Cryptogamic epiphytes and microhabitat diversity on non-native green ash (Fraxinus pennsylvanica Marsh., Oleaceae) in urban habitats
iForest - Biogeosciences and Forestry, Volume 14, Issue 5, Pages 393-399 (2021)
doi: https://doi.org/10.3832/ifor3739-014
Published: Sep 01, 2021 - Copyright © 2021 SISEF
Research Articles
Abstract
With the increased planting of non-native trees within urban environments there is a need for investigating the impacts they may have on the indigenous biodiversity. In this study, we explored the diversity of epiphytic lichens and bryophytes as well as the tree-related microhabitats on planted, non-native green ash Fraxinus pennsylvanica and compared it to that of indigenous Fraxinus excelsior and Quercus robur. We conducted sampling on trees of similar growing conditions and size within two cities of eastern Germany (Dresden and Dessau-Roßlau). In our analysis we did not find any significant differences in epiphyte diversity and abundance. By contrast, microhabitat diversity was significantly higher on F. pennsylvanica than on the indigenous tree species, which we attribute to the pioneer character of F. pennsylvanica with faster ageing. Our results underline a low impact of F. pennsylvanica on epiphytic lichen and bryophyte diversity, while indigenous animals might even benefit from the higher diversity and frequency of microhabitats on trees of this species. Therefore, its use as an ornamental tree should not be generally rejected in urban environments.
Keywords
Introduction
In urban environments, the propagation of non-native (i.e., alien) tree species is indispensable, as many indigenous species do no longer tolerate the extreme conditions of urban habitats ([47], [16]). As such, non-native tree species might safeguard important tree-related ecosystem services ([13]). However, some of these species may have serious impacts on indigenous communities, but evidence is rare ([51]). Likewise, the potentially beneficial effects are often unknown ([48], [35]). This specifically holds true for the impact of non-native trees on epiphytic lichens and bryophytes, a species group that often makes a significant contribution to urban biodiversity ([43]). It has been found that some non-native tree species can support rare epiphyte lichen and bryophyte species and harbour highly diverse epiphyte communities ([30], [21]). Though, systematic comparisons to indigenous host tree species in the same habitat are scarce and specifically lacking for urban habitats.
Even fewer data are available for further tree-inhabiting taxa such as fungi and insects ([25], [38], [5]). Investigation of these taxa can be difficult, time-consuming and expensive. This is also due to their high species diversity, demand for specialized and rare taxonomic expertise and often inconspicuous, seasonal appearances. Therefore, the trade-off between investigation effort and indicator value of such taxa can be disadvantageous ([33]). In the absence of direct data on many species groups, tree-related habitat structures have been suggested to serve as a proxy for biodiversity assessments ([41]). In urban environments microhabitats such as trunk cavities or crown deadwood are critical for many species ([57], [20]). These microhabitats are also supposed to occur on mature and over-aged non-native trees, but evidence is scarce ([57], [6]).
Among the trees that are increasingly planted in German cities, North American green ash (Fraxinus pennsylvanica Marsch., Oleaceae), including natural and cultivated varieties, has been proven to be tolerant to harsh environmental conditions ([5]). In addition, F. pennsylvanica is attractive for its appearance. Furthermore, F. pennsylvanica is discussed as an alternative to the indigenous common ash (F. excelsior L.), which is hardly affected by ash dieback ([38]). F. pennsylvanica was introduced to some central European countries in the 18th century as an ornamental tree and it was planted in alluvial hardwood forests ([50]). In near-natural habitats of Germany and other European countries this tree species is being considered as invasive ([44], [40], [11]). By contrast, the impact on indigenous epiphytic communities has not yet been assessed in urban habitats of central Europe (but see studies conducted in North America and Eastern Europe - [22], [23], [18], [29], [37]), and data for tree-related microhabitats are lacking.
Based on observations in two German cities (namely Dresden and Dessau-Roßlau), this study deals with two questions: (i) Does planted F. pennsylvanica have a negative impact on the diversity of indigenous epiphytic lichens and bryophytes? (ii) Which microhabitats do evolve on planted F. pennsylvanica, compared to indigenous broadleaved tree species?
Material and methods
Trees were investigated in the cities of Dresden (51° 03′ N, 13° 44′ E - Saxony Federal State) and Dessau-Roßlau (51° 52′ N, 12° 15′ E - Saxony-Anhalt Federal State) in 2018 and 2019. Climate is characterized as warm-temperate, with mean annual precipitation and mean annual temperature of 650 mm and 9.0 °C for Dresden and 542 mm and 9.2 °C for Dessau-Roßlau for the past decades ([10], [17], [3]). We compared F. pennsylvanica to two indigenous broadleaved tree species which are also common in alluvial hardwood forests in central Europe ([1]): F. excelsior L. and common oak (Quercus robur L., Fagaceae). Planted F. pennsylvanica individuals were selected based on unpublished inventories (see Acknowledgments) as well as a public online database on veteran trees (“Champion-Trees” - [24], [12]). The age could not be determined for all individual trees; the oldest F. pennsylvanica tree in Dresden (Botanical Garden) exceeded 130 years ([12]).
