Diurnal dynamics of water transport, storage and hydraulic conductivity in pine trees under seasonal drought
iForest - Biogeosciences and Forestry, Volume 9, Issue 5, Pages 710-719 (2016)
doi: https://doi.org/10.3832/ifor2046-009
Published: Aug 21, 2016 - Copyright © 2016 SISEF
Research Articles
Abstract
The temporal dynamics of water transport and storage in plants have major implications for plant functioning and survival. In trees, stress on the conductive tissue can be moderated by water storage. Yet, trees can survive high percent loss of conductivity (PLC, up to 80%), suggesting efficient recovery. We assess the role of tree water storage and PLC recovery based on simultaneous measurements of leaf transpiration, branch hydraulic conductivity, and stem sap-flow from different seasons in three study years in mature Pinus halepensis (Miller) trees in a semi-arid forest. During the wet season the rates of transpiration (T) and sap flow (SF) peaked at high morning and through the mid-day. During the dry season T peaked at ~9:00 and then decreased, whereas SF lagged T and fully compensated for it only in the evening, resulting in a mid-day water deficit of ~5 kg tree-1, and with up to 33% of daily T derived from storage. PLC of 30-40% developed during mid-day and subsequently recovered to near zero within 2-3 hr in the dry season (May, June, and September), but not in the wet season (January). The observed temporal decoupling between leaf water loss and soil water recharge is consistent with optimization of the trees’ water and gas exchange economy, while apparently facilitating their survival in the semi-arid conditions.
Keywords
Cavitation Reversal, Sap Flow, Semi-arid, Water Deficit, Xylem Embolism.
Introduction
Pinus halepensis is a major tree species in the Mediterranean region, where it is routinely exposed to a long summer drought. This has led to multiple hydraulic adjustments underlying its drought resistance ([37], [23]). Variable cavitation levels (percent loss of hydraulic conductivity - PLC, 0-80%) were measured in drought-stressed saplings in the greenhouse and in 20-year old trees in the field ([24]), depending on drought intensity and tree provenance. Remarkably, most individual trees that suffered from the higher cavitation (PLC = 80%) still survived, in line with other reports of conifer survival at water potentials approaching 88% PLC, and in contrast with angiosperms for which 50% PLC can be lethal ([7], [30]). Field measurements in P. halepensis under semi-arid conditions ([27]) showed leaf water potential, Ψl < -2.5 MPa throughout the day during the entire dry season, approaching the points of stomatal closure (Ψl = -2.8 MPa) and PLC50 (Ψl = -3.9 MPa). Carbon uptake continues throughout the dry season, in spite of the harsh conditions, mainly to support fresh needle growth ([27]). This “risky” behavior of P. halepensis was contrasted by relatively high survival, growth and productivity in a semi-arid forest site ([18], [27], [26]). Together, these observations suggest that the xylem of P. halepensis regularly experiences high cavitation levels during the dry season and that this species must have some immunity to the effects of cavitation, allowing its survival and continuous activity under drought.
Isohydric-like, low wood density species such as P. halepensis are expected to have a relatively high capacity to store water ([32]) and possibly also to refill cavitated xylem (as shown for angiosperm species - [42]). Some level of water storage is common in tree stems and is active in the daily water balance, which results in temporal decoupling of sap flow (SF) from transpiration (T). A short (~0.5 hr) T vs. SF lag is usually expected due to changes in Ψl, e.g., the release of water from leaves in early morning, when Ψl decreases from its pre-dawn value to an operational value. A T vs. SF lag was discussed ([11]) but not frequently tested, presumably due to the differentiation between studies using gas (T) and liquid phase (SF) measurement techniques. Two notable exceptions are Zweifel et al. ([51]) and Fisher et al. ([16]), who showed time lags between T and SF of 0.5-3 hr. Comparing SF rates at the bottom and high crown of large tropical trees, Meinzer et al. ([31]) found up to a 2 hr lag time. The role of tree water storage was shown in many studies ([51], [31], [29], [16], [32]), some showing a major storage contribution, e.g., 10-75% of daily T in potted, young Norway spruce (Picea abies - [51]), or a more minor contribution of 2-10% in adult yellow poplar (Lirodendron tulipifera - [29]) and down to zero stem storage in the woody monocot Bamboo suffering from cavitation ([49]).
Sap flow is usually under large negative pressures and subsequently trees live under the threat of cavitation ([45]). Cavitation breaks the continuity of water columns and hence the water supply to transpiring leaves. Xylem embolism and cavitation reduce hydraulic conductance and therefore bear major implications for plant function and survival. Cavitation can be fatal to trees ([45], [5], [23]) and irreversible ([45]). Among P. halepensis provenances, those with higher sensitivity to cavitation generally had higher mortality rates in the field ([24]). Since water is transported in the xylem under constant tension (i.e., water potential - Ψ < 0) the possibility of refilling was initially ruled out. However, empirical evidence for the reversibility of cavitation became available from experimental data on induced and native embolism ([46]). A proposed refilling mechanism overcame the tension enigma by segregation, i.e., the refilling process is preceded by isolation of the cavitated section from the water conducting system ([20]). Cavitation repair is usually allowed once trees are re-watered ([35]), e.g., between hot day and cooler night ([43]).
Cavitation removal was shown in grapevine (Vitis vinifera - [50]) where synchrotron x-ray microtomography (microCT) facilitated visualization of the refilling process, confirming the segregation hypothesis and showing water influx from surrounding living cells ([4]). The new microCT technology permits direct, nearly non-invasive embolism measurement. Yet in mature trees in the field, hydraulic conductivity measurements still offer the best way to detect changing levels of embolism.
The refilling process could result from osmotic gradients generated by sucrose in walls of embolized vessels ([40]). In the desert woody shrub Encelia farinosa, embolism repair occurs at night while stomata are open and transpiring, without the need to isolate the cavitated section ([14]). In Bamboo (Sinarundinaria nitida) nocturnal root pressure was sufficient to cause recovery from embolism in the leaves ([49]). In conifers, mechanisms of embolism repair are currently unclear and might differ from those proposed for angiosperms due to differences in xylem structure and chemical composition, but embolism repair has been demonstrated in at least eight conifer species ([28], [4] and references therein), although microCT showed no refilling in Sequoia sempervirens saplings ([8]). Additionally, some of the measurements involving the cutting of xylem under tension might have overestimated the levels of embolism and recovery ([48]) and hence must be taken with caution. In P. halepensis under drought, embolism repair was detected using acoustic emissions, a non-quantitative method ([1], [2]).
We hypothesize that water storage and maintenance of hydraulic conductivity must be integral components of the water management in P. halepensis to sustain activity and survival under continuous dry conditions of the summer. To test this hypothesis we applied a high temporal resolution sampling strategy to study in vivo the dynamics of hydraulic conductivity in mature P. halepensis trees in a semi-arid forest, where extreme seasonal drought is the norm.
