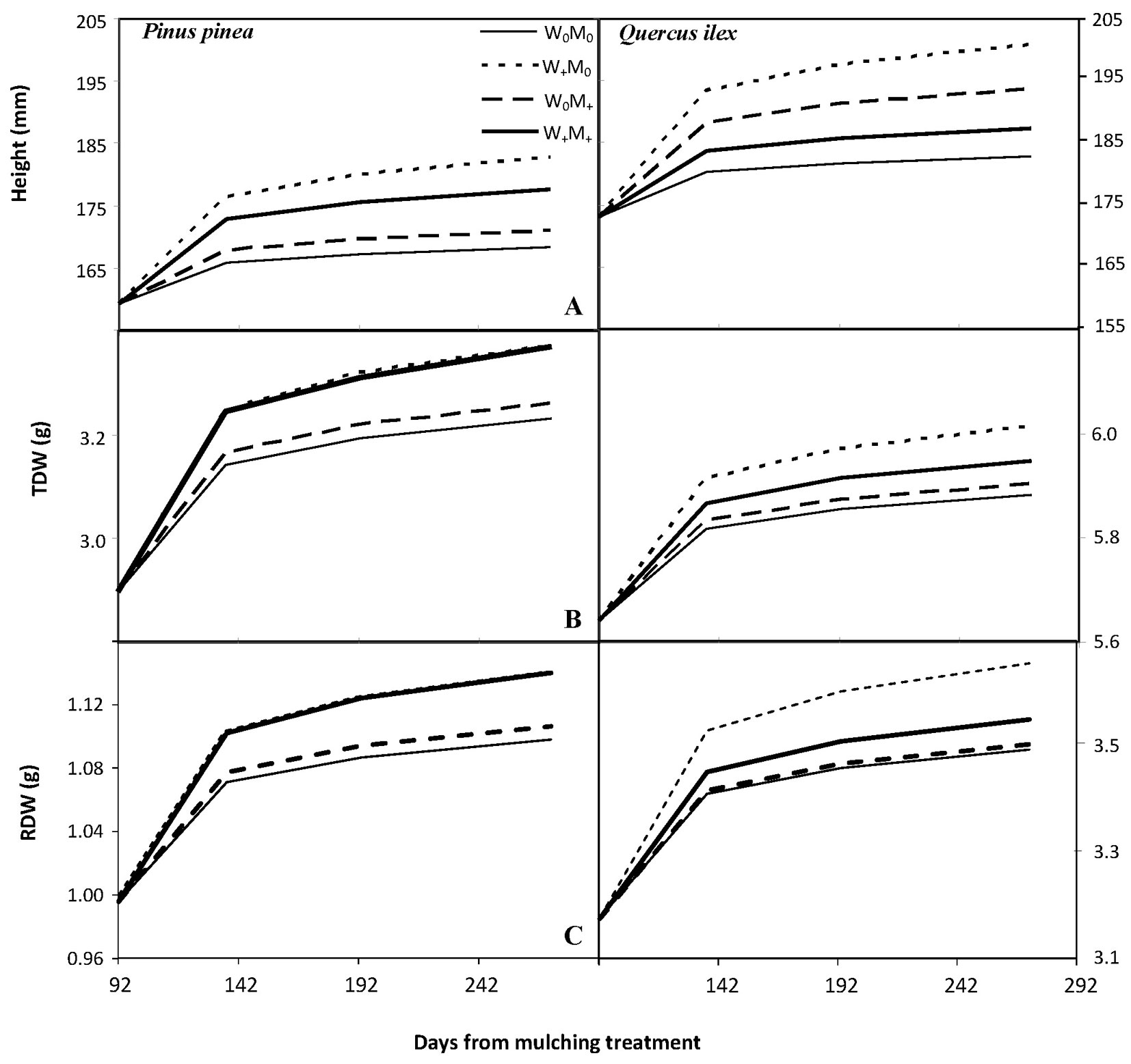
Significant increases (p<0.001, Tab. 1) were recorded in seedling height (between 5.6% and 14.7% in stone pine and between 5.6% and 16.1% in holm oak, Fig. 3) and root collar diameter (between 18.2% and 21.3% in stone pine and 12.7% and 20.1% in holm oak, data not shown) over time. The initial difference in height between species (159.3 mm for stone pine and 173.1 mm for holm oak, p<0.001) was maintained until the end of the experimental period. Irrigation had a significant and positive effect over time on stone pine shoot height growth, regardless of mulching (p<0.001, Tab. 1, Fig. 3). The effect of time (p<0.001) and the mulching × irrigation interaction (p<0.05) was significant for holm oak shoot height growth (Tab. 1). For both species, there was no significant treatment effect on root collar diameter growth over time (Tab. 1). For both species there was a significant positive effect of time (p<0.001) and a slight effect of irrigation over time (p<0.1) on total biomass (TDW), with no significant mulching effect (Tab. 1, Fig. 3). In addition, irrigated stone pine seedlings significantly increased (p< 0.001, Tab. 1, Fig. 3) their root biomass (RDW) over time (16.4% increase in irrigated seedlings vs 12% in non-irrigated seedlings). By the end of the experiment, holm oak seedlings showed higher RDW (p<0.001, RDWholm oak = 3.59 g, RDWstone pine = 1.19 g) and root mass ratio (RMR, p<0.001, RMRholm oak = 0.59 ± 0.07 g g-1, RMRstone pine = 0.350 ± 0.08 g g-1) than stone pine seedlings; however, by December 12, root growth rate (RGR) of stone pine seedlings was higher than that of holm oak (RGRholm oak = 0.00027 day-1, RGRstone pine = 0.00033 day-1, p<0.01).