For each individual of F. pennsylvanica we selected nearby individuals of F. excelsior and Q. robur at similar growing conditions and, preferably, similar size (i.e., diameter at breast height, DBH). Therefore, sample trees were not selected randomly and sample tree number was determined by the number of possible species triplets. As a consequence, six replicate groups in Dresden and five replicate groups in Dessau-Roßlau were established. The Dresden set mainly includes mature and old trees of DBH > 60 cm, while the sample trees in Dessau-Roßlau are much younger with a DBH < 40 cm.
Epiphytic lichens and bryophytes were recorded in the lower trunk section of the trees (0-2 m). Species abundance was quantified by modified percentage estimation referred to the trunk section area covered ([15]). For additionally exploring epiphytes in the (often overlooked) tree canopy ([43]) we made use of a peri-urban, over-aged, F. pennsylvanica specimen in Dessau-Roßlau, of which large parts of the unstable crown had been cut off and deposited nearby in July 2018. Of a sample tree of F. pennsylvanica in Dresden, we could also access downed canopy branches for a survey of canopy-inhabiting epiphyte species. Nomenclature of species is based on Wirth et al. ([56]) for lichens, Caspari et al. ([8]) for bryophytes and Buttler & Hand ([7]) for vascular plants.
For each tree, we determined the three features DBH, site hemeroby and bark pH (Tab. 1). DBH was measured with a measuring tape, and we classified hemeroby (i.e., the degree of human impact) of the growing site according to a scale of three classes: class 1 included sites of lowest hemeroby (e.g., abandoned parks and urban successional forests), class 2 represented sites of medium hemeroby (e.g., managed parks and sides of secondary roads), while class 3 included sites of highest hemeroby, i.e., main street roadsides. In the bark pH analyses, 1 g of sample tree bark was dried and pulverized. Re-hydrated with 20 ml of de-ionised water, these suspensions were horizontally shaken for 24 hours. The pH values were measured with the electronic analyser Toledo MP-220® (Mettler-Toledo, Greifensee, Switzerland) in the suspension (method after [36]). Tree-related microhabitats were determined after Kraus et al. ([31]), and their presence was noted for each sample tree.
Tab. 1 - Sample tree features in Dresden and Dessau-Roßlau (mean ± standard error). (Fp): Fraxinus pennsylvanica; (Fe): Fraxinus excelsior; (Qr): Quercus robur. Statistical testing of the data did not yield significant differences within the relevant study area (Kruskal-Wallis test, Wilcoxon test: p > 0.05).
| Feature | Dresden | Dessau-Roßlau | ||||
|---|---|---|---|---|---|---|
| Fp | Fe | Qr | Fp | Fe | Qr | |
| Replicates | 6 | 6 | 6 | 5 | 5 | 5 |
| Bark pH | 5.3 ± 0.2 | 5.6 ± 0.2 | 5.1 ± 0.3 | 5.8 ± 0.1 | 5.6 ± 0.3 | 5.5 ± 0.1 |
| Tree DBH (cm) | 66.2 ± 6.6 | 71.4 ± 9.5 | 69.3 ± 9.7 | 24.9 ± 2.1 | 26.9 ± 3.8 | 26.2 ± 4.9 |
| Site hemeroby | 2.0 ± 0.2 | 2.0 ± 0.2 | 1.8 ± 0.2 | 2.8 ± 0.2 | 2.8 ± 0.2 | 2.4 ± 0.2 |
Statistical analyses
We compared the frequency and mean cover of the epiphyte species as well as the frequency of microhabitats between the three tree species in the two cities. To detect a significant turnover in species composition between the trees, the epiphyte relevés were subjected to one-way ANOSIM ([9]) using species abundance ranks for group-wise comparisons. Significant differences in tree features and the single species cover were tested by Kruskal-Wallis test, as the data were not normally distributed (Shapiro-Wilks test, p < 0.05). Differences in microhabitat and species diversity were additionally tested for significance between the sample tree species with the Wilcoxon test. Most statistical analyses were done using the software R v. 3.6.3, especially the package “R commander” ([45], [19]), whereas ANOSIM was performed with the software PAST v. 4.01 ([26]). As climate and growing conditions of the sample tree species in the two regions did not strongly differ, we summarized all sample trees in analyses on the general trends (i.e., N = 11 replicates). Detailed epiphyte species composition and microhabitat spectra were analysed separately for the two cities and size classes, respectively.