Materials and methods
Site description and environmental conditions
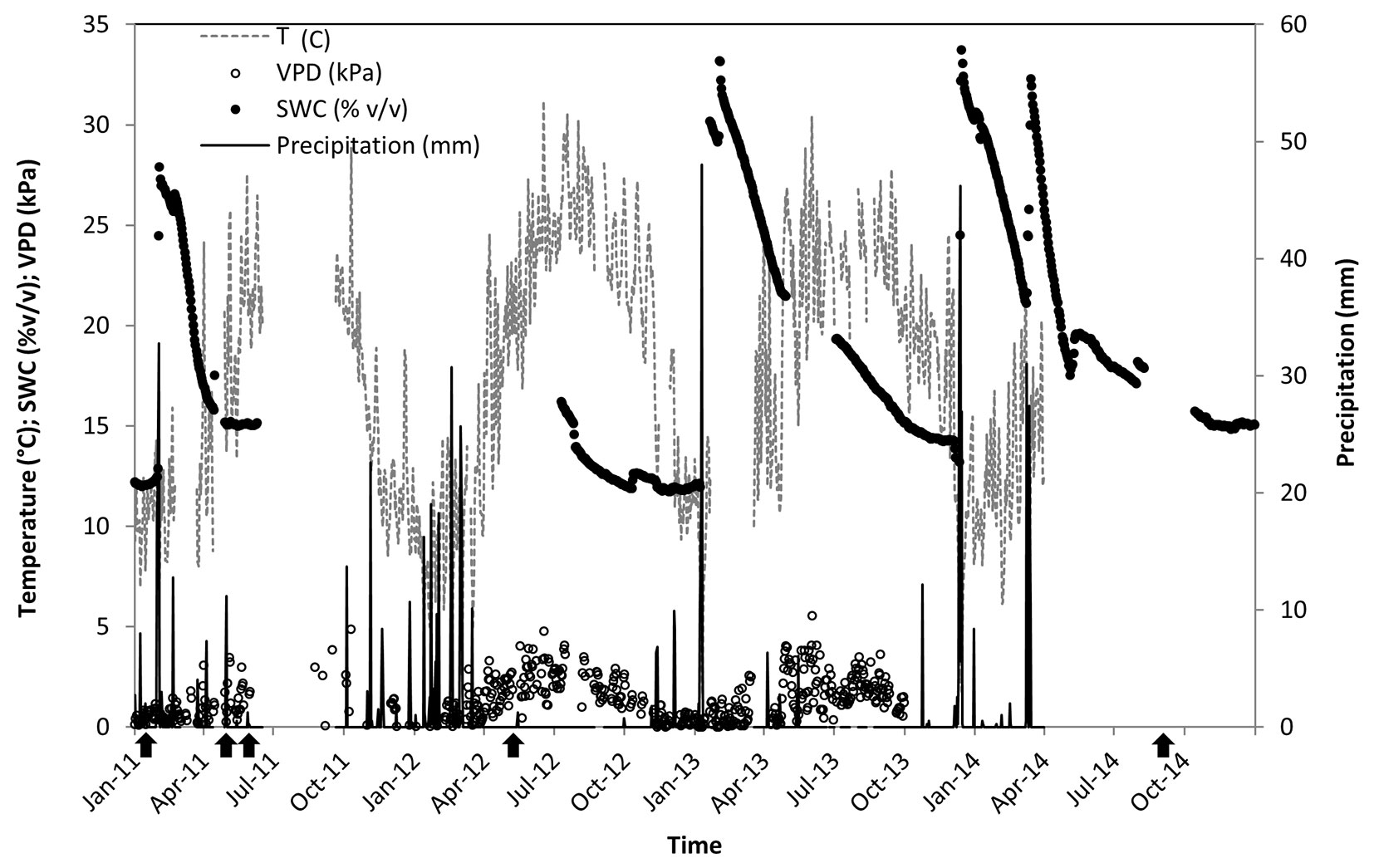
Our study was conducted in the Yatir forest, a 45-yr-old P. halepensis plantation located at the northern edge of the Negev desert, Israel (31° 20′ N, 35° 20′ E). The forest covers an area of 2800 ha and lies on a predominantly light brown Rendzina soil (79 ± 45.7 cm deep), overlying chalk and limestone bedrock. The climate is hot (40-yr average mean annual temperature is 18 °C) and dry (40-yr average mean annual precipitation is 285 ± 88 mm). Rainfall is restricted to the wet season between November/December and April/May, increasing soil water content from 12-15 to 32-33 % v/v (Fig. 1). Mean daily vapor pressure deficit is mostly below 1.0 kPa during the wet season, and well over 2.0 and up to 5.0 kPa during the long dry season. Stand density is ca. 300 trees ha-1, leading to an average leaf area index of about 1.50.
Fig. 1 - Site environmental conditions in 2011-2014. Daily precipitation (P); mean soil water content at 30 cm below surface (SWC); mean vapor pressure deficit (VPD); and mean air temperature over the forest canopy (T). Data are missing due to technical impediments for T and VPD during June-September 2011 and in 2014; for SWC during June 2011-July 2012; but not for P. Black arrows in the bottom indicate major field day campaigns.
Study trees
In 2000, an instrumented flux tower was installed in the geographic center of the forest. Across the forest, trees were planted around the same time and at homogeneous density, and hence the tower stand can be considered as a representative stand. In 2009, sap flow probes were installed on sixteen trees ca. 70 m from the tower, defined as the observation plot within the tower stand. A subset of 1-4 trees of these sixteen trees was selected for all other measurements and samples taken between 2009 and 2014 (hydraulic conductivity, needle gas exchange, water potential, and relative water content). These four trees had the longest and most stable sap flow record in the observation plot, and were arbitrarily selected as typical representative healthy trees; other trees in the same stand died following the drought years of 2008, 2009, and 2011 ([26]), or showed some level of drought stress ([25]). The forest inventory performed in 2010 in the tower stand showed that tree height and diameter at breast height (DBH) were 10.2 ± 0.2 m (mean ± SE) and 19.8 ± 0.4 cm, respectively (n = 177). In the observation plot, tree height and DBH were 9.5 ± 0.3 m and 18.2 ± 0.9 cm, respectively (n = 16). Among the subset of trees selected for intensive measurements, tree height and DBH were 9.0 ± 0.6 m and 21.5 ± 1.3 cm, respectively (n = 4). Therefore, the four selected trees had, on average, slightly higher DBH than their plot neighbors, but not significantly higher than the stand-level mean. Considering the relatively low stand density and LAI in Yatir forest, lower tree crowns are sunlit and active, facilitating measurements at 1.3-2.0 m aboveground.
Xylem hydraulic conductivity
Branch sections (~5 mm diameter, ~30 cm length) were sampled from lower crowns (1.3-2.0 m) of 1-4 P. halepensis trees every 1-2 hr during five field days between January 2011 and September 2014. In P. halepensis in Yatir forest, the outer 4 cm of the sapwood is conductive ([10]), which, according to tree-ring counting, was formed within 27 ± 1 years. The 5 mm diameter branches whose conductivity was measured were 13 ± 1 years old and hence fully conductive. All measurements were done according to the best practices at the time of measurement, which changed during the time-frame of this study, and we adapted accordingly. Therefore, the protocol used in 2011-2012 was replaced by an improved protocol in 2014, following the validations made by Delzon et al. ([12]) and by Wheeler et al. ([48]), which are detailed below. However, the results from the different protocols remained consistent.
In the first protocol, specific hydraulic conductivity (Ks) was measured in the lab, under low pressure (0.02 MPa) before and after perfusing the xylem tissue at a high pressure of 0.5 MPa. The branch section was cut in the field and resin secretion from the cuts was eliminated by placing both ends (5 cm from cut tips) in a water bath at 95 °C for 10 min (adapted from [47]). The resin treatment was essential ([33]) and also helped remove trapped gas from branch ends, which could otherwise create artifacts ([48]). Experimenting with branches of well-watered Olea europaea showed that the effect of this treatment on Ks was <5%. Next, stem sections were submerged in distilled water and transferred to the lab. In the lab, the upstream end of each branch was then fitted with a rubber gasket, while still submerged, to a latex pipe fed by a degassed 10 mM KCl solution reservoir. A hydrostatic pressure of 0.02 MPa was applied by placing the reservoir exactly 2 m above the stem section. For two hr, water dripping from the distal end of the branch section was collected and weighed every 20 min. The flow rate was steady, producing a linear increase in the efflux water mass (r2 of linear fits > 0.9) and indicating no rehydration of the branch. To test the effect of hydrostatic pressure on Ks, nine branches were exposed to hydrostatic pressures of 0.0015-0.0070 MPa. Ks increased from practically zero to 0.4 kg m-1 MPa-1 s-1 at pressure larger than 0.003 MPa (Fig. S1 in Supplementary material). The water flow rate (kg s-1) was divided by the pressure gradient (0.02 MPa) along the ~30 cm stem length to provide the hydraulic conductivity Kh (kg m MPa-1 s-1), as described by Tyree & Alexander ([44]). Kh was further divided by the xylem cross-sectional area to get specific hydraulic conductivity, Ks (kg m-1 MPa-1 s-1). Branch sections were then fitted with high-pressure valve to a high-pressure pipe fed by 10 mM KCl solution reservoir placed inside a Scholander pressure chamber. The segment was perfused at 0.5 MPa for 5 min and then a second measurement of Ks was made to get the maximum specific conductivity, Ks max. Measurements of Ks and Ks max were further used to identify loss of conductivity, which can be attributed to xylem cavitation according to (eqn. 1):
where PLC is the percent loss of conductivity (%) due to cavitation.
In the second protocol, resin secretion was stopped by immediate immersion in ice water. Branch sections were re-cut under degassed water in the lab to a length of 10 cm, to avoid potential artifacts related to induced cavitation due to cutting in the field ([48]). Ks was measured in a similar fashion but using a lower hydrostatic pressure of 0.007 MPa by placing a degassed 10 mM KCl solution reservoir 0.7 m above the stem section. Branches were not exposed to higher pressure, to avoid sealing of the torus-margo pit membrane ([12]). For the perfusion treatment, degassed water was drawn through the stem overnight by putting the stem in a beaker of degassed water and connecting the other side to a vacuum line whose negative pressure was ~0.07 MPa. Observation of the water level in the beaker showed that a substantial amount of water had perfused the stem overnight. A second measurement at 0.007 MPa provided the reference conductivity, Ks max.
Sap flow
Sap flow (SF) was measured continuously since September 2009 on sixteen trees in Yatir forest using lab manufactured thermal dissipation sensors ([17]) calibrated by comparison with commercial heat balance sensors (EMS, Brno, Czech Republic) in the same trees. All probes were installed at 1.3 m above the ground and 2 cm into the sapwood. Measurements were taken every 30 s and the 30 min average value was saved on a CR1000® data-logger (Campbell Scientific Inc., Utah, USA) and transmitted via internet to the lab at the Weizmann Institute of Science. Sap flow rates (kg hr-1) were calculated in relation to the minimum sap flux during the 24 hr period (typically at night), as shown in the empirical equation (eqn. 2) of Granier & Loustau ([17]), modified by Kanety et al. ([22]):
where SF is the half hourly sap flow rate; LCF is the correction factor for the 2 cm probes considering the radial distribution of sap velocity (for P. halepensis in Yatir - [10]) and calculated specifically for individual trees ([39], [22]); CF is a calibration factor of 2.5 ([41], [39], [22]), validated here by comparison with the heat balance sensors; ΔTr is the average half hourly temperature difference between heated and non-heated probes, and ΔTmax the maximum temperature difference measured during the 24 hr period (assumed to be at a negligible sap flow rate, typically at night).
Stem diameter variations
Delicate changes in stem diameter (down to ± 1 µm) were recorded continuously since September 2009 with high-resolution band dendrometer (EMS, Brno, Czech Republic) fitted at 1.9 m around one of the four P. halepensis trees used for intensive measurements. To detect diurnal variations, output data were set to zero at midnight of each 24 hr period. Stem diameter variations make a good proxy for tree water relations ([51]) because they reflect changes in the elastic tissues of the stem (i.e., phloem and bark), which have hydraulic connections to the xylem. The negative pressure associated with water transport in the xylem withdraws water from these elastic tissues, thereby decreasing stem diameter. This shrinkage stops as soon as the tension is relaxed, and stem diameter increases again.
Leaf gas exchange, water potential and relative water content
Leaf photosynthesis (A), transpiration (T) and stomatal conductance (gs) were measured using a LiCor 6400® photosynthesis system (Licor Inc., Lincoln, NE, USA) on the four trees every 30-60 min during five field days between April 2010 and June 2011. These were the trees used for hydraulic conductivity and sap flow measurements. Lower crown (1.3-2.0 m) cohorts of sunlit, 1-year old needles were marked for repeated measurement. Leaf chamber conditions were adjusted to ambient and all gas exchange parameters were expressed on a projected needle area basis of 3 cm2 based on the uniform area of twelve adjacent needles ([27]). Measurements were upscaled to tree-scale transpiration by multiplying by leaf area ([18]), since for the low LAI (1.5) it is fair to assume that all leaves can be considered as sunlit and equally active. Leaf water potential (Ψl) was measured using the pressure chamber technique. Small (5-7 cm long) twigs were cut from the same trees used for the gas exchange measurements at similar height and side and put in a pressure chamber (Arimad 2®, A.R.I, Kfar Charuv, Israel) fed by a Nitrogen gas cylinder and equipped with a lamp-carrying magnifying glass. Gas pressure within the chamber was gradually increased (~1 MPa min-1) until water emerged from the protruding cut branch surface, and the pressure value was recorded as leaf water potential (Ψl). Due to staff and equipment limitations, Ψl was only measured during a few field days. Needle relative water content (nRWC) was determined from three subsequent measurements: fresh leaf mass (in situ), water-saturated mass by immersion, and oven-dry mass (in the lab). Consecutive weightings verified complete saturation and drying until stable mass was achieved.
Statistical analysis
Diurnal changes in PLC were subjected to analysis of variance (ANOVA) using the JMP® software (Cary, NC, USA). Measurements of individual trees (or individual branches from one tree in the January and May 2011 campaigns) were used as observations and sampling time was defined as factor. Differences in PLC between sampling times were considered significant when type 3 sum of squares met the F-test criterion at probability < 0.05. Means were compared using the Student’s t test, where different letters indicated significant differences between sampling times.
Results
Daily and seasonal dynamics of sap flow and leaf transpiration
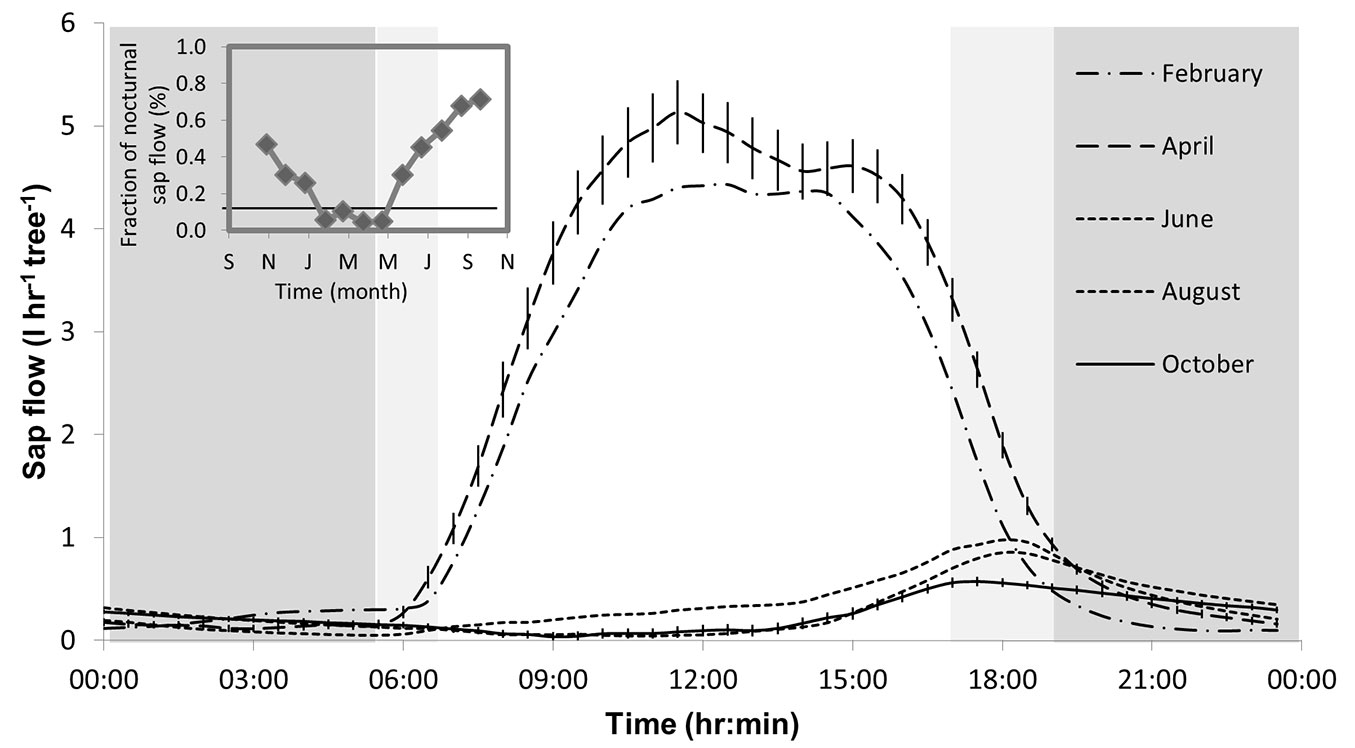
Sap flow rates showed strong interactions between daily and seasonal dynamics (Fig. 2). Moving from the wet season into the dry season, the diurnal peak in SF decreased from 4-5 kg hr-1 in February-April to less than 1 kg hr-1 in June-October. The timing of the SF peak - tmax(SF) - also changed, from ~11:00 in April to ~19:00 in June (Fig. 2, Tab. 1). As a result, while in April 96% of SF occurred during the daytime, during September-October as much as 70% of SF occurred at night (Fig. 2 inset). The dynamics of leaf scale transpiration did not reflect the major changes in SF. T was always restricted to daytime throughout the year, and its peak, tmax(T), was in the morning in all seasons (Tab. 1), and moved from mid-day in the wet season to mid-morning (~9:00) in the dry season. Consequently, the lag time between tmax(T) and the less distinct tmax(SF) increased from 30 min in April up to a maximum of 9.5 hr in July (Tab. 1).
Fig. 2 - Diurnal changes in the monthly average sap flow (SF) rate in Pinus halepensis in the Yatir forest, with the fraction of nocturnal sap flow shown in the inset (horizontal line represents the annual average, 0.16). Nighttime is indicated by a light or dark background, depending on the date. Error bars, shown for April and June for ease of reading, represent the standard error of the mean (n = 16 trees × 28-31 days), and are not observed when smaller than symbols.
Tab. 1 - Diurnal maxima of leaf transpiration [tmax(T)] and sap flow [tmax(SF)] along the year in the Yatir forest and the time difference between them [Lag time: tmax(SF)- tmax(T) (hr)].
| Date | tmax(T) |
tmax(SF) |
Lag time |
|---|---|---|---|
| 1-Oct | 11:00 | 19:00 | 8.0 |
| 1-Nov | 10:30 | 16:30 | 6.0 |
| 1-Dec | 10:00 | 12:30 | 2.5 |
| 1-Jan | 09:00 | 14:30 | 5.5 |
| 1-Feb | 09:30 | 13:30 | 4.0 |
| 1-Mar | 09:30 | 14:00 | 4.5 |
| 1-Apr | 09:30 | 10:00 | 0.5 |
| 1-May | 09:30 | 13:00 | 3.5 |
| 1-Jun | 09:30 | 18:30 | 9.0 |
| 1-Jul | 09:00 | 18:30 | 9.5 |
| 1-Aug | 09:00 | 18:00 | 9.0 |
| 1-Sep | 09:30 | 18:00 | 8.5 |
Daily and seasonal dynamics of hydraulic conductivity
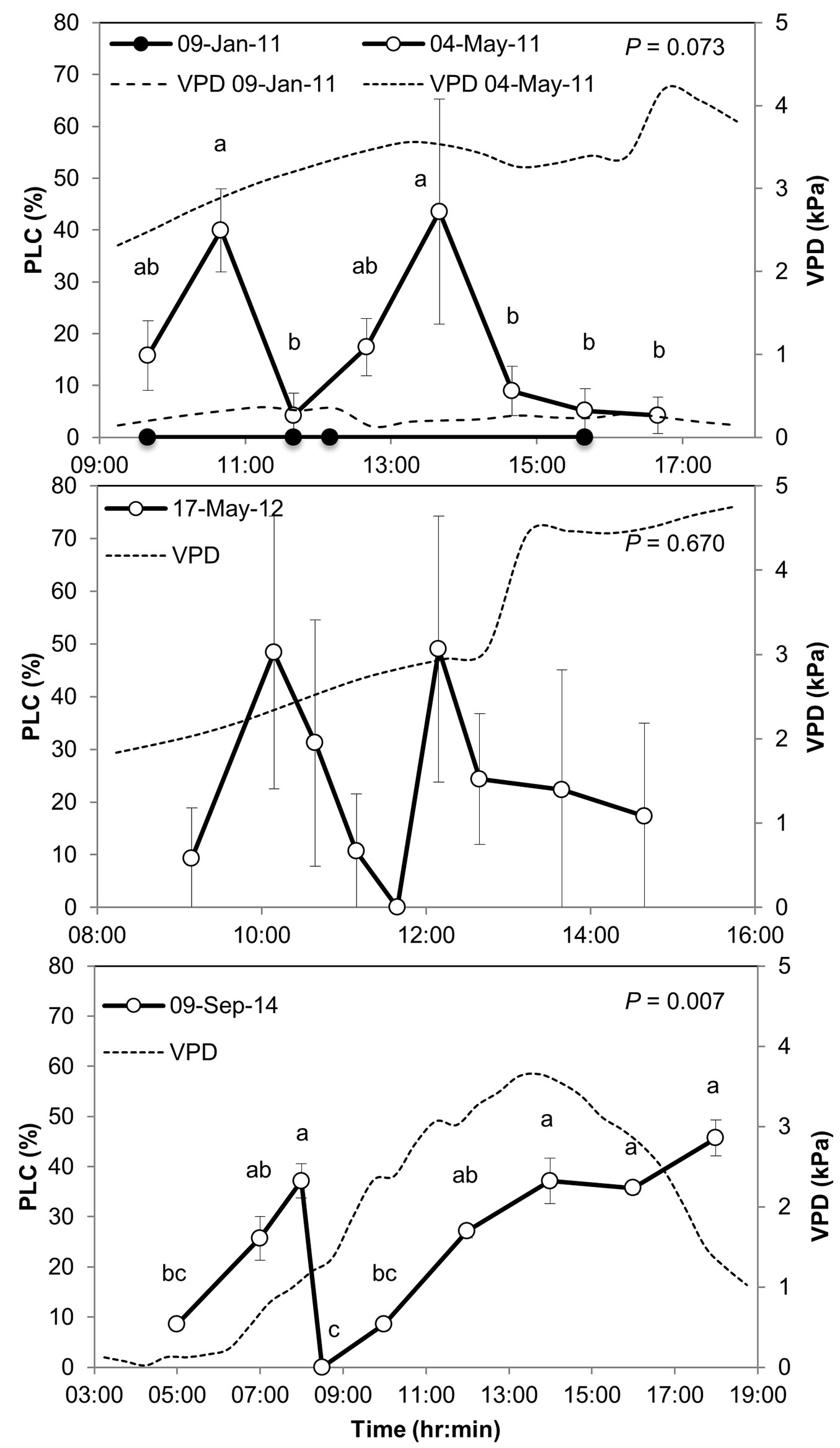
The daily course of PLC, often used as an indicator of xylem cavitation, showed a major shift from consistent zero PLC in the wet season (January) to highly fluctuating, increasing up to 50% in the dry season (Fig. 3). In the dry season PLC peaked two times during the day: first in the morning and later again in the afternoon. In between, PLC declined to 0-5% and xylem conductivity was fully restored. Morning and afternoon PLC peaked in 1-3 hr and consequently was reversed over a similar time period. The standard error among branches of the same tree was 7.4% on average, and 16.4% among branches of multiple trees in 2012. Such variations are expected from a highly dynamic process that may not occur uniformly throughout the entire xylem and among multiple trees. Nevertheless, using the improved protocol in 2014 the standard errors decreased to 2.2% on average while showing a similar pattern (i.e., there was still consistency between the old and newer methodologies, which add robustness to the results). In May 2011 and September 2014 differences in PLC in different times of the day were significant, e.g., between 10:40 and 11:40 in May 2011, and between 8:00 and 8:30 in September 2014. The exact timing of the PLC highs and lows changed between observation days, between the beginning of the dry season (May) and its end (September), consistent with previously observed seasonal shift in the peak of the daily activity cycle at the site ([27]). Xylem conductivity partially recovered in the afternoon of May 2011 and 2012, but not in September 2014. The lower vapor pressure deficit (VPD) in September vs. May meant that stomatal gas exchange was maintained throughout the afternoon hours in September despite low soil water availability, in turn inhibiting reversal of cavitation during the day.
Fig. 3 - Percent loss of conductivity, PLC (a measure of xylem cavitation) in Pinus halepensis branches sampled in the Yatir forest in 2011, 2012, and 2014 (upper, middle, and bottom panels, respectively). Changes in vapor pressure deficit (VPD) are indicated by dashed lines. PLC values are means from three branches of one tree (2011) or from three-four different trees (2012, 2014); PLC was determined using the first protocol in 2011 and 2012 (see Methods) or the second protocol in 2014. Different letters indicate significant differences among sampling times, with P-values indicated at upper right corners. Error bars represent the standard error of the mean.
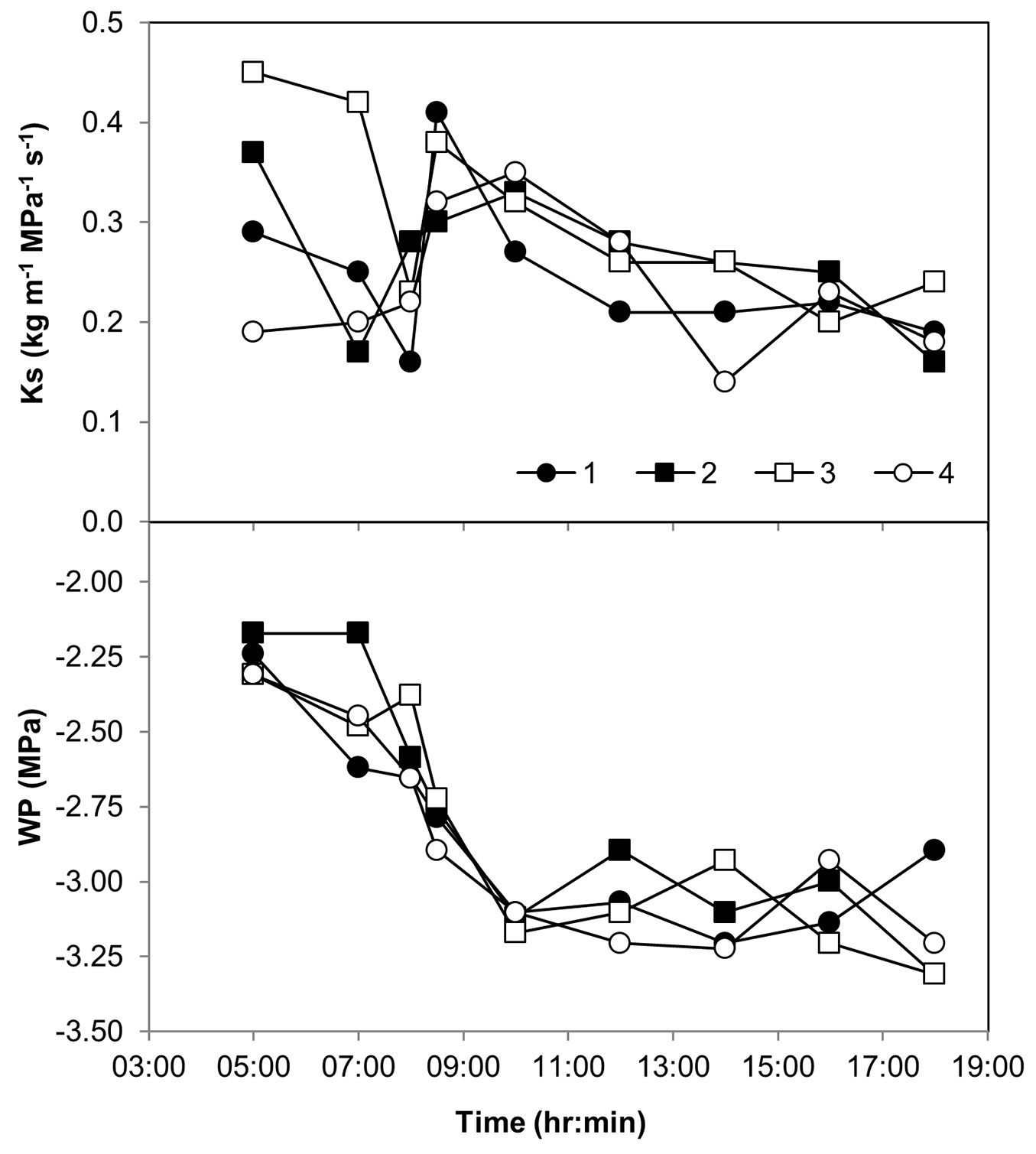
The dynamics of branch hydraulic conductivity (Ks) and water potential (Ψl) in neighboring trees were further examined. At predawn of the September 2014 field day trees had various levels of Ks and a uniform Ψl of -2.25 MPa (Fig. 4). During the next 5 hours Ks reached its minimum and subsequently, maximum values, while Ψl decreased monotonously by 1.0 MPa. During the rest of the day and until sunset, Ks continued to decline gradually, while Ψl remained around -3.15 MPa, a level associated with 33% PLC (Fig. S2 in Supplementary material).
Fig. 4 - Diurnal changes in xylem specific hydraulic conductivity (Ks) and shoot water potential (WP) in four neighboring Pinus halepensis trees in the Yatir forest during 9 Sep 2014.
Contemporary changes in tree hydraulics and gas exchange
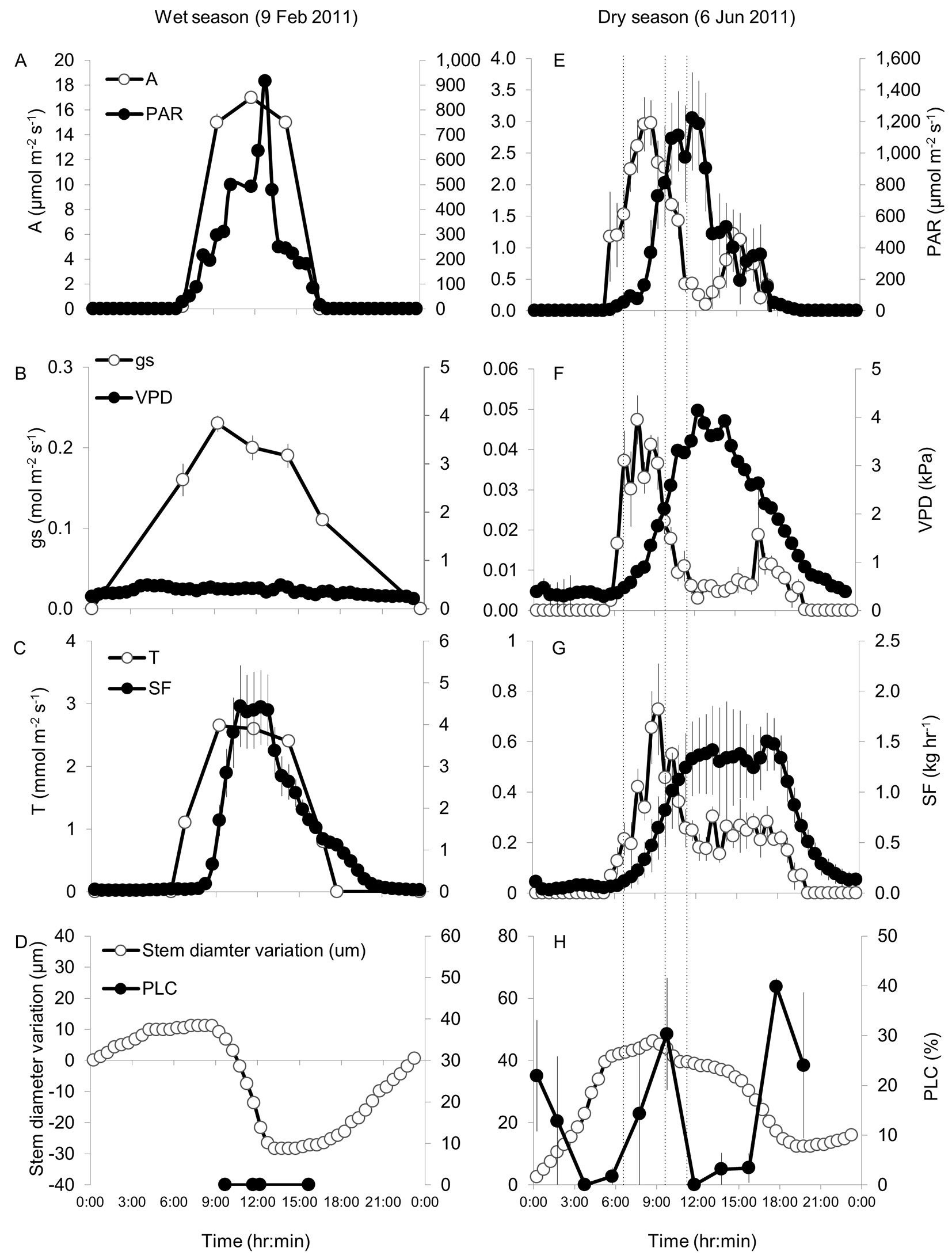
More detailed 24-hr gas exchange and hydraulic measurements in both the wet and dry periods were used to help understand the dynamics of the interactions reported above. In the wet season, photosynthetically active radiation (PAR) was between 200 and 900 µmol m-2 s-1 during most of the light hours (8:15-15:15), and VPD was low (Fig. 5A-B). Under these favorable conditions, net carbon assimilation (A) reached 17 µmol m-2 s-1 at noontime and stomatal conductance (gs) was >0.1 mol m-2 s-1 throughout the entire day. Water transport rates in the xylem (SF) and from the leaf (transpiration, T) were relatively synchronized (Fig. 5C) and the short lag could be explained by the effect of changes in Ψl (see Introduction). Stem diameter decreased by 40 µm between 8:15 and 12:15, when SF was increasing, or at its peak of 4.5 kg hr-1 (Fig. 5C-D). No cavitation was observed at any of the four sampling times during the day.
Fig. 5 - Diurnal changes in net assimilation (A), photosynthetically active radiation (PAR), stomatal conductance (gs), vapor pressure deficit (VPD), leaf transpiration (T), sap flow (SF), stem diameter variation, and percent loss of hydraulic conductivity (PLC) in Pinus halepensis during wet season (A-D) and dry season (E-H) days in the Yatir forest. Note different scales for the same parameters in wet and dry seasons. Dry season curves were measured on 6 Jun 2012; wet season curves on 9 Feb 2011, except for A, gs, T (mean curves for Jan-Feb) and PLC (9 Jan 2011). Error bars represent the standard error of the mean (n = 1-4, depending on the parameter), and are not observed when smaller than symbols. The dashed vertical lines in the right-hand panels denote the times 6:30, 9:30, and 11:30 discussed below.
Under the regular dry season drought, net assimilation A was observed in the light hours but was not synchronized with PAR (Fig. 5E), probably due to the development of high VPD around midday (>3 kPa - Fig. 5F; see also [27]) The increased VPD resulted in reduced gs from ~0.05 mol m-2 s-1 at 7:45 to below 0.01 mol m-2 s-1 at 12:15 (Fig. 5F). In the afternoon, around 16:00, VPD decreased below 3 kPa and gs increased two folds to ~0.02 mol m-2 s-1, still lower than the morning peak, presumably due to the low light levels approaching sunset (when PAR = 50 µmol m-2 s-1 by 17:45). Consistent with this pattern, the diurnal curve of A also showed a morning peak (3 µmol m-2 s-1) followed by a midday depression and a smaller afternoon peak (1 µmol m-2 s-1, Fig. 5E). In contrast to A, the observed diurnal changes in T reflected the combined effects of gs and VPD, where variations in T can be approximated by variations in gs × VPD. Therefore T peaked at 0.73 mmol m-2 s-1 at 9:15 and declined to 0.15 mmol m-2 s-1 at 13:45 (Fig. 5G). Between 14:00 and 18:30 T stabilized at around 0.25 mmol m-2 s-1, reflecting the inverse relationship between gs and VPD. Comparing the dynamics in T and SF (Fig. 5G) indicated that: (1) T had large fluctuations, while changes in SF were more gradual; and (2) T ceased at night whereas SF continued long after sunset. Stem shrinkage of 30 µm matched the time-frame between the start of SF increase (8:15) and decrease (18:45), reflecting the longer SF time-window in dry vs. wet season. But unlike the constant shrinkage rate of 10 µm hr-1 in January, the dry season shrinkage rate changed from 3 µm hr-1 (8:15-10:15) to a moderate 1 µm hr-1 (10:15-15:15) and then to 6 µm hr-1 (15:15-18:45). Stem contraction during the dry season day did not reach negative values due to higher relaxation at night. Fluctuations in xylem conductivity dynamics were associated with the decoupling between T and SF, reflected by rapid changes in the PLC (Fig. 5H), with two transient peaks of 30% and 40% at 9:45 and 17:45, respectively. The PLC dynamics on 6 June 2011 were generally similar to those observed in May 2011 and 2012 (Fig. 3), albeit conductivity did not fully recover in the evening. PLC and transient decreases in needle relative water content (nRWC, from 77 to 63% - data not shown) occurred simultaneously with high gs, while the recovery in PLC and nRWC was at decreasing gs, which may indicate that PLC reversal was dependent on reduced rates of water loss and increasing nRWC.
Seasonal changes were observed in the T vs. SF dynamics, between wet and dry season (Fig. 5C and Fig. 5G, respectively). The increase in T from 6:00 to 9:00 occurred in both seasons, but the SF supply of water for T was substantially delayed in the dry season. Upscaling leaf-scale transpiration measurements (mmol m-2 s-1) to tree-scale transpiration (kg hr-1), using individual tree leaf area estimates based on our own site-specific allometric equations ([18]), showed that large (3-5 kg) water deficits developed in the tree during the day, but the trees also re-hydrated daily, i.e., tree water content did not decline during the season. Integrating the dry season diurnal water use of an average tree in Yatir forest yields a value of 15 kg day-1. This implies that during the light hours of a typical dry season day 20-33% of the transpiration came from water storage.
Discussion
Temporal decoupling between leaf transpiration and stem sap flow
In the studied semi-arid forest ecosystem, leaf transpiration and xylem sap flow are never quantitatively synchronized (Fig. 5C), and even at the time of peak activity, when sap fluxes surpassed 5 kg hr-1 (April) there was still a 30 min lag of SF peak behind that for T (Tab. 1). Such short lags are expected due to changes in tree capacitance or water content as Ψl decreases during the morning (Fig. 4). The interaction of leaf conductance with water potential can involve long response times, sometimes producing sustained stomatal cycling with an average period of approximately 70 min ([13]). In our study, diurnal peaks of T and SF (the latter peak was not as distinctive) were 9.5 hr apart, compared to 0.5-3 hr reported in other studies ([51], [31], [16]). These are exceptionally long delays, which might be expected in this case of extreme drought. The lag time was inversely related to the water flux, peaking at 9.5 hr in the height of the dry season (July - Tab. 1), when peak sap fluxes never exceeded 1 kg hr-1. This could be partly explained by a higher contribution of a few sinker roots to the tree water use in the dry season, thereby reducing the overall flow and increasing the mean water path length. The inverse relationship noted above is in agreement with measurements in young spruce ([51]), where the lag increased from 30 min on high-flux days to 110 min on low-flux days. Brodersen et al. ([4]) showed in grapevine that delays are related to water deficits, where cavitated vessels could not refill until additional water was introduced. Here we show that SF was often delayed into the night (Fig. 2), and consequently up to 70% of the total diurnal SF occurred after sunset. This high nocturnal SF proportion, which occurs when nighttime leaf transpiration is negligible, is much larger than the values of up to 35% observed in a California oak species, Quercus douglasii ([16]), and up to 25% in other trees.
The calculated water storage capacity, contributing 20-33% of daily T, is within the range of 10-75% reported for potted, young Norway spruce ([51]), but much higher than the 2-10% reported for yellow poplar ([29]). This also agrees with the hypothesis that isohydric-like, low wood density species like P. halepensis should have relatively high capacity to store water ([32]). This reliance on daily water storage is equivalent to other forms of water redistribution, such as hydraulic lift and soil water storage ([36]).
Dynamics of xylem hydraulic conductivity
We detected high loss of hydraulic conductivity, which is usually associated with cavitation (PLC of 40%) in the xylem of P. halepensis under drought, which was reversed on a time scale in the order of several hours (Fig. 3). These observations were consistent across four field days along three dry seasons, and across the two measurement protocols, with minor differences due to the day in the dry season. Measured PLC levels were also in agreement with the P. halepensis branch vulnerability curve, predicting 33% embolism at Ψl = -3.15 MPa (Fig. S2 in the Supplementary material). Measurements in grapevine stems revealed a diurnal cycle of cavitation and recovery, with a 3-5-hr refilling time ([4], [50], respectively). Shorter refilling times (~2 hr) were reported for poplar (Populus trichocarpa) stems under induced embolism in the lab ([40]). However, we noticed that some of the measurements involving the cutting of xylem under tension might have overestimated the levels of embolism and recovery ([48]) and hence must be taken with caution. However, our observations of two consecutive sub-diurnal cycles in PLC may be, to the best of our knowledge, the most dynamic cavitation/ recovery cycle reported for trees in the field.
Although embolism repair has been reported in conifers ([2], [28]) and in P. halepensis specifically ([1]), its mechanism is yet to be resolved. If ray parenchyma cells and non-structural carbohydrates (NSCs) are involved, as suggested by Brodersen & McElrone ([3]), P. halepensis has both components at notable levels ([15], [25], respectively). Live parenchyma cells are also expected in the young branches studied here (13 ± 1 years, see Methods). The kinetics of xylem refilling depends on the volume of the embolized lumen, and hence on species-specific traits like conduit diameter ([4]). With its narrow tracheids (9.4 µm - [37]), P. halepensis makes a good candidate for rapid refilling. Frequent cavitation and recovery cycles can induce “cavitation fatigue”, decreasing the ability of the xylem to recover ([19]). However, Taneda & Sperry ([42]) reported repeated overnight xylem refilling throughout most of the summer in an oak species, similar to our observations.
Interestingly the cavitation dynamics did not always correlate with the level of water potential (Ψl) in the studied branches (Fig. 4), although in situ measurements were in line with the vulnerability curve (Fig. S2 in the Supplementary material). Cavitation occurred at decreasing Ψl but its reversal was not followed by increased Ψl as might have been expected. Hölttä et al. ([21]) proposed that Ψl might increase during cavitation due to a capacitive effect. It is possible that such an effect, together with a 0.5-1.5 hr delay, was masked by cavitation reversal.
Stomata, VPD, leaf water potential and cavitation
For a first approximation, we validate our observations of cavitation and recovery cycles semi-quantitatively based on the basic flux equations for approximate transpiration (T) and sap flow (SF - eqn. 3, eqn. 4):
where K is the overall plant hydraulic conductivity. Under wet season conditions Ψl = -1.5 MPa (Tab. 2), T greatly exceeds the tree capacitance, and T and SF are continuous and nearly temporally coupled (T ~ SF at the same point in time - Fig. 5C). In the dry season, the soil water potential (Ψs) decreases from the wet season value of -0.1 MPa to -0.9 MPa (Tab. 2 - [26]), thereby decreasing ΔΨsoil-leaf from 1.4 in the wet season to 0.6 MPa in the early morning of a dry season day and, using eqn. 4, decreasing the driving force for SF. Together with the observed decrease in K for the same time-frame, from 3.0 to 0.5 kg hr-1 MPa-1, SF decreases from 4.5 kg hr-1 in wet season to 0.1 kg hr-1 in the early morning of a dry season day and up to 1.3 kg hr-1 at 11:30 (Tab. 2).
Tab. 2 - Summary of changes in vapor pressure deficit (VPD), leaf water potential (Ψl), soil water potential (Ψs), stomatal conductance (gs), hydraulic conductivity (K), percent loss of conductivity (PLC), leaf transpiration (T), and sap flow (SF) in Pinus halepensis in the Yatir forest. Observed T and SF rates are compared with values calculated using eqn. 3 and eqn. 4, respectively.
| Values | Parameters | Wet season | Dry season | ||
|---|---|---|---|---|---|
| 11:30 | 6:30 | 9:30 | 11:30 | ||
| Observed | VPD (kPa) |
1.5 | 0.4 | 1.9 | 3.4 |
Ψl (MPa) |
-1.5 | -1.5 | -3.0 | -3.0 | |
Ψs (MPa) |
-0.1 | -0.9 | -0.9 | -0.9 | |
gs (mol m-2 s-1) |
0.18 | 0.030 | 0.030 | 0.007 | |
K (kg hr-1 MPa-1) |
3.00 | 0.50 | 0.35 | 0.55 | |
PLC (%) |
0 | 7 | 30 | 0 | |
T (mmol m-2 s-1) |
3.15 | 0.13 | 0.58 | 0.25 | |
SF (kg hr-1) |
4.50 | 0.10 | 0.73 | 1.30 | |
| Calculated | T = gs × VPD (mmol m-2 s-1) |
2.70 | 0.12 | 0.57 | 0.24 |
SF = K × ΔΨs-l (kg hr-1) |
4.20 | 0.30 | 0.73 | 1.16 | |
Conditions change rapidly during the dry season mornings, as in our measurement campaign. VPD increases from 0.4 kPa at 6:30 to 1.9 kPa at 9:30 (Tab. 2). This seems to trigger two parallel processes with contrasting consequences: (1) Ψl decreases from -2.3 to -3.3 MPa (Fig. 4), meaning the development of xylem embolism; and (2) T increases (eqn. 3) from 0.13 mmol m-2 s-1 at 6:30 to 0.58 mmol m-2 s-1 at 9:30. This increase reflects the small change in gs (0.03 mol m-2 s-1 - Tab. 2) while VPD increased.
Evidently, while process (1) decreases the tree hydraulic conductance, process (2) increases the tree hydraulic demand. This, in turn, seems to trigger the next sequence of events: the cavitation of part of the xylem (30% - Tab. 2) decreases overall K from 0.50 to 0.35 kg hr-1 MPa-1 (Tab. 2). This further decouples SF from T, increases the water deficit (~3 kg tree-1 by 9:30) and decreases nRWC (from 77% to 63% in our case). Only then, stomatal closure follows, reducing gs from 0.030 mol m-2 s-1 at 9:30 to 0.007 mol m-2 s-1 at 11:30 (Tab. 2). This over-compensates for the VPD increase (from 1.9 to 3.4 kPa), and hence T decreases from 0.58 mmol m-2 s-1 at 9:30 to 0.25 mmol m-2 s-1 at 11:30 (eqn. 3) and nRWC increases. The parallel decrease in T, increase in SF, and recovery in hydraulic conductance by 11:30 permit the closure of the hydraulic deficit that developed earlier.
Oscillations in gs in water-stressed woody plants have been long related to the feedback loop between T and gs spanning over 2-4 hr in orange trees ([9]). High VPD may be one of the signals that lead to stomatal closure ([38]), thereby facilitating recovery from cavitation. The decrease in gs in mid-morning and its subsequent increase in the afternoon seem to correspond to similar trends in VPD and inverse trends in water deficit. The time course of change in gs, in turn, may influence that of the cavitation/ recovery cycles. For example, in May maximum daily VPD in the Yatir forest is usually below 3.0 kPa ([27]) and hence the midday depression in gs is restricted to 10:30-13:30, compared to 10:00-16:00 in June (Fig. 5F). This can explain the shorter cavitation/recovery cycles observed in May (Fig. 3) than in June. The lower vapor pressure deficit (VPD) in September vs. May meant that stomatal gas exchange was maintained throughout the afternoon hours, which can explain the lack of hydraulic recovery during the day in our September field day (Fig. 3 and Fig. 4). Causality is of course difficult to infer from these patterns. While some studies claim that stomatal activity protects the xylem ([11], [49]), others propose that the xylem imposes a limit on gs and water loss ([5], [50]). Nardini & Salleo ([34]) concluded that in very dry conditions some cavitation could not be avoided, because it acts as a signal regulating gs. This is consistent with the observation that stomatal responses both at 9:15 and 17:15 (Fig. 5F) coincided with a PLC (cavitation level) of ~30% (Fig. 3) and indicates a possible threshold for stomatal response, with the value for initiation of closure corresponding to a water deficit of ~2.5 kg tree-1. Based on these observations, we speculate that while there are complex feedbacks between gs and PLC, actual changes in leaf conductance do not correlate with threshold or cumulative changes in PLC, such that moderate levels of cavitation and recovery of xylem in our extremely dry conditions can be routine during daytime, thus permitting or maximizing leaf gas exchange.
Interactions between hydraulic and physiological adjustments
The pine trees in the semi-arid study site have relatively high productivity and a range of adjustments to the dry conditions. Furthermore, these trees maintain photosynthetic activity throughout the long annual dry season, sufficiently to support the growth of new needles during this period ([27]). At the same time xylem resistance to embolism is not high in P. halepensis (ΨPLC50 > -4.0 MPa) similarly to other pine species ([6]).
Based on the results reported above we speculate that the observed temporal decoupling between water loss from the leaves and water recharge from the soil (Fig. 5G) helps to optimize the trees’ water and gas exchange economy, facilitating their survival in the semi-arid conditions. Such a system operates with a relatively narrow safety margin, at the cost of significant loss in hydraulic conductivity, relying, in turn, on rapid recovery (Fig. 3). The results seem to indicate that high vulnerability to cavitation does not necessarily reflect low levels of resistance to drought, but may be part of the overall optimization between reduced water availability and the requirement for maintaining gas exchange under dry conditions. Equally important, the redistribution of water at both soil and tree levels is an essential component.
Further research is needed to focus on the different aspects of the cavitation/recovery cycle process. In addition, better tools are needed to measure cavitation in the field, facilitating more frequent sampling, which could give insight into the different environmental and physiological factors involved in forest sustainability under increasingly dry conditions in the Mediterranean and other regions.
List of Abbreviations
The following abbreviations have been used throughout the paper:
A: net carbon assimilation;gs: stomatal conductance;K: hydraulic conductivity;nRWC: needle relative water content;PAR: photosynthetic active radiation;PLC: percent loss of conductivity;SF: sap flow;T: transpiration;VPD: vapor pressure deficit.
Acknowledgments
Measurements were performed jointly by all authors. TK conceived the study and wrote the first draft of the manuscript, with all authors contributing to the writing.
TK acknowledges the Karshon foundation grant provided through KKL-JNF and the Rieger foundation grant. TK thanks Dr. Jiri Kucera of EMS Ltd., Czech Republic, and Prof. Katarina Strelcova of Zvolen Technical University, Slovakia, for training with the sap flow measurement system; and Tal Kanety of ARO Volcani, Israel, for assistance in sap flow sensor manufacturing. We thank Prof. Harvey Scher of the Weizmann Institute of Science for helpful discussions.
Funding
This work was supported by KKL-JNF and the Israel ministry of Agriculture; the Sussman Center and the Cathy Wills and Robert Lewis Program in Environmental Science of the Weizmann Institute; the Israel ministry of Science (France-Israel High Council for Research Scientific and Technological Cooperation, project 3-6735); the Minerva Foundation, The Israeli Science Foundation (ISF), and the COST FORMAN (Forest Management and the Water Cycle, FP0601) program.
The authors declare no conflict of interest.
Data Accessibility
All data are included in the manuscript and supporting information.
References
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Yakir Preisler
Eyal Rotenberg
Dan Yakir
Department of Earth and Planetary Sciences, Weizmann Institute of Science, Rehovot 76100 (Israel)
Shabtai Cohen
Indira Paudel
Institute of Soil, Water and Environmental Sciences, ARO Volcani Center, Beit Dagan 50250 (Israel)
Yakir Preisler
Robert H. Smith Institute of Plant Sciences and Genetics in Agriculture, Faculty of Agricultural, Food and Environmental Quality Sciences, the Hebrew University of Jerusalem, Rehovot (Israel)
Corresponding author
Paper Info
Citation
Klein T, Cohen S, Paudel I, Preisler Y, Rotenberg E, Yakir D (2016). Diurnal dynamics of water transport, storage and hydraulic conductivity in pine trees under seasonal drought. iForest 9: 710-719. - doi: 10.3832/ifor2046-009
Academic Editor
Silvano Fares
Paper history
Received: Mar 08, 2016
Accepted: Jul 19, 2016
First online: Aug 21, 2016
Publication Date: Oct 13, 2016
Publication Time: 1.10 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2016
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 54535
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 43790
Abstract Page Views: 4124
PDF Downloads: 5181
Citation/Reference Downloads: 62
XML Downloads: 1378
Web Metrics
Days since publication: 3459
Overall contacts: 54535
Avg. contacts per week: 110.36
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2016): 35
Average cites per year: 3.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Research Articles
Electrochemical in-situ studies of solar mediated oxygen transport and turnover dynamics in a tree trunk of Tilia cordata
vol. 10, pp. 355-361 (online: 07 March 2017)
Technical Reports
Detecting tree water deficit by very low altitude remote sensing
vol. 10, pp. 215-219 (online: 11 February 2017)
Review Papers
Revisiting the Heat Field Deformation (HFD) method for measuring sap flow
vol. 11, pp. 118-130 (online: 07 February 2018)
Research Articles
Alterations on flow variability due to converting hardwood forests to pine
vol. 5, pp. 44-49 (online: 02 April 2012)
Research Articles
Classification of xeric scrub forest species using machine learning and optical and LiDAR drone data capture
vol. 18, pp. 357-365 (online: 07 December 2025)
Research Articles
Sap flow, leaf-level gas exchange and spectral responses to drought in Pinus sylvestris, Pinus pinea and Pinus halepensis
vol. 10, pp. 204-214 (online: 01 November 2016)
Research Articles
Oak sprouts grow better than seedlings under drought stress
vol. 9, pp. 529-535 (online: 17 March 2016)
Research Articles
Sodium and potassium allocation under drought stress in Atlas mastic tree (Pistacia atlantica subsp. mutica)
vol. 6, pp. 90-94 (online: 07 February 2013)
Research Articles
Establishment of a planted field with Mediterranean shrubs in Sardinia and its evaluation for climate mitigation and to combat desertification in semi-arid regions
vol. 2, pp. 77-84 (online: 10 June 2009)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword