Red wood ants shape epiphytic lichen assemblages in montane silver fir forests
iForest - Biogeosciences and Forestry, Volume 15, Issue 1, Pages 71-76 (2022)
doi: https://doi.org/10.3832/ifor3897-014
Published: Feb 22, 2022 - Copyright © 2022 SISEF
Research Articles
Abstract
The Formica rufa group comprises several ant species which are collectively referred to as “red wood ants” (hereafter RWA). These species have key roles in forest ecosystems, where they are ecologically dominant and greatly influence the dynamics of the habitat they colonise. Various studies have shown how their trophic activity may affect other organisms, which include both other invertebrates and plants. We can therefore hypothesize that their presence could affect the taxonomic and functional composition of epiphytes, despite clear information on such an effect is lacking. This study aimed to fill this research gap by evaluating whether the presence of red wood ants could affect the structure and composition of lichen communities. We selected two sites on the Apennine Mountains in Italy, where the red wood ant F. paralugubris was introduced from the Alps more than 50 years ago. In each site, lichen assemblages on Abies alba trees located within the colonised areas were compared to those from nearby, non-occupied areas. The results allowed for the identification of significant effects of F. paralugubris on the structure of lichen communities. Although there was no detectable impact on lichen species richness, a significant difference in their community composition between colonised and control sites was detected. Furthermore, ant presence seemed to be associated with specific lichen functional traits such as asexual reproduction. We argue that RWA could affect the lichen community either directly, e.g., by actively dispersing the species capable of asexual reproduction through their movements on trees (ant-mediated dispersion), or indirectly through herbivore exclusion. Finally, we also observed differences in β-diversity among the colonised and non-colonised sites.
Keywords
Formica paralugubris, Red Wood Ants, Lichen Diversity, Impact, Introduced Species, Functional Diversity
Introduction
Animal-plant interactions are widespread and extend beyond the known consumer-resource association in a wide array of relationships. As such, these interactions have long ago attracted the interest of scientists and today constitute an important field of research ([24], [10]). Among the many documented examples, those involving arthropods, particularly ants species, are the most intriguing ([42]). Ants are one of the most diverse, abundant, and ecologically dominant animal groups worldwide, and their impact on ecosystem function is correspondingly significant ([27]). Most of the available literature is biased towards angiosperms, and comparatively little is known about other organisms such as lichens.
Lichens are symbiotic poikilohydric organisms formed by a fungus, the mycobiont, and one or more algae, the photobiont ([34]). Together with bryophytes, these cryptogamic organisms represent an important component of forest ecosystems and biodiversity. They are key organisms in ecosystem functioning, since they are primary producers involved, for example, in water and nutrient cycles ([37], [40]). Several interactions are known to occur between lichens and animals ([4]). Lichens can be a feeding substrate for both some vertebrates and several invertebrates. For example, gastropods are known to feed on lichens ([8]) and some species are specialised lichen-feeders ([26]). On the other hand, lichens provide a microhabitat where an organism could find particular temperature or moisture conditions. Invertebrates could find protection and food in the interface between the thallus and substrate, especially in the case of foliose lichens ([3]). Moreover, although lichens rely mainly on wind for dispersion, several forms of zoochory by both invertebrates and vertebrates have been documented. In particular, ants can disperse both lichen soredia ([29]) and thallus fragments ([23]). Soredia can attach to ants’ bodies during their passage on the surface of lichen thalli, or in some cases, wind-borne spores are carried in contact with ant body ([5]). In the case of thallus fragments, some ants use them to build nests, collecting fragments in the surrounding areas and thus providing maintenance of epigeous lichen diversity ([23]). This harvesting behaviour has also been investigated to explain the negative correlation between epiphytic macrolichen richness and ants ([46]). Nevertheless, to the best of our knowledge, the effect of ants on the overall lichen epiphytic community has never been investigated.
Red wood ants (hereafter: RWA) are ecologically dominant species belonging to the Formica rufa group, with seven species described in Europe and at least 19 species reported in North America ([45]). RWA are cold-adapted species inhabiting coniferous woodlands ([43]), and in the southern part of their range they are restricted to mountain areas, rarely occurring below 900 m a.s.l. In Italy, these species are widespread along the Alpine chain and only the more thermophilic Formica pratensis (Retzius, 1783) naturally occurs south of the Alps in the Apennine mountains. Moreover, starting in the mid-1900s, colonies of Formica lugubris (Zetterstedt, 1838), F. polyctena (Foerster, 1850) and F. aquilonia (Yarrow, 1955) were repeatedly transplanted as a biocontrol agent for forest insect pests from their original areas in the Alps to other sites in the Apennines, where they were formerly absent. The current status of most of the introduced populations is unknown. In a few cases, local extinctions have been documented, especially in less suitable and warmer areas, whereas some of the introduced populations have grown considerably ([15], [6]).
Most RWA have a strong influence on forest ecosystems ([47]), affecting both nutrient cycling and ecosystem function ([17]). They also affect a wide range of co-occurring animal taxa, which includes other ant species ([45]), herbivorous insects ([41]), predatory arthropods ([44]) and birds ([1]). Moreover, RWA can affect plant communities both indirectly, mediated by their action on aphids or other plant parasites ([13]), and directly through seed dispersion or soil enrichment ([36]). In particular, RWA can be effective seed dispersers for plants with elaiosome-bearing seeds or fruits that represent a valuable trophic resource ([21]). More recently, Thunes et al. ([46]) suggested that F. aquilonia colonies can impact lichen species richness, removing them from the tree bark along their foraging trails to aphids in the canopy, and sometimes collecting them as nest material. Because of these effects, RWA are interesting candidates to explore ant-lichen interactions.
In this study, we investigated the effects of Formica paralugubris populations on lichen assemblages at two sites in the Central Apennine Mountains, in Italy. In particular, we compared the lichen species richness, lichen assemblage composition, community functional traits, and β-diversity between areas within and outside of the range occupied by F. paralugubris. The estimation of β-diversity has proven to a be an effective tool to better understand mechanisms determining differences between communities ([33]).
Materials and methods
Study areas and sampling
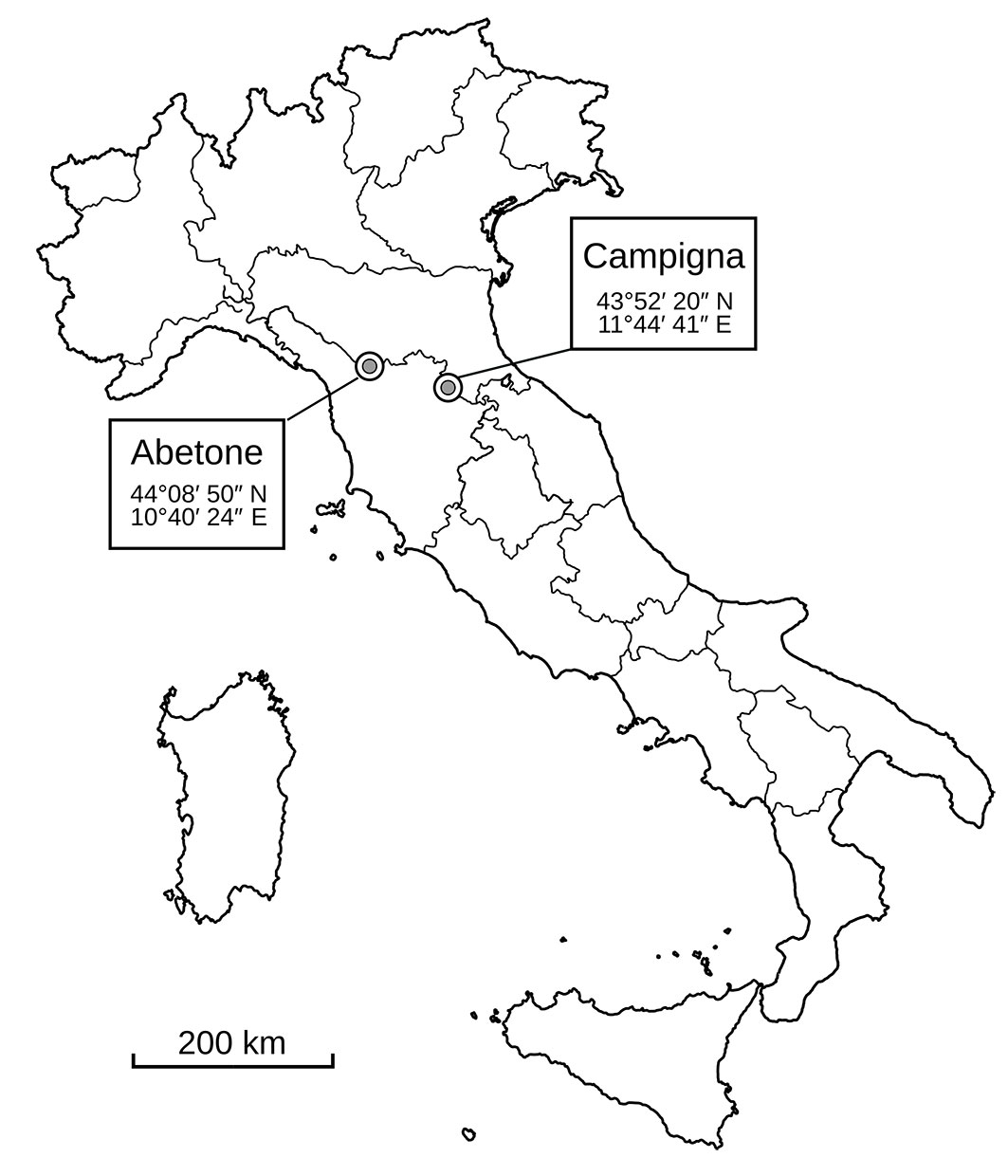
The study was carried out in Abetone forest (AB - 44° 08′ 50″ N, 10° 40′ 24″ E) and in the Campigna Biogenetic Nature Reserve (hereafter referred to as Campigna, CA - 43° 52′ 20″ N, 11° 44′ 41″ E), where RWA populations have been transplanted since the late ’50 ([22] - Fig. 1). According to IV level Corine Land Cover for the Tuscany region, both sites can be classified as coniferous woodlands (CLC code 3123) dominated by white fir (Abies alba). Both sites have similar elevational range (1200-1350 m a.s.l.) and the mean annual precipitation is 2325 mm and 1682.1 mm for Abetone ([7]) and Campigna ([20]), respectively.
In Campigna, the RWA population is subdivided into several independent sub-populations displaced in the area, one of which is located in Avorniolo Alto, occupies an area of about 8 ha, and was chosen for this study ([16]). In the area of Abetone, a single population exists and covers an area of about 10 ha. Despite previous identifications described RWA populations in both areas as F. lugubris based on morphological characteristics ([22]), here the target populations were identified as F. paralugubris following the methods described in Masoni et al. ([31]). The nest density per hectare was similar between sites (AB: ~13 nest ha-1, CA: ~12 nests ha-1). For each area, we randomly selected three F. paralugubris nests spaced at least 150 metres from each other. Six silver fir (Abies alba Mill., 1759) trees located within 20 m from each nest were chosen to sample lichen communities. The fir trees were as evenly spaced in all directions around the nests as possible. Additionally, we randomly selected as control sites three points from nearby non-occupied areas, where F. paralugubris was known to be never introduced or present ([22]). As a result of their reproductive strategy based on colony budding, populations of this ant have sharp, easily recognisable boundaries ([11]). Moreover, as these populations are under study since 2015, their boundaries are well mapped ([15]). The control areas shared similar environmental features of the occupied areas (i.e., altitude, type of forest stand, undergrowth, orientation, and slope). For each site, the lichen communities present on the randomly selected Abies trees were assessed.
Epiphytic lichens were sampled using four standard frames of 10 × 50 cm, subdivided into five 10 × 10 cm quadrants as sampling grids, which were vertically attached to the tree trunk at three different heights above the soil (with the lower edge at 0, 50, and 100 cm). At 0 and 100 cm, the frames were attached at the cardinal points, while at 50 cm, they were rotated by 45° with respect to the cardinal points. All lichen species occurring within the frames were listed, and their frequency was recorded as the number of quadrats in which the species occurred. Most species were identified in the field, while critical specimens were collected and identified in the laboratory using standard stereo- and light microscopy techniques and chemical reagents. Following Nimis & Martellos ([35]), we described lichen diversity considering three functional traits evaluated for each species: growth form (GF), reproductive strategy (RS), and presence of lichenic acids (LA - Tab. S1 in Supplementary material), which have been previously used to compare lichen communities ([19], [25]).
Data analysis
Differences in observed lichen species density (defined as the number of species observed in each sampled tree), carried out by comparing trees with and without RWA at both sites, were assessed using Generalised Linear Mixed Models (GLMM) with Poisson error distribution, including the nest as a random variable ([48]).
Compositional differences within sites were investigated using multivariate techniques. The multivariate distances among samples were computed using the Bray-Curtis dissimilarity index after fourth root transformation to reduce the influence of extreme values. We then performed non-Metric Multidimensional Scaling (nMDS) ordination analysis on the resulting distance matrix, according to Clarke & Warwick ([12]). As described in Anderson ([2]), differences in species composition were tested within each site using a permutation-based non-parametric Multivariate Analysis of Variance (npMANOVA), using the presence of the ants as the main fixed factor and the plot as a nested random factor.
For the analysis of functional traits, we computed the Community Weighted Mean (CWM), which represents the weighted mean trait in a community and accounts for the abundance of the species that carries the considered trait ([38]). The CWM of categorical traits was measured as the relative abundance of the category or group in the community, while the CWM of continuous traits was calculated as the trait average value ([28]). The computed CWM values were fitted onto the ordination axes using the function “envfit” of the Vegan/R package.
β-diversity was computed to evaluate the lichen species diversity and turnover among the areas tested. We applied the SDR simplex approach ([39]), comparing for both areas the plots with and without F. paralugubris. We computed the three additive components of β-diversity: the relativized species replacement (R), the relativized richness difference (D), and similarity (S). This method proceeded by comparing all pairs of plots and computing these three components of β-diversity to analyse lichen species presence.
All analyses were carried out in R v. 3.6 using the packages “vegan”, “lme4”, “FD”, and “BiodiversityR”.
Results
On the 72 A. alba sampled trees we found 65 lichen species (Tab. S1 in Supplementary material), belonging to 40 genera, whose density in both sites did not vary according to RWA presence (z = -0.572, P = 0.567).
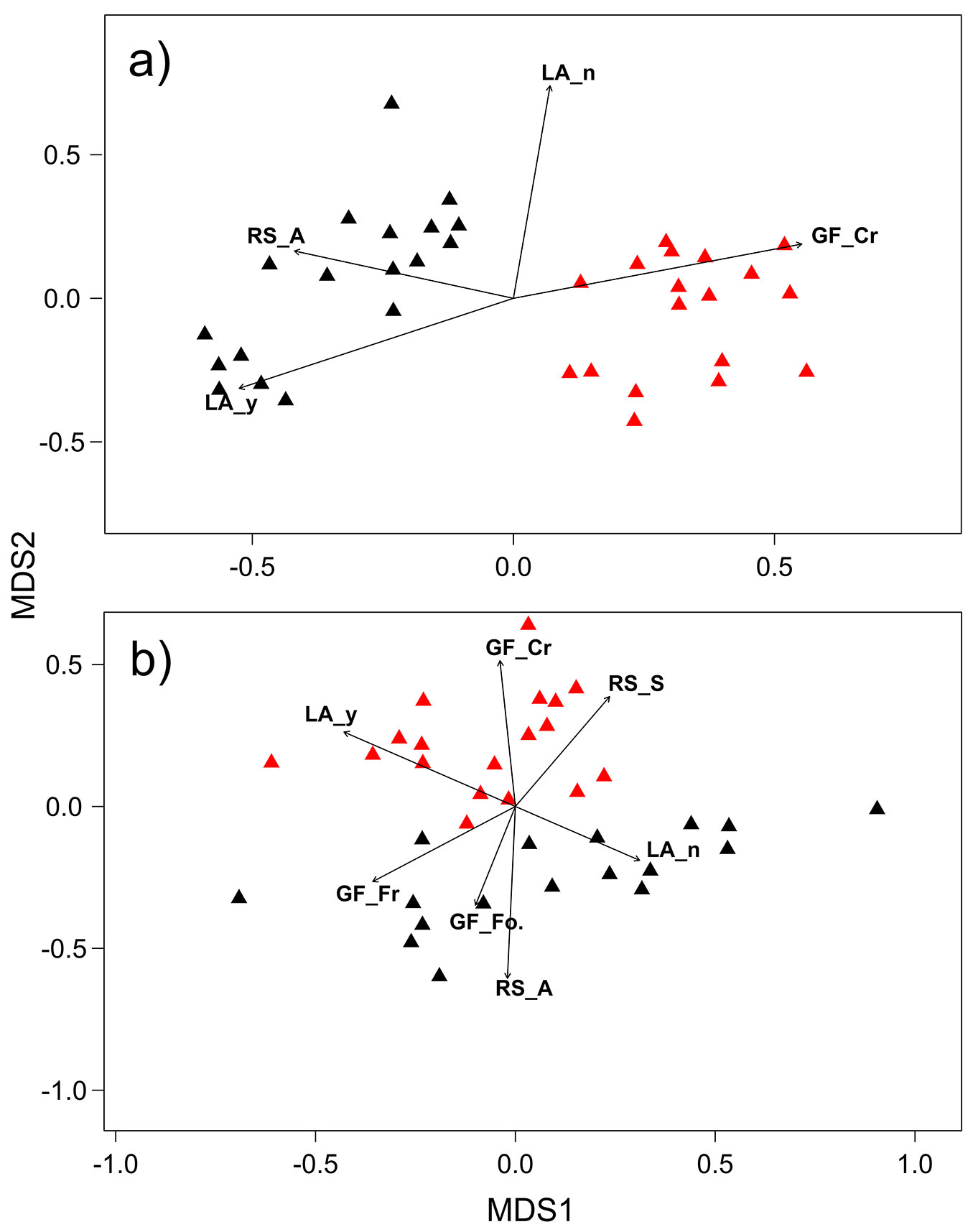
Focusing on lichen species composition within each site, nMDS ordination plots (Fig. 2) for both AB (stress = 0.18) and CA (stress = 0.13) showed a clear separation of ant-visited trees compared to those from control sites. In both AB and CA sites, npMANOVA revealed a significant effect of ant presence (AB: F[1, 2] = 27.77, P = 0.001; CA: F[1, 2] = 11.15, P = 0.001), but also significant variability among plots within each site (AB: F[1, 4] = 5.50, P = 0.001; CA: F[1, 4] = 6.68, P = 0.001).
Fig. 2 - Non-metric multidimensional scaling plot of tree lichen composition sample dissimilarities in Abetone (a) and Campigna (b), according to ant presence. Black symbols: tree within the ant range; red symbols: control trees. The significant functional traits are plotted as vectors. Growth form: (GF_F) fruticose; (GF_Fo) foliose; (GF_Cr) crustose. Reproductive strategy: (RS_A) asexual; (RS_S) sexual. Presence of lichenic acids: (LA_y) presence; (LA_n) absence. The orientation of vectors represents the correlation direction with ant presence.
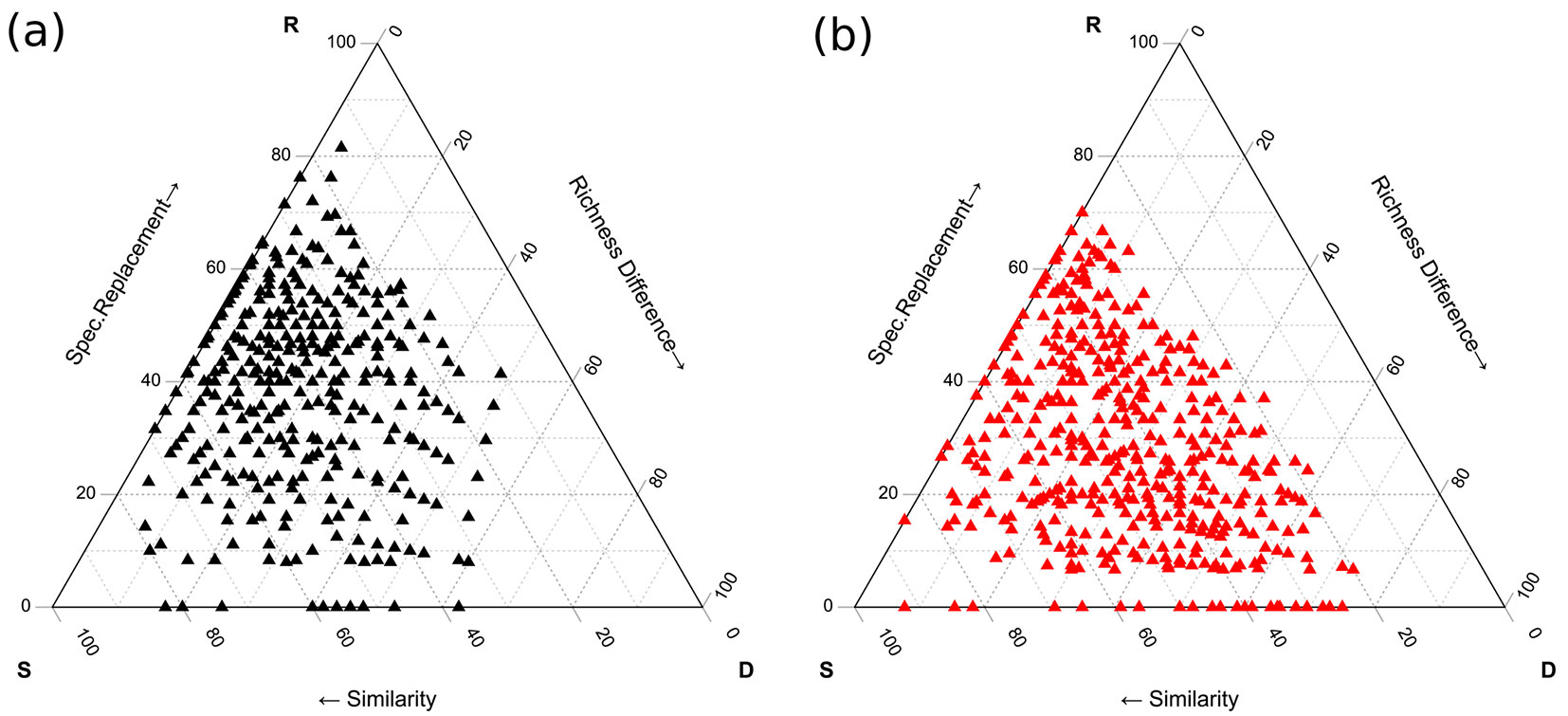
CWM values varied according to ant presence (Tab. 1, Fig. 2). At Abetone, the crustose growth form (GF_Cr) was negatively associated with RWA presence, while the asexual reproductive strategy (RS_A) and the presence of lichenic acids (LA_y) were positively associated with ants. This was also evident in the nMDS plot, where their vectors pointed toward the RWA-sites (Fig. 2a). In Campigna (Fig. 2b), the sites with ants were related with a higher abundance of lichen species with an absence of lichenic acids (LA_n), asexual reproductive strategy (RS_A) and a foliose (GF_Fo) and fruticose (GF_Fr) growth form. On the contrary, these sites were negatively related to crustose growth form (GF_Cr), presence of lichenic acids (LA_y), and sexual reproductive strategy (RS_S). β-diversity results (Fig. 3) indicated that plots with RWA were more variable, with a higher value of species replacement (R = 37.4) and lower values of similarity (S = 41.5) and relative richness difference (D = 20.9) compared to control areas (R = 29.1, S = 43.8, D = 27.1).
Tab. 1 - Correlation values between Community Weighted Mean (CWM) of each functional trait and ordination axes at both sites. For traits significantly correlated with axes, the type of effect summarizes whether RWA presence has a positive (+), negative (-) or no influence (n) on the trait. (0): not tested. Growth form: (GF_F) fruticose; (GF_Fo) foliose; (GF_Cr) crustose. Reproductive strategy: (RS_A) asexual; (RS_S) sexual. Presence of lichenic acids: (LA_y) presence; (LA_n) absence.
| Site | Trait | r2 | P | Direction |
|---|---|---|---|---|
| Abetone | GF_Cr | 0.3706 | 0.001 | - |
| GF_Fo | 0.0422 | 0.514 | 0 | |
| GF_Fr | 0.0001 | 0.998 | 0 | |
| RS_A | 0.2208 | 0.021 | + | |
| RS_S | 0.0100 | 0.859 | 0 | |
| LA_n | 0.6009 | 0.001 | n | |
| LA_y | 0.4068 | 0.001 | + | |
| Campigna | GF_Cr | 0.3658 | 0.002 | - |
| GF_Fo | 0.1805 | 0.041 | + | |
| GF_Fr | 0.2723 | 0.004 | + | |
| RS_A | 0.5062 | 0.001 | + | |
| RS_S | 0.2833 | 0.006 | - | |
| LA_n | 0.1829 | 0.035 | + | |
| LA_y | 0.3484 | 0.004 | - |
Fig. 3 - Three β-diversity components estimated in the areas with (a) and without F. paralugubris (b). (R): relative species replacement; (D): relative richness difference; (S): similarity.
Discussion
Overall, our results showed that the lichen epiphytic communities significantly varied according to RWA presence in the two surveyed areas. Ants affected lichen communities in terms of species composition. In particular, they seemed to affect lichen functional traits, favouring species with asexual reproduction. One possible explanation is that they unintentionally transport lichen propagules during their movements, acting as a vector for lichen asexual dispersion in surrounding trees ([29]).
In our sites, lichen richness was not affected by ant presence. Lichen epiphytic diversity, in terms of species number, has been shown to be influenced more by macroclimatic factors than specific habitat features ([32]). Thus, the overall epiphytic lichen diversity may not be a good proxy for assessing possible effects on lichen diversity carried out by ants. Our results differed from previous studies that found a negative influence of ant presence on lichen species richness ([29], [46]). However, these studies focused on both lichens growing on trees and on other substrates. Thus, ant effects may differ depending on which lichen community is considered (epiphytic vs. terricolous). Thalli fragments of terricolous lichen species were usually found in ant mounds, supporting this hypothesis ([23]). Moreover, Thunes et al. ([46]) considered F. aquilonia, which could have a distinct behavioural patterns compared to F. paralugubris, thus influencing cryptogam communities differently. F. paralugubris has been introduced in the studied forests, so we could not exclude different behavioural patterns compared to the original ants’ populations in the Alps or in other autochthonous areas of Europe.
The major influence of ants on lichen epiphytic communities seemed to be mediated by functional traits. Plots with ants displayed a greater presence of lichens with asexual reproduction as soredia and isidia and fewer crustose species. Previous research has reported that soredia could attach to ants when the latter pass over lichen thalli ([29]). Thus, our finding corroborates the hypothesis that ants could act as a dispersion vector of lichen soredia, transporting them onto other trees and enhancing their colonisation. To date, evidence of ant interaction with isidia is lacking, but we argue that continuous movements over lichen thalli could enhance the detachment of these propagules, favouring their dispersion. Wood ants establish complex networks of foraging routes that radiate to nearby trees and connect different nests ([14]). These trail networks change dynamically to track temporal changes in resource availability, mainly aphid colonies, and allow an efficient and almost uniform exploitation of the area surrounding the colony ([9]). As a consequence, isidia and soredia could travel through this network, potentially dispersing to other trees in the area surrounding a nest. In this way, ants may affect lichen species composition due to temporal changes of the foraging trees according to aphid availability.
Regarding the growth form, control sites had a greater presence of crustose lichen species. Epiphytic lichens are often predated by invertebrates such as snails ([8]), and RWA presence may negatively affect invertebrate assemblages. Frizzi et al. ([15]) have previously found in the same forest of this study that F. paralugubris negatively impacts invertebrates’ presence, describing a clear decrease in their abundance in colonised areas. This could suggest that the decreased abundance of invertebrates feeding on lichens may positively influence the presence of foliose and fruticose lichens that may be more likely predated ([18]).
It is also worth reporting that the geographic position seemed to have an effect in influencing lichen communities. Several studies have shown that epiphytic lichen species richness and composition are mainly influenced by microscale factors such as bark pH, roughness, water holding capacity, and tree species, and macroscale factors such as temperature ([30]). Moreover, the difference in lichen species could be affected by the wide variety of growth forms, reproductive strategies, and photobionts that could allow the replacement of lost species. In our work, we tried to avoid the effect of these factors by comparing sites with similar pedoclimatic conditions. Additionally, the influence of the phorophyte was not an issue here, as only one tree species was present in both sites. However, it is evident that some microscale factors (i.e., at the plot scale) could not be controlled for, despite our efforts.
Our study also revealed a significant difference in β-diversity between sites with and without RWA. β-diversity was higher where RWA were present, and all its components (i.e., replacement, similarity and richness) were affected. The specific mechanism behind this pattern are not known and surely deserve further research, but in principle, the same mechanisms discussed to explain the observed differences in lichen functional diversity may apply here.
Conclusion
Our study revealed a significant effect of the presence of F. paralugubris on lichen communities and extended our knowledge on the impact of this introduced species on autochthonous communities. Our results suggest both a direct effect, with a physical dispersal of lichen propagules by ant workers, and an indirect effect, by ant predatory pressure on lichen herbivores. Our study is the first attempt to unveil the possible effects of RWA on lichen epiphytic diversity using a functional approach. We stressed that possible effects of the presence of RWA could be concentrated on species with specific functional traits. Nevertheless, further studies are needed to clarify the mechanisms involved in the interactions between RWA and lichen communities.
Author contributions
RB and GS conceived and designed the experiments. LDN, EB, RB,AM,FF, GS, MBC, FM, YS, CV and PB collected the data. FF, LDN and AM performed data analysis. LDN and AM wrote the first draft and contribute equally. All authors contributed extensively to the ideas, writing and discussions. Comments from two anonymous reviewers greatly improved the manuscript. The work was funded by grants of the University of Florence to GS and RB.
Acknowledgements
We are grateful to the Reparto Carabinieri per la Biodiversità di Pratovecchio e Pistoia for their assistance during field work.
References
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Supplementary Material
Authors’ Info
Authors’ Affiliation
Alberto Masoni 0000-0001-5473-4649
Filippo Frizzi 0000-0002-1419-0445
Elisabetta Bianchi 0000-0003-1197-4081
Maria Beatrice Castellani 0000-0003-4355-7733
Paride Balzani 0000-0003-1549-7139
Federica Morandi
Ylenia Sozzi
Giacomo Santini 0000-0001-8159-6632
Renato Benesperi 0000-0003-4296-3393
Department of Biology, University of Florence, v. la Pira 4, I-50121 Florence (Italy)
University of South Bohemia in Ceske Budejovice, Faculty of Fisheries and Protection of Waters, South Bohemian Research Center of Aquaculture and Biodiversity of Hydrocenoses, Zátiší 728/II, 389 25 Vodnany (Czech Republic)
Biodiversity and Macroecology Group, Department of Biological, Geological and Environmental Sciences, Alma Mater Studiorum - University of Bologna, v. Irnerio 42, I-40126 Bologna (Italy)
Corresponding author
Paper Info
Citation
Di Nuzzo L, Masoni A, Frizzi F, Bianchi E, Castellani MB, Balzani P, Morandi F, Sozzi Y, Vallese C, Santini G, Benesperi R (2022). Red wood ants shape epiphytic lichen assemblages in montane silver fir forests. iForest 15: 71-76. - doi: 10.3832/ifor3897-014
Academic Editor
Massimo Faccoli
Paper history
Received: Jun 10, 2021
Accepted: Dec 27, 2021
First online: Feb 22, 2022
Publication Date: Feb 28, 2022
Publication Time: 1.90 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2022
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 32446
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 27558
Abstract Page Views: 2502
PDF Downloads: 1853
Citation/Reference Downloads: 4
XML Downloads: 529
Web Metrics
Days since publication: 1440
Overall contacts: 32446
Avg. contacts per week: 157.72
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2022): 6
Average cites per year: 1.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Advantages of the point-intercept method for assessing functional diversity in semi-arid areas
vol. 8, pp. 471-479 (online: 31 October 2014)
Research Articles
Variability of ant community composition in cork oak woodlands across the Mediterranean region: implications for forest management
vol. 10, pp. 707-714 (online: 27 July 2017)
Review Papers
Biodiversity assessment in forests - from genetic diversity to landscape diversity
vol. 2, pp. 1-3 (online: 21 January 2009)
Research Articles
Relationships between overstory and understory structure and diversity in semi-natural mixed floodplain forests at Bosco Fontana (Italy)
vol. 9, pp. 919-926 (online: 21 August 2016)
Research Articles
Functional turnover from lowland to montane forests: evidence from the Hyrcanian forest in northern Iran
vol. 8, pp. 359-367 (online: 16 September 2014)
Research Articles
Endangered and endemic species increase forest conservation values of species diversity based on the Shannon-Wiener index
vol. 9, pp. 469-474 (online: 02 January 2016)
Research Articles
Cryptogamic epiphytes and microhabitat diversity on non-native green ash (Fraxinus pennsylvanica Marsh., Oleaceae) in urban habitats
vol. 14, pp. 393-399 (online: 01 September 2021)
Research Articles
Effects of different silvicultural measures on plant diversity - the case of the Illyrian Fagus sylvatica habitat type (Natura 2000)
vol. 9, pp. 318-324 (online: 22 October 2015)
Research Articles
Long-term outcome of precommercial thinning on floristic diversity in north western New Brunswick, Canada
vol. 1, pp. 145-156 (online: 25 November 2008)
Research Articles
Diversity of saproxylic beetle communities in chestnut agroforestry systems
vol. 13, pp. 456-465 (online: 07 October 2020)
iForest Database Search
Search By Author
- L Di Nuzzo
- A Masoni
- F Frizzi
- E Bianchi
- MB Castellani
- P Balzani
- F Morandi
- Y Sozzi
- C Vallese
- G Santini
- R Benesperi
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
- L Di Nuzzo
- A Masoni
- F Frizzi
- E Bianchi
- MB Castellani
- P Balzani
- F Morandi
- Y Sozzi
- C Vallese
- G Santini
- R Benesperi
Search By Keywords
PubMed Search
Search By Author
- L Di Nuzzo
- A Masoni
- F Frizzi
- E Bianchi
- MB Castellani
- P Balzani
- F Morandi
- Y Sozzi
- C Vallese
- G Santini
- R Benesperi
Search By Keyword