Results
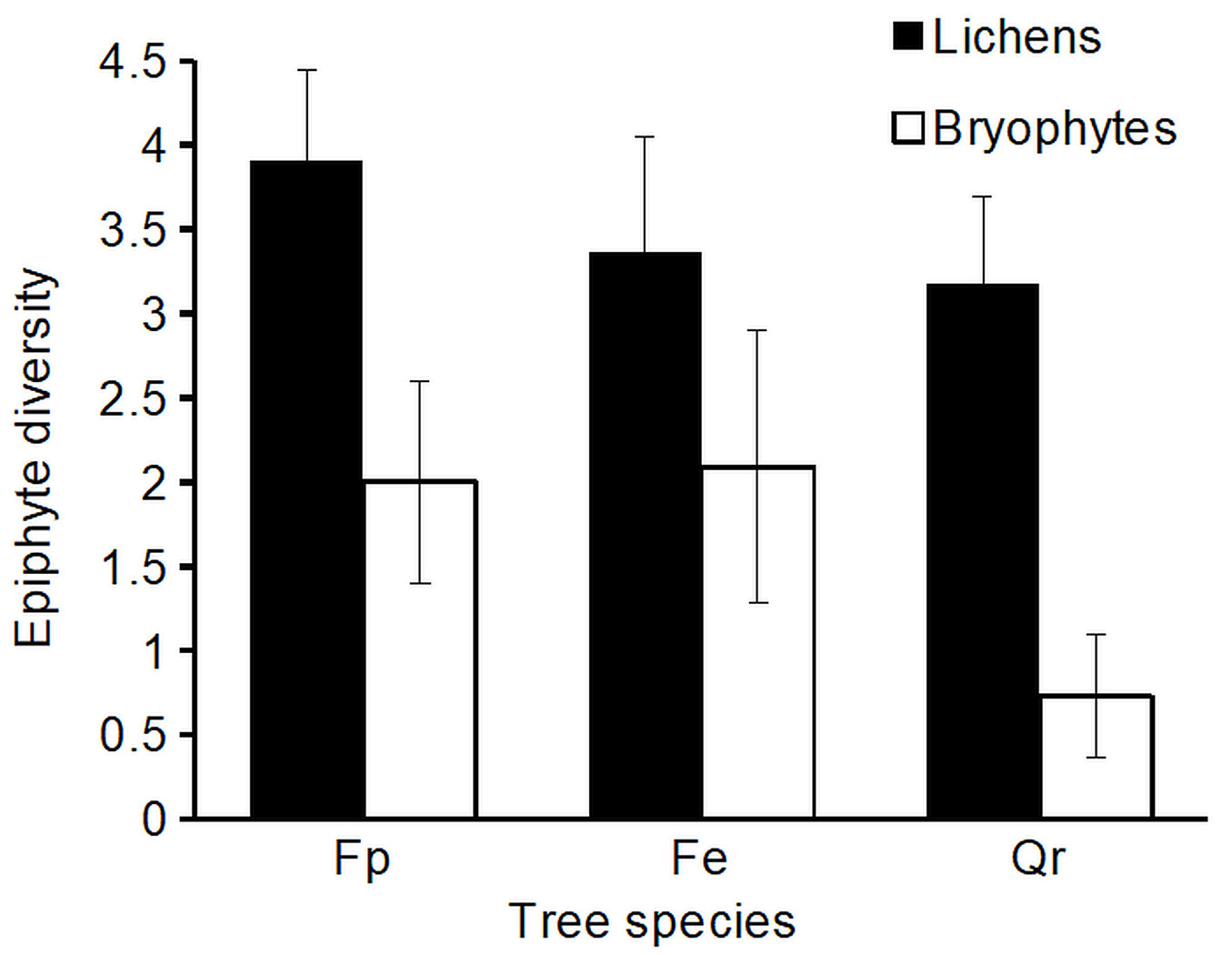
In the entire tree set, epiphyte diversity was not significantly different between F. pennsylvanica, F. excelsior and Q. robur (Wilcoxon test, p > 0.05 - Fig. 1). However, diversity of epiphytic bryophytes was lowest in Q. robur individuals. Trends were similarly expressed in both study areas (Kruskal-Wallis test, p > 0.05; Wilcoxon test, p > 0.05 - Tab. 2). Furthermore, no significant differences were found in the epiphyte community composition (ANOSIM, p > 0.05), single tree features and mean cover of the single lichen and bryophyte species (Kruskal-Wallis test, p > 0.05; Wilcoxon test, p > 0.05). These trends were similar both in all sample trees as well as in the different regions and size classes. Few, and rare, lichen or bryophyte species were confined to one tree species in one of the study areas (Tab. 2). Lichen cover and lichen diversity was higher on all sample trees in Dessau-Roßlau, while we observed higher cover and higher diversity of bryophytes in Dresden. The complete epiphyte survey of two F. pennsylvanica specimen revealed a much higher lichen diversity in the tree crown than on the lower trunk. Three epiphyte species found in the canopy of two F. pennsylvanica specimen were completely absent from the lower trunk of the other sample trees (Orthotrichum stramineum, Phaeophyscia nigricans, Scoliciosporum chlorococcum - Tab. 2, Tab. 3).
Fig. 1 - Diversity of epiphytic lichens and bryophytes on the sample tree species (number of species, mean ± standard error); (Fp): F. pennsylvanica; (Fe): F. excelsior; (Qr): Q. robur.
Tab. 2 - Epiphyte species (frequency, mean cover ± standard error) on sample trees in Dresden and Dessau-Roßlau. (Fp): Fraxinus pennsylvanica; (Fe): Fraxinus excelsior; (Qr): Quercus robur; (+): mean cover <0.1 %. Statistical testing of the data did not yield significant differences within the relevant study area and between the sample tree species (p >0.05, Kruskal-Wallis-Test; Wilcoxon test). Rare species: Dresden - Fp: crustose lichen indet., Phlyctis argena, Brachthecium salebrosum, Climacium dendroides, Ptychostomum moravicum, Pylaisia polyantha, Ulota bruchii; Fe: Candellariella aurella, Flavoparmelia soredians, Ptychostomum capillare; Qr: Physcia spec. Dessau-Roßlau - Fp: Candellariella xanthostigma; Fe: Arthonia radiata, Candellariella reflexa; Qr: Candellariella spec., Parmotrema perlatum.
| Taxa | Features/Species | Dresden | Dessau-Roßlau | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fp | Fe | Qr | Fp | Fe | Qr | ||||||||
| - | Cover lichens | 11.0 ± 5.0 | 8.5 ± 2.2 | 8.6 ± 3.6 | 31.0 ± 8.7 | 26.4 ± 10.9 | 12.8 ± 3.1 | ||||||
| - | Cover bryophytes | 5.3 ± 3.7 | 4.0 ± 2.1 | 0.6 ± 0.3 | 0.4 ± 0.2 | 1.8 ± 1.0 | 0.1 ± 0.1 | ||||||
| - | Lichen species | 2.2 ± 0.6 | 3.8 ± 0.8 | 2.8 ± 0.8 | 4.8 ± 1.0 | 4.0 ± 0.8 | 3.6 ± 0.6 | ||||||
| - | Bryophyte species | 3.0 ± 1.3 | 2.0 ± 0.6 | 1.2 ± 0.6 | 1.0 ± 0.4 | 2.0 ± 1.1 | 0.2 ± 0.2 | ||||||
| Lichens | Physcia tenella | 33 | 3.4 ± 3.0 | 33 | 2.2 ± 1.5 | 33 | 0.3 ± 0.2 | 100 | 17.8 ± 7.6 | 100 | 20.2 ± 10.8 | 100 | 10.7 ± 3.7 |
| Xanthoria parietina | 33 | 0.2 ± 0.1 | 33 | 0.7 ± 0.1 | 33 | 0.2 ± 0.1 | 100 | 1.1 ± 0.3 | 80 | 1.5 ± 0.8 | 80 | 0.6 ± 1.7 | |
| Phaeophyscia orbicularis | 17 | 1.3 ± 1.2 | 67 | 2.7 ± 1.5 | 33 | 6.7 ± 3.9 | 60 | 11.0 ± 7.7 | 40 | 2.1 ± 1.8 | 80 | 1.3 ± 0.6 | |
| Candellariella vitellina | - | - | 33 | 0.7 ± 0.1 | 17 | 0.1 ± 0.1 | 20 | 0.1 ± 0.1 | 20 | 0.1 ± 0.1 | 20 | 0.1 ± 0.1 | |
| Lepraria finckii | 17 | 4.2 ± 3.8 | 17 | 1.0 ± 0.9 | 33 | 0.2 ± 0.1 | 20 | 0.2 ± 0.2 | - | - | - | - | |
| Physconia grisea | - | - | 33 | 0.8 ± 0.6 | 17 | 0.1 ± 0.1 | 20 | 0.1 ± 0.1 | 20 | 0.1 ± 0.1 | - | - | |
| Amandinea punctata | - | - | 17 | 0.1 ± 0.1 | 33 | 0.1 ± 0.1 | 40 | 0.2 ± 0.1 | 40 | 0.3 ± 0.2 | - | - | |
| Lecanora carpinea | 33 | 0.2 ± 0.1 | 33 | 0.2 ± 0.1 | - | - | 40 | 0.2 ± 0.1 | - | - | - | - | |
| Physcia adscendens | 33 | 1.8 ± 1.5 | 17 | 0.8 ± 0.8 | 17 | 1.7 ± 1.5 | - | - | - | - | - | - | |
| Parmelia sulcata | 17 | + | 17 | 0.1 ± 0.1 | - | - | 20 | + | - | - | - | - | |
| Cladonia spec. | - | - | 17 | + | 33 | 0.1 ± 0.1 | - | - | - | - | - | - | |
| Zwackhia viridis | - | - | 17 | 0.3 ± 0.3 | 17 | 0.1 ± 0.1 | - | - | - | - | - | - | |
| Lecanora spec. | - | - | 17 | + | - | - | - | - | - | - | 20 | 0.1 ± 0.1 | |
| Lecidella elaeochroma | - | - | - | - | - | - | 20 | 0.1 ± 0.1 | 40 | 0.9 ± 0.7 | 20 | 0.2 ± 0.2 | |
| Rinodina pityrea | - | - | - | - | - | - | 20 | 0.1 ± 0.1 | 20 | 0.1 ± 0.1 | - | - | |
| Masjukiella polycarpa | - | - | - | - | - | - | 20 | + | 20 | 0.1 ± 0.1 | - | - | |
| Bryophytes | Orthotrichum diaphanum | 50 | 0.3 ± 0.1 | 33 | 0.2 ± 0.1 | - | - | 20 | 0.1 ± 0.1 | 40 | 0.2 ± 0.1 | 20 | 0.1 ± 0.1 |
| Orthotrichum affine | 33 | 0.7 ± 0.4 | 33 | 0.2 ± 0.1 | 17 | 0.1 ± 0.1 | 40 | 0.2 ± 0.1 | 40 | 0.2 ± 0.1 | - | - | |
| Hypnum cupressiforme | 50 | 1.5 ± 1.2 | 17 | 0.8 ± 0.8 | 17 | 0.2 ± 0.2 | - | - | 20 | 0.2 ± 0.2 | - | - | |
| Brachythecium rutabulum | 33 | 0.6 ± 0.5 | 33 | 0.4 ± 0.3 | 17 | 0.1 ± 0.1 | 40 | 0.2 ± 0.1 | - | - | - | - | |
| Platygyrium repens | 17 | 0.5 ± 0.5 | 50 | 1.8 ± 1.5 | 50 | 0.3 ± 0.2 | - | - | 20 | 0.1 ± 0.1 | - | - | |
| Amblystegium serpens | 17 | 0.5 ± 0.5 | 17 | 0.1 ± 0.1 | - | - | - | - | - | - | - | - | |
| Grimmia pulvinata | 17 | + | - | - | - | - | - | - | 20 | 0.1 ± 0.1 | - | - | |
| Dicranoweisia cirrata | - | - | - | - | 17 | 0.1 ± 0.1 | - | - | 20 | + | - | - | |
Tab. 3 - Complete, section-wise epiphyte surveys of two F. pennsylvanica specimen. (B): bryophytes; (L): lichens; (+): present. (1) Segment division after John & Stapper ([30]): I, 0-40 cm; II, 40-200 cm; III, upper trunk above lowest canopy branch, includes branches ≥5 cm diameter; IV, canopy twigs <5 cm diameter; (2) Locations: Dresden, “Bienert-Garten” (successional forest), tree age ~123 yrs ([12]); Dessau-Roßlau / Großkühnau, solitary tree on grass lawn; (3): break-off branches surveyed in November 2020.
| Div. (1) | Taxa | Variable/Species | Location (2) | |
|---|---|---|---|---|
| Dresden | Dessau-Roßlau | |||
| - | - | Survey date | Nov 2018 (3) | July 2018 |
| DBH (cm) | 66 | 73 | ||
| Height (m) | 22 | >10 | ||
| Bark pH | 4.75 | 5.9 | ||
| No. Bryophyte species | 5 | 6 | ||
| No. Lichen species | 8 | 4 | ||
| Site hemeroby | 1 | 2 | ||
| I | B | Brachythecium rutabulum | + | - |
| B | Hypnum cupressiforme | + | - | |
| II | B | Brachythecium rutabulum | - | + |
| B | Hypnum cupressiforme | - | + | |
| B | Orthotrichum diaphanum | - | + | |
| L | Phaeophyscia orbicularis | - | + | |
| L | Physcia adscendens | - | + | |
| B | Pylaisia polyantha | - | + | |
| III | B | Orthotrichum affine | + | + |
| L | Phaeophyscia orbicularis | + | + | |
| L | Xanthoria parietina | + | + | |
| L | Masjukiella polycarpa | + | - | |
| B | Orthotrichum stramineum | + | - | |
| L | Parmelia sulcata | + | - | |
| L | Phaeophyscia nigricans | + | - | |
| L | Physcia tenella | + | - | |
| B | Platygyrium repens | + | - | |
| B | Brachythecium rutabulum | - | + | |
| B | Hypnum cupressiforme | - | + | |
| L | Physconia grisea | - | + | |
| B | Ptychostomum capillare | - | + | |
| B | Pylaisia polyantha | - | + | |
| IV | L | Xanthoria parietina | + | + |
| L | Candellariella reflexa | + | - | |
| L | Masjukiella polycarpa | + | - | |
| L | Parmelia sulcata | + | - | |
| L | Phaeophyscia orbicularis | + | - | |
| L | Physcia tenella | + | - | |
| L | Scoliciosporum chlorococcum | + | - | |
| L | Physcia adscendens | - | + | |
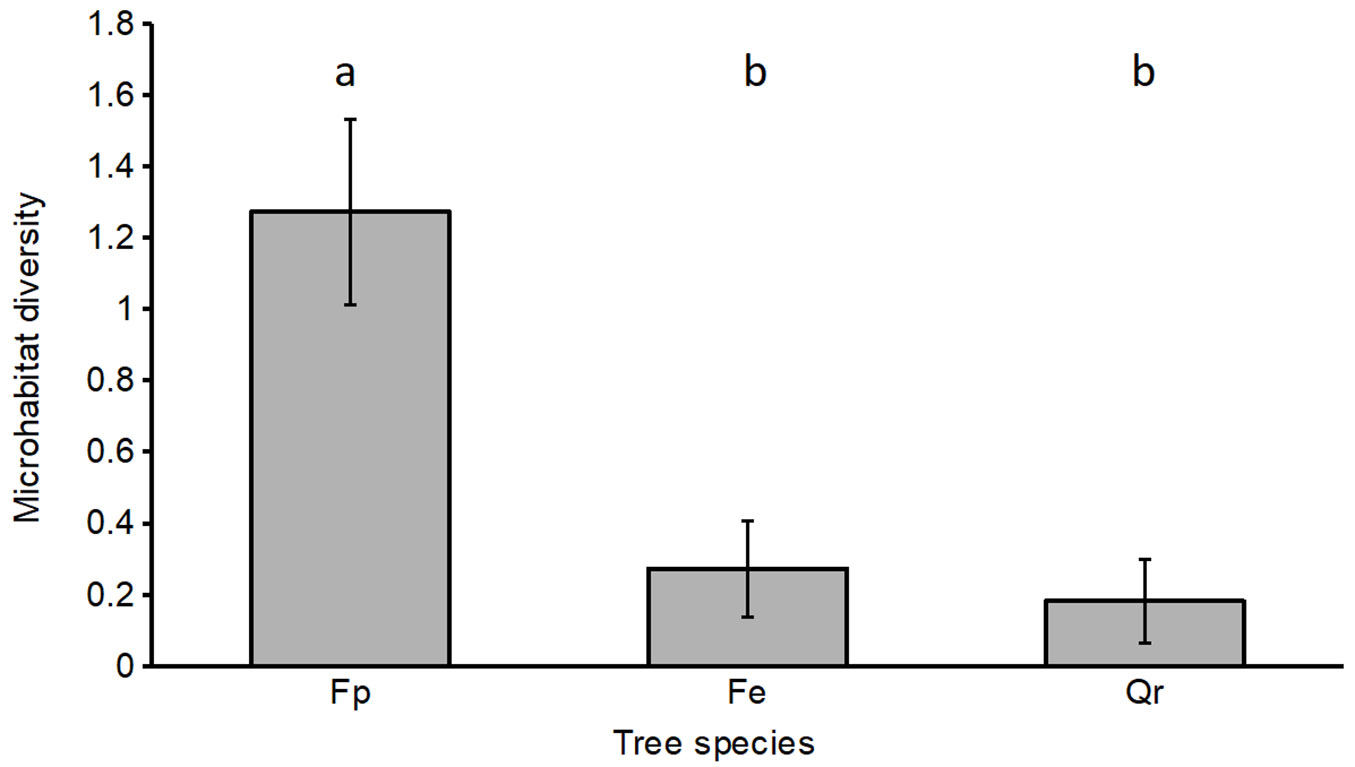
In contrast to the epiphytic species, differences in microhabitats were more pronounced between the three tree species. Microhabitat diversity was significantly higher in F. pennsylvanica than in the two indigenous tree species in all sample trees (Fig. 2) and in Dessau-Roßlau. The same pattern was found in Dresden, though not significant (Tab. 4). Additionally, the frequency of trees with microhabitats was considerably higher in F. pennsylvanica than in F. excelsior and Q. robur in both cities (Tab. 4). Thereby, mistletoes (Viscum album L.) and different types of crown deadwood were the most frequent microhabitats found in Dresden. In Dessau-Roßlau, microhabitats were even exclusively found on F. pennsylvanica. Here, mistletoes and high lichen cover were the only microhabitats present (Tab. 4).
Fig. 2 - Diversity of microhabitats on the sample tree species (number of microhabitats, mean ± standard error); (Fp): F. pennsylvanica; (Fe): F. excelsior; (Qr): Q. robur. Different lower-case letters above the bars indicate significant differences (Wilcoxon test, p < 0.05).
Tab. 4 - Frequency (%) of microhabitats found on the sample trees in the two cities (classification and coding after [31]). (Fp): F. pennsylvanica; (Fe): F. excelsior; (Qr): Q. robur. Different lower-case letters indicate significant differences in the microhabitat diversity within the relevant study area (Wilcoxon test, p<0.05).
| Feature | Dresden | Dessau-Roßlau | Code | ||||
|---|---|---|---|---|---|---|---|
| Fp | Fe | Qr | Fp | Fe | Qr | ||
| % sample trees with microhabitats | 83 | 50 | 33 | 60 | 0 | 0 | - |
| Diversity of microhabitats | 1.5 ± 0.4 a | 0.5 ± 0.2 a | 0.3 ± 0.2 a | 1.0 ± 0.3 a | 0 b | 0 b | - |
| Mistletoe (Viscum album) | 50 | - | - | 40 | - | - | EP35 |
| Trunk cavity with ground contact | 33 | 17 | - | - | - | - | CV21 |
| Dead branches / crown deadwood | 33 | - | 17 | - | - | - | DE11+DE13 |
| Small trunk cavities | 17 | 17 | - | - | - | - | CV13+CV22 |
| Annual polypores (cf. Laetiporus spec.) | 17 | - | - | - | - | - | EP11 |
| Small vertebrate nest (songbird) | 17 | - | - | - | - | - | NE12 |
| Branch hole / rot hole | - | 17 | - | - | - | - | CV31 |
| Gallery of bore holes (cf. Hylesinus fraxini) | - | 17 | - | - | - | - | CV51 |
| Liana cover >25% (Hedera helix) | - | - | 17 | - | - | - | EP33 |
| Epiphytic foliose lichen cover >25% | - | - | - | 60 | - | - | EP32 |
Discussion
In both cities and the entire dataset of all sample trees, the impact of F. pennsylvanica on urban epiphyte diversity was not significant. In contrast to Mitchell et al. ([38]) we found that most epiphytes growing on F. excelsior also occurred on F. pennsylvanica and Q. robur. At a low level of acidifying pollution, differences between tree species and even between live and dead trees are generally less pronounced ([2], [27]). While the abundance of some lichens can strongly shift due to slight variations in bark pH ([28]), the ecological amplitude of many other epiphyte species enables their colonization even of trees with strongly different bark chemistry and structure ([2], [39]). Therefore, the small bark pH variations in both cities across the three tree species did not affect epiphyte community composition either, as well as their naturalization status ([39]).
Few epiphyte species preferably occurred on one tree species. Based on the total number of epiphytic lichen species, however, a cross-regional comparison between different cities and host-trees points to larger differences between the tree species but also to a high variation across the different study areas (Tab. 5). Such results, including our own study, support the findings of Richter et al. ([46]) that the importance of different host tree species for epiphyte diversity also depends on the surrounding local habitat or urban landscape type. Besides, none of the available previous studies provided detailed information on the age and number of the tree specimens studied, which might contribute to the different local diversity patterns (Tab. 5).
Tab. 5 - Number of lichen species on the investigated host tree species in different cities of Europe and North America. (Total): entire lichen species on the three sample tree species; (Fp): F. pennsylvanica; (Fe): F. excelsior; (Qr): Q. Robur; (1): based on the description of the investigated sites; (2): F. pennsylvanica only in one location; (3): F. pennsylvanica included F. p. var. subintegerrima; F. excelsior: F. excelsior “Hessei”; no specimens of Q. robur.
| City | Total | Fp | Fe | Qr | Site hemeroby (1) |
Reference |
|---|---|---|---|---|---|---|
| Lomza (PL) | 24 | 3 | 24 | 11 | 2-3 | Matwiejuk & Chojnowska ([37])(2) |
| Szarvas (HUN) | 20 | 6 | 11 | 11 | 2 | Gallé ([23]) |
| Dresden (D) | 19 | 9 | 15 | 11 | 1-3 | This study |
| Dessau-Roßlau (D) | 18 | 13 | 11 | 8 | 2-3 | This study |
| Chicago (USA) | 16 | 15 | 7 | - | 2 | Hyerczyk ([29])(3) |
| Szeged (HUN) | 10 | 7 | 5 | 3 | 2 | Gallé ([22]) |
Some additional species found in the canopy of two F. pennsylvanica trees also point on an incomplete epiphyte assessment by our trunk-based investigation, at least in Dresden. But this methodical limitation applies to all sampled trees included in the comparison. Furthermore, species confined to the tree canopy in closed forests can be expected to occur at lower trunk sections of tree individuals outside forests ([55]). This is also substantiated by the different vertical epiphyte stratification on the two completely surveyed trees at different growing conditions (Tab. 3). Therefore, the share of neglected epiphyte species in urban areas may be lower than in closed forest stands ([4]). Consequently, surveys of the lower tree trunk sections can be sufficient for analyses of the diversity and indicator value of cryptogamic epiphytes in urban areas ([43]).
Most of the lichen and bryophyte species found can be classified as pollution-tolerant, i.e., toxitolerant, acidophytic or nitrophytic ([14], [56]). They are common in settlement areas and have partly been found in previous studies on urban F. pennslyvanica in Eastern Europe ([22], [23], [18], [37]). Locally noteworthy are Climacium dendroides and Zwackhia viridis, which rarely occur in urban areas and are confined to the larger trees in Dresden (Tab. 2). Rarely found Flavoparmelia soredians and Parmotrema perlatum indicate climate warming ([54]). Much more than host tree identity, the epiphytes found depict the imprint of (past) pollution, together with recent eutrophication and over-warming of urban areas. These factors lead to both impoverishment and homogenization of epiphyte communities ([53], [34]).
While epiphytic lichens and bryophytes were documented by direct surveys in this study, the possible occurrences of other taxa can be estimated from structural tree attributes ([41]). So far, the higher frequency and significantly higher diversity of microhabitats found on urban F. pennsylvanica compared to the two indigenous tree species (Fig. 2) points to a certain ecological significance for tree-bound biota. The (overall) higher microhabitat diversity on sample trees in Dresden than in Dessau-Roßlau can mainly be attributed to their larger dimensions and, thus, higher age ([42]).
In comparison to the two indigenous tree species, the higher frequency and diversity of microhabitats may be due to the pioneer character and shorter life span of F. pennsylvanica (125-150 yrs - [49]). This could lead to faster ageing and more timely creation of microhabitats compared to the intermediate F. excelsior and the long-lived Q. robur. This is also substantiated by the results for Dessau-Roßlau. Here, among the younger-aged sample trees, only F. pennsylvanica had evolved any microhabitats at all. Within the Dresden dataset, microhabitat frequency and diversity was higher in F. pennsylvanica (though not statistically significant in the case of microhabitat diversity) than on the two indigenous tree species.
However, data on indigenous animal species which actually use such microhabitats on F. pennsylvanica in general, are widely lacking ([38]). In Dresden, we at least encountered an active wasp hive in a trunk cavity of F. pennsylvanica and a bird nest on twigs. The aforementioned over-aged F. pennsylvanica specimen in Dessau-Roßlau (Tab. 3) had a hollow trunk and accommodated a Hornet hive (Vespa cabro L.) in 2017. On declining specimen in Dresden, not included in the sampling, we observed woodpecker holes (Dendrocopus major L.). In central German alluvial hardwood forests, F. pennsylvanica is also used by several cave-nesting bird species ([32]). However, in alluvial hardwood forests, indigenous taxa might yet use indigenous trees to a disproportionately higher degree than non-native tree species ([52]).
Conclusions
Our study provides evidence on a low impact of the non-native tree species F. pennsylvanica on the diversity of epiphytic lichens and bryophytes in urban habitats, when compared to the two common indigenous tree species F. excelsior and Q. robur. The higher frequency of tree-related microhabitats, with significantly higher microhabitat diversity found for F. pennsylvanica compared to the two indigenous tree species, could even be potentially beneficiary to the urban fauna. Therefore, in contrast to near-natural habitats of high conservation value such as alluvial hardwood forests, the use of F. pennsylvanica as ornamental tree in urban environments of central Europe should not be generally rejected. However, cross-regional conclusions are limited and further research should focus on its interaction with other taxonomic groups. The spontaneous establishment and potential spread of F. pennsylvanica in urban environments and beyond should, nonetheless, be monitored and managed.
Acknowledgements
Field work in Dessau-Roßlau was funded by a grant from the German Federal Institute of Hydrology (Bundesanstalt für Gewässerkunde, BfG) within the project “The impact of Fraxinus pennsylvanica on the biodiversity of River Elbe and River Oder”. Christian Bodamer (city administration, Dessau-Roßlau) and Steffen Löbel (city administration, Dresden) provided data on planted F. pennsylvanica and other sample trees. Barbara Ditsch granted access to Dresden botanical garden for epiphyte assessments. Laboratory analyses were done at the Institute of Soil Science and Site Ecology (Forestry, TU Dresden). Klaus Max Stetzka (Institute of Forest Zoology and Forest Botany, TU Dresden) checked some critical lichen samples.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Online | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Online | Gscholar
Online | Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Elke Thiem
Birte Marie Albrecht
Goddert von Oheimb 0000-0001-7408-425X
Technische Universität Dresden, Institute of General Ecology and Environmental Protection, Chair of Biodiversity and Nature Conservation, Pienner Str. 7, 01735 Tharandt (Germany)
German Centre of Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, Deutscher Platz 5E, 04103 Leipzig (Germany)
Corresponding author
Paper Info
Citation
Dittrich S, Thiem E, Albrecht BM, von Oheimb G (2021). Cryptogamic epiphytes and microhabitat diversity on non-native green ash (Fraxinus pennsylvanica Marsh., Oleaceae) in urban habitats. iForest 14: 393-399. - doi: 10.3832/ifor3739-014
Paper history
Received: Jan 08, 2021
Accepted: Jul 06, 2021
First online: Sep 01, 2021
Publication Date: Oct 31, 2021
Publication Time: 1.90 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2021
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 33432
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 27976
Abstract Page Views: 2675
PDF Downloads: 2212
Citation/Reference Downloads: 1
XML Downloads: 568
Web Metrics
Days since publication: 1640
Overall contacts: 33432
Avg. contacts per week: 142.70
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2021): 2
Average cites per year: 0.40
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Spread intensity and invasiveness of sycamore maple (Acer pseudoplatanus L.) in Lithuanian forests
vol. 8, pp. 693-699 (online: 19 March 2015)
Research Articles
Dead wood quality influences species diversity of rare cryptogams in temperate broadleaved forests
vol. 9, pp. 276-285 (online: 28 September 2015)
Research Articles
Exploring patterns, drivers and structure of plant community composition in alien Robinia pseudoacacia secondary woodlands
vol. 11, pp. 586-593 (online: 25 September 2018)
Research Articles
Background, main results and conclusions from a test phase for biodiversity assessments on intensive forest monitoring plots in Europe
vol. 2, pp. 67-74 (online: 18 March 2009)
Research Articles
Single-tree influence on understorey vegetation in five Chinese subtropical forests
vol. 5, pp. 179-187 (online: 02 August 2012)
Research Articles
Essential environmental variables to include in a stratified sampling design for a national-level invasive alien tree survey
vol. 12, pp. 418-426 (online: 01 September 2019)
Review Papers
The forest biodiversity artery: towards forest management for saproxylic conservation
vol. 9, pp. 205-216 (online: 26 October 2015)
Research Articles
Biodiversity conservation and wood production in a Natura 2000 Mediterranean forest. A trade-off evaluation focused on the occurrence of microhabitats
vol. 12, pp. 76-84 (online: 24 January 2019)
Research Articles
The effects of forest management on biodiversity in the Czech Republic: an overview of biologists’ opinions
vol. 15, pp. 187-196 (online: 19 May 2022)
Book Reviews
National forest inventories: contributions to forest biodiversity assessments (2010)
vol. 4, pp. 250-251 (online: 05 November 2011)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword