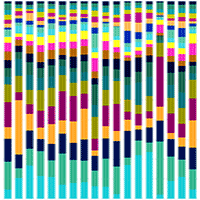
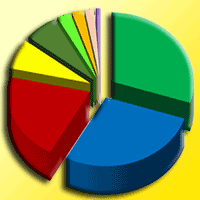
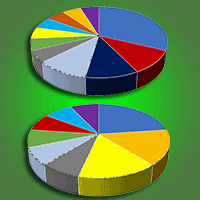
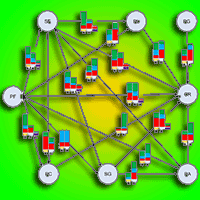
Mexico is a center of diversity for the genus Pinus, with 44% of pine species being endemic to the country. Mexican pine forests are recognized as hotspots for ectomycorrhizal fungi and bacteria due to the extensive interactions that take place between microorganisms and plants in their roots. These microorganisms play a vital role in the survival of pine species. This study aims to identify fungal and bacterial communities in a relict Mexican pine forest and evaluate the influence of soil physicochemical parameters on microbial composition. Sampling was conducted along a 145 m transect in an isolated natural relict of P. pseudostrobus var. coatepecensis, which is located within a commercial plantation of Pinus patula. A total of 18 soil samples were collected at predetermined distances along the transect, with replicated sampling points as follows: six samples at 20 cm intervals, four samples at 1 m intervals, four samples at 10 m intervals, and four samples at 25 m intervals. The results indicate that fungal composition varies even at short distances and is influenced by the C:N ratio, total carbon (C), total phosphorus (P), and total hydrogen ion concentration (H+). Ectomycorrhizal fungi (EcM) exhibited a higher relative abundance compared to saprotrophic and pathogenic fungi. A total of 69 EcM ASVs (Amplicon Sequence Variants) were identified, being the dominant genera Tomentella, Clavulina, Suillus, Russula, and Elaphomyces. Bacterial communities did not show significant variation in relation to the distance from the sampling points, but soil pH was identified as the main factor of bacterial composition. Dominant bacterial genera included Burkholderia, Bryobacter, Acidobacterium, and Acidothermus. Additionally, it was observed that current soil conditions influenced β diversity. Overall, the results demonstrate that soil fungal and bacterial communities associated with P. pseudostrobus exhibit a unique composition compared to other natural forest systems in the Neotropics.
Keywords
, , , , ,
Citation
Baeza-Guzmán Y, Camargo-Ricalde SL, Trejo Aguilar D, Montaño NM (2023). Fungal and bacterial communities in a forest relict of Pinus pseudostrobus var. coatepecensis. iForest 16: 299-306. - doi: 10.3832/ifor4284-016
Academic Editor
Alberto Santini
Paper history
Received: Dec 07, 2022
Accepted: Sep 13, 2023
First online: Nov 09, 2023
Publication Date: Dec 31, 2023
Publication Time: 1.90 months
© SISEF - The Italian Society of Silviculture and Forest Ecology 2023
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.

Breakdown by View Type
(Waiting for server response...)
Article Usage
Total Article Views: 17276
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 13152
Abstract Page Views: 2295
PDF Downloads: 1480
Citation/Reference Downloads: 1
XML Downloads: 348
Web Metrics
Days since publication: 828
Overall contacts: 17276
Avg. contacts per week: 146.05
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2023): 2
Average cites per year: 0.67
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
(1)
Aceves-Rangel LD, Méndez-González J, García-Aranda MA, Nájera-Luna JA (2018)Distribución potencial de 20 especies de pinos en México [Potential distribution of 20 pine species in Mexico]. Agrociencia 52 (7): 1043-1057.
Online |
Gscholar
(2)
Aguirre F, Nouhra E, Urcelay C (2021)Native and non-native mammals disperse exotic ectomycorrhizal fungi at long distances from pine plantations. Fungal Ecology 49: 101012.
CrossRef |
Gscholar
(3)
Alvarez-Manjarrez J, Villegas-Ríos M, Garibay-Orijel R, Contreras-Pacheco M, Kõljalg U (2016)Tomentella brunneoincrustata, the first described species of the Pisonieae-associated Neotropical Tomentella clade, and phylogenetic analysis of the genus in Mexico. Mycological Progress 15 (1): 1-11.
CrossRef |
Gscholar
(4)
Argüelles-Moyao A, Garibay-Orijel R, Márquez- Valdelamar LM, Arellano-Torrez E (2016)Clavulina-Membranomyces is the most important linage within the highly diverse ectomycorrhizal fungal community of
Abies religiosa. Mycorrhiza 27: 53-65.
CrossRef |
Gscholar
(5)
Avolio M, Müller T, Mpangara A, Fitz M, Becker B, Pauck A, Kirsch A, Wipf D (2012)Regulation of genes involved in nitrogen utilization on different C/N ratios and nitrogen sources in the model ectomycorrhizal fungus
Hebeloma cylindrosporum. Mycorrhiza 22: 515-524.
CrossRef |
Gscholar
(6)
Baeza-Guzmán Y, Medel-Ortiz R, Garibay-Orijel R (2017)Caracterización morfológica y genética de los hongos ectomicorrízicos asociados a bosques de
Pinus hartwegii en el Parque Nacional Cofre de Perote, Veracruz [Morphologic and genetic characterization of the ectomycorrhizal fungi associated to
Pinus hartwegii forests from Cofre de Perote National Park, Veracruz]. Revista Mexicana de Biodiversidad 88 (1): 41-48. [in Spanish]
CrossRef |
Gscholar
(7)
Baselga A (2010)Partitioning the turnover and nestedness components of beta diversity. Macroecological Methods 19 (1): 134-143.
CrossRef |
Gscholar
(8)
Bennett JA, Maherali H, Reinhart KO, Lekberg Y, Hart MM, Klironomos J (2017)Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 355 (6321): 181-184.
CrossRef |
Gscholar
(9)
Boeraeve M, Honnay O, Mullens N, Vandekerkhove K, De Keersmaeker L, Thomaes A, Jacquemyn H (2018)The impact of spatial isolation and local habitat conditions on colonization of recent forest stands by ectomycorrhizal fungi. Forest Ecology and Management 429: 84-92.
CrossRef |
Gscholar
(10)
Bray RH, Kurtz LT (1945)Determination of total organic and available forms of phosphorus in soils. Soil Science 59 (1): 39-45.
CrossRef |
Gscholar
(11)
Bremner JM, Mulvaney CS (1982)Nitrogen-Total. In: “Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties” (Page AL, Miller RH, Keeney DR eds). American Society of Agronomy, Soil Science Society of America, Madison, WI, USA, pp. 595-624.
Gscholar
(12)
Byers AK, Condron L, Donavan T, O’Callaghan M, Patuawa T, Waipara N, Black A (2020)Soil microbial diversity in adjacent forest systems-contrasting native old growth kauri (
Agathis australis) forest with exotic pine (
Pinus radiata) plantation forest. FEMS Microbiology Ecology 96 (5): fiaa047.
CrossRef |
Gscholar
(13)
Callahan BJ, McMurdie PJ, Holmes SP (2017)Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. The ISME Journal 11: 263-264.
CrossRef |
Gscholar
(14)
Cataldo DA, Maroon M, Schrader LE, Youngs VL (1975)Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Communications in Soil Science and Plant Analysis 6: 71-80.
CrossRef |
Gscholar
(15)
Chapman HD, Pratt PF (1962)Methods of analysis for soils, plants, and waters. Soil Sciences 93 (1): 68.
Gscholar
(16)
Clausing S, Likulunga LE, Janz D, Feng HY, Schneider D, Daniel R, Krüger J, Lang F, Polle A (2021)Impact of nitrogen and phosphorus addition on resident soil and root mycobiomes in beech forests. Biology and Fertility of Soils 57 (8): 1031-1052.
CrossRef |
Gscholar
(17)
Correia M, Espelta JM, Morillo JA, Pino J, Rodríguez-Echeverría S (2021)Land-use history alters the diversity, community composition and interaction networks of ectomycorrhizal fungi in beech forests. Journal of Ecology 109 (8): 2856-2870.
CrossRef |
Gscholar
(18)
Domínguez-Castillo C, Alatorre-Cruz JM, Castañeda-Antonio D, Munive JA, Guo X, López-Olguín JF, Fuentes-Ramírez LE, Carreño-López R (2021)Potential seed germination-enhancing plant growth-promoting rhizobacteria for restoration of
Pinus chiapensis ecosystems. Journal of Forestry Research 32 (5): 2143-2153.
CrossRef |
Gscholar
(19)
Druebert C, Lang C, Valtanen K, Polle A (2009)Beech carbon productivity as driver of ectomycorrhizal abundance and diversity. Plant, Cell and Environment 32: 992-1003.
CrossRef |
Gscholar
(20)
IUSS Working Group WRB (2015)Base referencial mundial del recurso suelo 2014, Actualización 2015 [Global Soil Resource Reference Database 2014, Update 2015]. FAO, Rome, Italy, pp. 106. [in Spanish]
Gscholar
(21)
Fernandez CW, See CR, Kennedy PG (2020)Decelerated carbon cycling by ectomycorrhizal fungi is controlled by substrate quality and community composition. New Phytologist 226 (2): 569-582.
CrossRef |
Gscholar
(22)
Gavito ME, Leyva-Morales R, Vega-Pena EV, Arita H, Jairus T, Vasar M, Opik M (2019)Local-scale spatial diversity patterns of ectomycorrhizal fungal communities in a subtropical pine-oak forest. Fungal Ecology 42: 100860.
CrossRef |
Gscholar
(23)
Heredia-Acuña C, Almaraz-Suarez JJ, Arteaga-Garibay R, Ferrera-Cerrato R, Pineda-Mendoza D (2018)Isolation characterization and effect of plant-growth-promoting rhizobacteria on pine seedlings (
Pinus pseudostrobus Lindl). Journal of Forestry Research 30: 1727-1734.
CrossRef |
Gscholar
(24)
Högberg MN, Skyllberg U, Högberg P, Knicker H (2020)Does ectomycorrhiza have a universal key role in the formation of soil organic matter in boreal forests? Soil Biology and Biochemistry 140: 107635.
CrossRef |
Gscholar
(25)
Jimu L, Nyakudya IW, Magogo C, Mureva A (2020)Impact of pine plantation establishment on soil properties and fungal communities of natural forests in Zimbabwe. Southern Forests 82 (3): 263-270.
CrossRef |
Gscholar
(26)
Kataoka R, Siddiqui ZA, Kikuchi J, Ando M, Sriwati R, Nozaki A, Futai K (2012)Detecting nonculturable bacteria in the active mycorrhizal zone of the pine mushroom
Tricholoma matsutake. The Journal of Microbiology 50: 199-206.
CrossRef |
Gscholar
(27)
Kielak AM, Barreto CC, Kowalchuk GA, Van Veen JA, Kuramae EE (2016)The ecology of Acidobacteria: moving beyond genes and genomes. Frontiers in Microbiology 7: 744.
CrossRef |
Gscholar
(28)
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006)World map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift 15 (3): 259-263.
CrossRef |
Gscholar
(29)
Lammel D, Barth G, Ovaskainen O, Cruz L, Zanatta J, Ryo M, De Souza E, Pedrosa F (2018)Direct and indirect effects of a pH gradient bring insights into the mechanisms driving prokaryotic community structures. Microbiome 6: 106.
CrossRef |
Gscholar
(30)
Lladó S, López-Mondéjar R, Baldrian P (2017)Forest soil bacteria: diversity, involvement in ecosystem processes, and response to global change. Microbiology and Molecular Biology Reviews 81 (2): e00063-16.
CrossRef |
Gscholar
(31)
Nguyen NH, Bruns TD (2015)The microbiome of
Pinus muricata ectomycorrhizae: community assemblages, fungal species effects, and
Burkholderia as important bacteria in multipartnered symbioses. Microbial Ecology 69 (4): 914-21.
CrossRef |
Gscholar
(32)
Ni Y, Yang T, Ma Y, Zhang K, Soltis PS, Soltis DE, Gilbert JA, Zhao Y, Fu C, Chu H (2021)Soil pH determines bacterial distribution and assembly processes in natural mountain forests of eastern China. Global Ecology and Biogeography 30 (11): 2164-2177.
CrossRef |
Gscholar
(33)
Nilsson R, Larsson K, Taylor A, Bengtsson-Palme J, Jeppesen T, Schigel D, Kennedy P, Picard K, Glöckner F, Tedersoo L, Saar I, Kõljalg U, Abarenkov K (2019)The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Research 47 (D1): D259-D264.
CrossRef |
Gscholar
(34)
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2013)The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Research 41: D590-D596.
CrossRef |
Gscholar
(35)
R Core Team (2020)R: a language and environment for statistical computing. The R Foundation for Statistical Computing, Vienna, Austria.
Online |
Gscholar
(36)
Rivera VR, Navarro-Noya YE, Dendooven L, Guido ML (2022)Land-use change alters the bacterial community structure, but not forest management. Folia Microbiologica 68: 277-290.
CrossRef |
Gscholar
(37)
Reis F, Magalhães AP, Tavares RM, Baptista P, Lino-Neto T (2021)Bacteria could help ectomycorrhizae establishment under climate variations. Mycorrhiza 31 (3): 395-401.
CrossRef |
Gscholar
(38)
Reverchon F, Del Pilar Ortega-Larrocea M, Pérez-Moreno J (2012)Soil factors influencing ectomycorrhizal sporome distribution in neotropical forests dominated by
Pinus montezumae Mexico. Mycoscience 53 (3): 203-210.
CrossRef |
Gscholar
(39)
Rodríguez-Ramos JC, Cale JA, Cahill Jr JF, Simard SW, Karst J, Erbilgin N (2021)Changes in soil fungal community composition depend on functional group and forest disturbance type. New Phytologist 229 (2): 1105-1117.
CrossRef |
Gscholar
(40)
Rog I, Rosenstock NP, Körner C, Klein T (2020)Share the wealth: trees with greater ectomycorrhizal species overlap share more carbon. Molecular Ecology 29 (13): 2321-2333.
CrossRef |
Gscholar
(41)
Rosinger C, Sandén H, Matthews B, Mayer M, Godbold DL (2018)Patterns in ectomycorrhizal diversity, community composition, and exploration types in European beech, pine, and spruce forests. Forests 9 (8): 445.
CrossRef |
Gscholar
(42)
Santini NS, Adame MF, Nolan RH, Miquelajauregui Y, Piñero D, Mastretta-Yanes Cuervo-Robayo P, Eamus D (2019)Storage of organic carbon in the soils of Mexican temperate forests. Forest Ecology and Management 446: 115-125.
CrossRef |
Gscholar
(43)
Sinclair L, Osman OA, Bertilsson S, Eiler A (2015)Microbial community composition and diversity
via 16S rRNA gene amplicons: evaluating the illumina platform. PLoS One 10(2): e0116955.
CrossRef |
Gscholar
(44)
Spiesman BJ, Stapper AP, Inouye BD (2018)Patch size isolation and matrix effects on biodiversity and ecosystem functioning in a landscape microcosm. Ecosphere 9 (3): e02173.
CrossRef |
Gscholar
(45)
Tedersoo L, Bahram M, Põlme S, Kõljalg U, Yorou NS, Wijesundera R, Villarreal Ruiz L, Vasco-Palacios AM, Thu PQ, Suija A, Smith ME, Sharp C, Saluveer E, Saitta A, Rosas M, Riit T, Ratkowsky D, Pritsch K, Põldmaa K, Piepenbring M, Phosri C, Peterson M, Parts K, Pärtel K, Otsing E, Nouhra E, Njouonkou AL, Nilsson RH, Morgado LN, Mayor J, May TW, Majuakim L, Lodge DJ, Lee SS, Larsson KH, Kohout P, Hosaka K, Hiiesalu I, Henkel TW, Harend H, Guo LD, Greslebin A, Grelet G, Geml J, Gates G, Dunstan W, Dunk C, Drenkhan R, Dearnaley J, De Kesel A, Dang T, Chen X, Buegger F, Brearley FQ, Bonito G, Anslan S, Abell S, Abarenkov K (2014)Fungal biogeography. Global diversity and geography of soil fungi. Science 346 (6213): 1256688.
CrossRef |
Gscholar
(46)
Villarreal-Ruiz L, Neri-Luna C (2018)Testing sampling effort and relative abundance descriptors of belowground ectomycorrhizal fungi in a UK planted scots pine woodland. Mycology 9 (2): 106-115.
CrossRef |
Gscholar
(47)
Villegas-Jiménez D, Rodríguez-Ortiz G, Chávez-Servia J, Enríquez-Del-Valle J, Rodríguez J (2016)Variación del crecimiento en vivero entre procedencias de
Pinus pseudostrobus Lindl [Growth variation in nursery among provenances of
Pinus pseudostrobus Lindl]. Gayana Botánica 73 (1): 113-123. [in Spanish]
CrossRef |
Gscholar
(48)
Viswanathan A, Ghazoul J, Honwad G, Kumar N, Bagchi R (2019)The effects of rainforest fragment area on the strength of plant-pathogen interactions. Biology Letters 15: 20180493.
CrossRef |
Gscholar
(49)
Yu F, Liang JF, Song J, Wang S, Lu J (2020)Bacterial community selection of
Russula griseocarnosa mycosphere soil. Frontiers in Microbiology 11: 347.
CrossRef |
Gscholar
(50)
Zavišić A, Nassal P, Yang N, Heuck C, Spohn M, Marhan S, Pena R, Kandeler E, Polle A (2016)Phosphorus availabilities in beech (
Fagus sylvatica L.) forests impose habitat filtering on ectomycorrhizal communities and impact tree nutrition. Soil Biology and Biochemistry 98: 127-137.
CrossRef |
Gscholar
(51)
Zhang W, Wang X, Li Y, Wei P, Sun N, Wen X, Liu Z, Li D, Feng Y, Zhang X (2022)Differences between microbial communities of
Pinus species having differing level of resistance to the pine wood nematode. Microbial Ecology 84 (4): 1245-1255.
CrossRef |
Gscholar