Tab. 1 - Results of the shoot height, root collar diameter growth, total dry weight and root dry weight over time analyzed by a general mixed model for repeated measurements in Pinus pinea and Quercus ilex. The general structure for the general mixed model is shown in eqn. 1: Yij = β0i + β1i·tj + εij, where tj indicates the number of days from the start of the mulching treatment [92 days from plantation; tj = log(measuring date - 91)]. (W): irrigation treatment; (M): mulch treatment; (df): degrees of freedom in denominator.
| Variable | Effects | Pinus pinea | Quercus ilex | ||||
|---|---|---|---|---|---|---|---|
| df | F-value | P-value | df | F-value | P-value | ||
| Height | t j |
105 | 123.27 | <0.0001 | 60 | 46.46 | <0.0001 |
tj × W |
75 | 14.61 | 0.0003 | 32 | 1.25 | 0.2724 | |
tj × M |
75 | 0.17 | 0.6823 | 32 | 0.06 | 0.8112 | |
tj × W × M |
75 | 2.03 | 0.1587 | 32 | 5.29 | 0.0281 | |
| Root collar diameter | t j |
105 | 93.24 | <0.0001 | 60 | 81.47 | <0.0001 |
tj × W |
75 | 0.08 | 0.7763 | 32 | 1.26 | 0.2703 | |
tj × M |
75 | 0.25 | 0.6171 | 32 | 0.45 | 0.5061 | |
tj × W × M |
75 | 0.01 | 0.9125 | 32 | 0.65 | 0.4250 | |
| Total Dry Weight | t j |
105 | 147.9 | <0.0001 | 60 | 127.13 | <0.0001 |
tj × W |
75 | 3.61 | 0.0611 | 32 | 2.9 | 0.0980 | |
tj × M |
75 | 0.05 | 0.8270 | 32 | 0.2 | 0.6613 | |
tj × W × M |
75 | 0.06 | 0.8048 | 32 | 0.71 | 0.4069 | |
| Root Dry Weight | t j |
105 | 150.25 | <0.0001 | 60 | 118.82 | <0.0001 |
tj × W |
75 | 3.73 | 0.0571 | 32 | 2.35 | 0.1348 | |
tj × M |
75 | 0.04 | 0.8378 | 32 | 0.47 | 0.4966 | |
tj × W × M |
75 | 0.05 | 0.8190 | 32 | 0.72 | 0.4021 | |
Fig. 3 - Population marginal response of (A) shoot height (mm), (B) total biomass (TDW, g) and (C) root biomass (RDW, g) over time for each treatment and species. The general structure of the mixed model is shown in eqn. 1, while population marginal response is given by Yi= β0 +β1 log(tj), where tj indicates the number of days from the start of the mulching treatment (92 days from plantation, tj = measuring date -91). Scale of the y-axis for TDW and RDW is different on both species for clarity.
Gas exchange and chlorophyll fluorescence
Gas exchange and chlorophyll fluorescence measurements (Fig. 2) were intimately related to environmental conditions at the time of measurement (Fig. 1). In July, the effect of irrigation on stone pine seedling physiology was significant irrespective of mulching. Net photosynthetic rate (An) was the most responsive parameter to irrigation, being 97% lower in non-irrigated than in irrigated stone pine seedlings (1.22 in W+ vs -0.13 µmol m-2 s-1 in W0). Values of the physiological parameters in non-irrigated stone pine seedlings were 88% lower for transpiration rate (E: 0.75 in W+ vs 0.09 mmol m-2 s-1 in W0), 86% lower for stomatal conductance (gs: 0.033 in W+ vs 0.0048 mol m-2 s-1 in W0), and 38% lower for the yield of photochemistry in PSII (ΦPSII: 0.52 in W+ vs 0.33 in W0), in comparison to irrigated seedlings. Irrigation in July affected positively An (137% increment compared to non-irrigated seedlings, 0.98 in W+ vs -0.1346 µmol m-2 s-1 in W0), gs (82% increment, 0.032 in W+ vs 0.006 mol m-2 s-1 in W0), ΦPSII (45% increment, 0.434 in W+ vs 0.238 in W0) and apparent photosynthetic electron transport rate (ETR: 45% increment, 18.7 in W+ vs 7.7 in W0) in holm oak seedlings. Holm oak showed higher transpiration rates (62% higher, p<0.01) and stomatal conductance (64% higher, p<0.05) than stone pine in the absence of treatment (i.e., W0M0). By September, net photosynthetic rates were positive for all seedlings measured. The irrigated stone pine seedlings grown with mulching showed significantly higher values of E (0.44 in M+ vs 0.084 mmol m-2 s-1 in M0) and gs (0.032 in M+ vs 0.006 mol m-2 s-1 in M0) than irrigated seedlings with no mulching. The effect of mulching on irrigated holm oak seedlings was significant for gs (0.05 in M+ vs 0.003 mol m-2 s-1 in M0). No differences between species were found in September.
Seedling survival
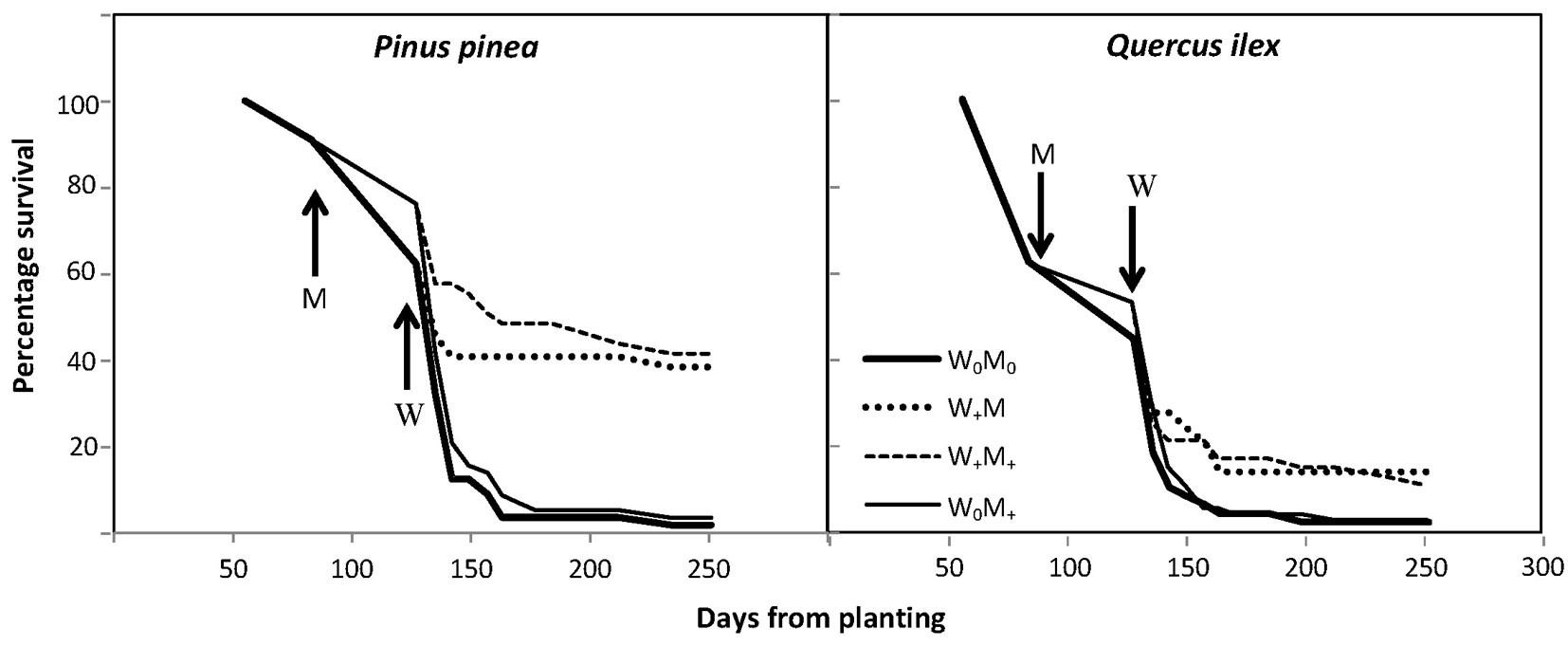
Seedling survival over time was higher in the irrigated seedlings, mainly in stone pine. When the irrigation treatment commenced, survival ranged from 44 to 53% in holm oak and from 62 to 76% in stone pine, the higher values always corresponding to seedlings grown with mulching. Final survival rates in December 2011 were 12.2% in irrigated seedlings and 2.4% in non-irrigated seedlings for holm oak, and 39.9% in irrigated seedlings and 2.6% in non-irrigated seedlings for stone pine (Fig. 4, Tab. 2). The few non-irrigated seedlings of each species that survived were located in a more favorable microenvironment, with GSF ≈ 0.5-0.7. In those holes where only one of the species survived and grew, no effect of the lack of competition for nutrients and water was observed.
Fig. 4 - Percentage of survival of Pinus pinea and Quercus ilex over time, according to treatment. Arrows indicate when the mulching (M) and irrigation (W) treatments started.
Tab. 2 - Parameter estimates, standard errors, Wald tests and hazard ratios of the Weibull seedling survival model for both species. (GSF): Global Site Factor; Diameter (mm); Height (mm); (Tmax): daily maximum temperature (°C); (VPD): vapor pressure deficit (KPa); (RH): air relative humidity (%); (SWH): soil water humidity calculated gravimetrically.
| Species | Parameter | Estimate | Standard Error |
Wald | Hazard Ratio |
|
|---|---|---|---|---|---|---|
| χ2 | p | |||||
| P. pinea | Intercept | -7.4683 | 4.7063 | 2.5182 | 0.1125 | 0.0006 |
| Irrigation | -1.6929 | 0.2532 | 44.6987 | <0.0001 | 0.184 | |
| GSF | 1.0677 | 0.7407 | 2.0779 | 0.1494 | 2.9087 | |
| Height | -0.0168 | 0.0051 | 10.9652 | 0.0009 | 0.9834 | |
| Diameter | -0.2163 | 0.1614 | 1.7974 | 0.18 | 0.8055 | |
| Tmax | 0.274 | 0.0668 | 16.8237 | <0.0001 | 1.3153 | |
| VPD | -0.9734 | 0.8598 | 1.2817 | 0.2576 | 0.3778 | |
| RH | 0.0695 | 0.0522 | 1.7748 | 0.1828 | 1.072 | |
| SWH | 0.0198 | 0.0615 | 0.1039 | 0.7472 | 1.02 | |
| Q.ilex | Intercept | -13.127 | 10.6246 | 1.5265 | 0.2166 | 0 |
| Irrigation | -0.7592 | 0.3096 | 6.0123 | 0.0142 | 0.468 | |
| GSF | 1.2146 | 0.3498 | 12.0578 | 0.0005 | 3.369 | |
| Height | -0.0054 | 0.0034 | 2.5391 | 0.1111 | 0.9947 | |
| Diameter | 0.2792 | 0.1579 | 3.1266 | 0.077 | 1.322 | |
| Tmax | 0.1024 | 0.3402 | 0.0905 | 0.7635 | 1.1078 | |
| VPD | 1.1204 | 1.3683 | 0.6705 | 0.4129 | 3.066 | |
| RH | 0.0677 | 0.0768 | 0.7769 | 0.3781 | 1.0701 | |
| SWH | 0.1283 | 0.2804 | 0.2094 | 0.6473 | 1.1369 | |
The generalized log-rank test results confirmed a statistically significant difference between treatments, which was more significant in stone pine (p<0.001, for both tests) than in holm oak (p<0.01 for the test of Zhao & Sun ([70]), and p<0.01 for the test of Sun et al. ([62])). Results show that mulching was not a significant predictor of survival once irrigation was applied. The Weibull scale parameters were significantly greater than 1 for both species, which means that the rate of death decreases with time (Tab. 2). Besides the irrigation treatment, stone pine seedling survival was significantly influenced by initial height and daily maximum temperature. The value of the coefficient of daily maximum temperature was 0.2740, which meant that the risk of death is 1.31-fold [=exp (0.2740)] higher per unit increase in daily mean temperature, while all other covariates are held constant. Initial seedling height had a smaller effect on risk, with 0.98-fold decrease in risk per unit increase in height. For holm oak, irrigation treatment and GSF had a significant effect on the risk of death, the greatest effect being exerted by GSF, with a 2.84-fold increase in risk per unit increase in GSF, all else remaining equal.
Vigor status versus photosynthetic rate (An) and soil moisture
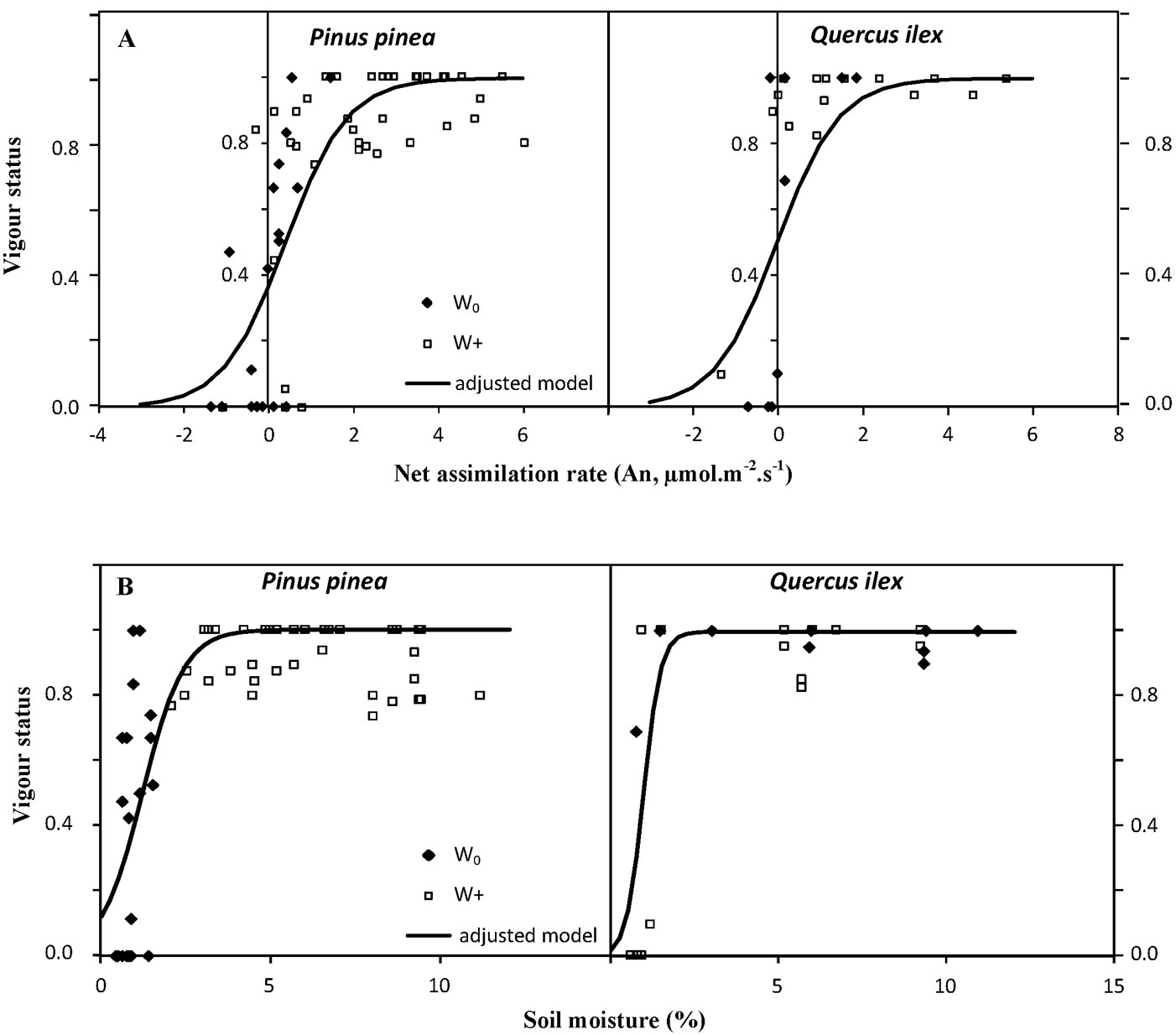
The photosynthetic rate (An) measured on July 22 and September 23 was significantly (p<0.001) related to the vigor status of seedlings in the following weeks, the response being similar for both species (p>0.5). Values of An greater than 1 µmol m-2 s-1 for holm oak and greater than 1.5 µmol m-2 s-1 for stone pine guaranteed a vigor status of 0.8, although these An values were only reached in irrigated seedlings (Fig. 5A). Conversely, negative rates of net assimilation led to a decrease in the vigor status to values below 0.3-0.4, endangering seedling survival. Soil moisture on July 22 and September 23 was also significantly (p<0.05) related to the vigor status of seedlings in the following weeks. As with An, values of soil moisture in the planting hole of around 2% guaranteed a vigor status of 0.8, which again, was only achieved in irrigated planting holes (Fig. 5B).
Fig. 5 - Relationship of percentage of vigor with: (A) net assimilation rate (An) measured in Pinus pinea and Quercus ilex seedlings; and (B) soil moisture measured in the planting holes on July 22 and September 23. Each point represents a measurement of An or soil moisture for irrigated (W+, squares) and non-irrigated (W0, diamonds) seedlings. All pairs of data for each species were adjusted to a logistic function.
Discussion
Effect of irrigation and mulching on growth, biomass, and survival
Our study revealed contrasting performance and survival responses to irrigation and mulching in stone pine and holm oak seedlings. A clear benefit of irrigation on first summer seedling survival was observed in both species, while the harsh site conditions in the absence of irrigation severely limited seedling survival and left seedlings susceptible to other environmental stresses, which led to reduced seedling growth ([41]). Furthermore, irrigation alone produced similar results to those obtained when both irrigation and mulching were employed. The scarce effectiveness of mulching alone as a means to improve seedling survival and performance under the water limiting conditions of Mediterranean environments has previously been reported ([31], [56], [59]). Similarly, stone pine seedlings were more responsive to the partial alleviation of summer drought than holm oak seedlings.
The limiting conditions usually found under a Mediterranean summer climate were further aggravated by the exceptionally drought summer conditions in 2011, which was drier than the previous 12-year average, summer rainfall events totaling less than 10 mm, which might be considered too little for the plants to be able to exploit ([64]). Consequently, survival through the summer was almost entirely limited to irrigated seedlings, and particularly in stone pine seedlings (39.9% survival), the roots of which seemed to display greater effectiveness in initiating growth and “coupling” with into the forest ecosystem than those of holm oak (12.2% survival). The ability of seedlings to quickly reach relatively deep and sufficiently moist soil layers reflects seedling functional integrity and vigor ([25], [64]). As seedling survival in Mediterranean environments is linked to root growth achieved by the summer ([66], [67]) and prior to drought occurrence ([40]), it seems that both species (particularly holm oak) suffered from a combination of high temperature, drought and shallow rooting, which together contributed to high seedling mortality. As with moisture availability, root volume and nutrient uptake are linked; thus, plant water stress may in turn lead to reduced plant nutrient uptake ([16]).
Effects of irrigation and mulching on gas exchange and chlorophyll fluorescence
Irrigation enabled stone pine and holm oak seedlings to photosynthesize, while negative carbon assimilation rates were shown in non-irrigated seedlings due to stomata closure. The maintenance of positive, albeit low, mid-morning net photosynthesis in July in irrigated seedlings compared to the negative values in the non-irrigated seedlings indicate that the drought-tolerance mechanisms of both species were intimately related to the low water availability by mid-summer. Likewise, high VPD and increased susceptibility to high radiation loads provoked stomatal closure in non-irrigated seedlings, avoiding excessive water loss at mid-morning ([42], [14], [45]). Although transpiration is an efficient way to reduce plant temperature, if it is permanently low on summer days with extremely high temperatures, stress from leaf overheating can occur ([33]). Non-irrigated holm oak seedlings reached higher gs and E values in July than stone pine seedlings. These results may suggest a better adaptation of the species to drought and warm conditions ([18], [23]) as well as a more rapid recovery after drought ([20]). However, gs and E values were not high enough to cope with the drought and heat through the summer of 2011, which led to high mortality in holm oak. The effect of irrigation on fluorescence parameters was in accordance with the An, E, and gs responses (i.e., drought and light exceeded the capacity of seedlings for carbon assimilation). This energy excess may cause photodamage to the photosynthetic apparatus, which can further compromise the short and mid-term survival of plants growing under full sun ([39]). Damage to PSII as a result of high temperatures can be more severe when plants are drought stressed ([69]), as occurred in this study. The persistence of such limiting drought conditions until October did not allow the recovery of PSII in non-irrigated seedlings, suggesting that photoinhibition was probably a secondary response to water stress and heat ([44]), causing the mortality of nearly all (97%) of the non-irrigated seedlings by September. The alleviation of drought through the summer in irrigated seedlings led to a recovery in PSII. Furthermore, by September, irrigation and mulching had produced an additive and positive effect on seedling physiology.
The severity of summer conditions was great enough to consistently reduce leaf-level photosynthesis in both species, regardless of their functional group or successional status. Stone pine behaves similarly to other pine species, which are considered good examples of water use regulation, reducing their stomatal conductance as soil water content declines and atmospheric conditions become drier, while maintaining relatively constant midday water potential values regardless of drought conditions ([8], [28]). On the other hand, the water regulation pattern in holm oak indicates an anisohydric behavior ([2]), maintaining higher stomatal conductance and allowing midday water potential to decline as soil water content decreases. Measurements of water potential carried out on nearby stone pine and holm oak natural regeneration during the summer of 2011 (July and August) confirmed this behavior (ΔΨ = midday water potential - predawn water potential = 0.37 MPa for stone pine; ΔΨ = 1.69 MPa for holm oak, unpublished data). Hence, the alleviation of these dry conditions through irrigation was the only way to guarantee seedling survival, stone pine seedlings being more responsive to this treatment. Nevertheless, the physiological responses in mid-summer, though favored by irrigation, rendered limited growth in both species. Summer net photosynthetic rates greater than 1 μmol m-2 s-1 in holm oak and greater than 1.5 μmol m-2 s-1 in stone pine were necessary to guarantee a vigor status of 0.8. However, these values were only reached in the irrigated seedlings, as non-irrigated seedlings showed negative assimilation rates, leading to the death of seedlings within weeks. Interestingly, a gravimetrically measured soil moisture as low as 2% (that was found in irrigated seedlings) is enough to guarantee a vigor status as high as 0.8. In our experiment, this soil moisture value could be a more efficient indicator of soil water availability for the dry soil during the 2011 summer drought conditions than predawn water potential, which can differ between plants due to soil heterogeneity ([5]).
Functional and practical implications for seedling survival and performance
Seedling survival and growth under a typical dry, sun-drenched summer in the Mediterranean climate is related, among other factors, to canopy cover ([55]), as excessive light can be a stress factor ([21]). The concurrence of high radiation and drought during the summer exacerbates the negative impact of drought on plant performance, leading to photoinhibition and thereby further limiting carbon assimilation ([63]), which can induce starvation-related dieback, cause hydraulic failure and finally plant death ([7]). Under the particularly harsh environmental conditions present in summer 2011, the few non-irrigated seedlings of each species that survived were located in a more favorable microenvironment, benefiting from some shade provided by the scarce understory vegetation. Under such severe conditions, the differing water status regulation behavior of each species (isohydric behavior in stone pine vs. anisohydric behavior in holm oak) did not appear to confer an advantage of one species over the other. Based on our results, the optimal niche for both species must maximize survival during the summer, avoiding high irradiance and extreme temperatures. These optimal conditions can be achieved most efficiently through a combination of first-year summer irrigation, artificial shading or by planting in specific microsites with partial-shade conditions in summer, avoiding the harsh midday sun, while being exposed to full sun during the moist spring period ([7]). From our results, first-year summer irrigation appears to be a good and not excessively expensive option, given that once a value of a gravimetrically measured soil moisture as low as 2% is achieved, survival will be assured. In addition, the beneficial effect of the below-crown environment is part of the regeneration ecology of stone pine ([35], [36], [22]). Likewise, shrubs, particularly rockrose and broom, play an important role in facilitating holm oak seedling survival, not only by protecting the seedlings from herbivores but also as buffers to stressful factors ([51]), thereby improving the abiotic conditions. However, this may be insufficient to prevent desiccation in the summer ([4]).
The repercussions for the potential persistence of both species in the area will be longer lasting in the case of stone pine because the resprouting capacity of holm oak provides it with an ecological advantage over the seeded stone pine seedlings. Resprouted individuals can use underground reserves and display an increased capacity to assimilate available water and nutrients as a result of their extensive root systems and small resprouting shoot ([38], [26]). Hence, it is likely that in the long term, the climatic constraints (increased probability of drought, heat stress and rising atmospheric CO2 concentration) will influence changes in species distribution ([43]). Under these new climatic scenarios, first year summer irrigation of outplanted seedlings (to reach 2% soil moisture) combined with the establishment of specific shaded microsites for planting that increase spatial heterogeneity in tree pattern should be considered as measures of adaptive management to promote resilient and resistant forested ecosystems ([61]).
Acknowledgments
This study was financed by the national project AGL2010-15521 and the project S2009AMB-1668 from the Regional Government of Madrid (Spain). We thank the Regional Forest Service for helping with the plantation.
References
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Online | Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Rafael Calama
Carolina Mayoral
Guillermo Madrigal
Mariola Sánchez-González
INIA-CIFOR, Crtra Coruña Km 7.5, E-28040 Madrid (Spain)
Corresponding author
Paper Info
Citation
Pardos M, Calama R, Mayoral C, Madrigal G, Sánchez-González M (2015). Addressing post-transplant summer water stress in Pinus pinea and Quercus ilex seedlings. iForest 8: 348-358. - doi: 10.3832/ifor1256-007
Academic Editor
Gianfranco Minotta
Paper history
Received: Jan 29, 2014
Accepted: Jul 20, 2014
First online: Sep 16, 2014
Publication Date: Jun 01, 2015
Publication Time: 1.93 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 57387
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 47495
Abstract Page Views: 3869
PDF Downloads: 4500
Citation/Reference Downloads: 30
XML Downloads: 1493
Web Metrics
Days since publication: 4182
Overall contacts: 57387
Avg. contacts per week: 96.06
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 10
Average cites per year: 0.91
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Tropical seedling performance under drought: a functional trait approach for species selection in restoration
vol. 19, pp. 9-17 (online: 10 January 2026)
Research Articles
Multi-temporal influence of vegetation on soil respiration in a drought-affected forest
vol. 11, pp. 189-198 (online: 01 March 2018)
Research Articles
Sap flow, leaf-level gas exchange and spectral responses to drought in Pinus sylvestris, Pinus pinea and Pinus halepensis
vol. 10, pp. 204-214 (online: 01 November 2016)
Research Articles
Optimum light transmittance for seed germination and early seedling recruitment of Pinus koraiensis: implications for natural regeneration
vol. 8, pp. 853-859 (online: 22 May 2015)
Review Papers
Climate change impacts on spatial distribution, tree-ring growth, and water use of stone pine (Pinus pinea L.) forests in the Mediterranean region and silvicultural practices to limit those impacts
vol. 14, pp. 104-112 (online: 01 March 2021)
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Research Articles
Matching seedling size to planting conditions: interactive response with soil moisture
vol. 12, pp. 220-225 (online: 25 April 2019)
Short Communications
Growth of Stone pine (Pinus pinea L.) European provenances in central Chile
vol. 10, pp. 64-69 (online: 29 August 2016)
Research Articles
Germination and seedling growth of holm oak (Quercus ilex L.): effects of provenance, temperature, and radicle pruning
vol. 7, pp. 103-109 (online: 18 December 2013)
Research Articles
Effect of drought stress on some growth, morphological, physiological, and biochemical parameters of two different populations of Quercus brantii
vol. 11, pp. 212-220 (online: 01 March 2018)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword