Long-term outcome of precommercial thinning on floristic diversity in north western New Brunswick, Canada
iForest - Biogeosciences and Forestry, Volume 1, Issue 5, Pages 145-156 (2008)
doi: https://doi.org/10.3832/ifor0470-0010145
Published: Nov 25, 2008 - Copyright © 2008 SISEF
Research Articles
Abstract
The Green River spacing trials were established between 1959 and 1961 to study the long-term growth and development of balsam fir (Abies balsamea (L.) Mill.) and red spruce (Picea rubens Sarg.) in response to precommercial thinning (PCT). Three nominal spacings (1.2 m, 1.8 m, 2.4 m) and an unthinned control were applied in a randomized complete block design with 5 replicates to regenerating stands, an average of 8 years after harvest. Our study examines floristic diversity associated with these treatments approximately four decades later. Floristic diversity was assessed with several alpha diversity indices as well as multivariate analysis to compare community composition. Specific a-priori contrasts compared plant diversity among a) control and average of the wider spacings (1.8 m and 2.4 m), b) control and the narrowest spacing (1.2 m), and c) the narrowest spacing and the widest spacing. Our results indicate that there were no appreciable differences among the treatments across all measures of plant diversity investigated. As such, we conclude that the forest understory, as represented by the unthinned plots, was analogous in the thinned plots at time of stand maturity. Vegetation response to PCT treatments is inconsistent in the published literature, but this can be attributed to differences in thinning intensities, recovery age or the type of forest ecosystem studied. We conclude that PCT is a variable silvicultural tool that could be used to attain both economic productivity and biodiversity conservation goals.
Keywords
Biodiversity, Forest ecology, Precommercial Thinning, Silviculture, Tree Spacing, Plant Diversity
Introduction
Modern forest management practitioners are increasingly taking forest diversity into consideration. Consideration should be given for the trade-off between past silviculture practices, which almost exclusively aimed to promote the growth of crop species ([17], [18]) and the contemporary silviculture goals of managing ecosystems and sustaining biodiversity ([36], [38]). A reasonable goal is to have the floristic diversity in a managed stand comparable to the diversity in a natural or unmanaged stand of similar age and in comparable environmental conditions ([4], [50]). The prospective tools for restoration include treatments that favour the composition and structure of the desired crop species, with the expectation that other components of biodiversity will follow ([57], [74]). Although precommercial thinning is a common silvicultural tool, the effects of tree species and spacing on the restoration of biodiversity have largely remained untested ([50], [51], [66]).
Precommercial thinning (PCT) is widely used to manipulate tree species composition and spacing for the purpose of accelerating natural forest succession and growth ([65]). As such, PCT could be used as a tool to restore biodiversity in forest maturity. A strategy aiming to maximize forest productivity by means of PCT treatments could contribute to, maintain, or even increase stand- and forest-level community diversity, and thus help restore forest plant diversity ([31], [74]). PCT is used extensively in eastern North America as a means of reducing the density of young conifer stands that have developed from prolific natural regeneration ([55]). Approximately 2 million hectares of young forests have been precommercially thinned from Ontario eastward in Canada during the past 15 years, and efforts continue in this region at a rate approaching 200 thousand hectares per year ([16]).
Despite its wide use, the effects of PCT on the local floristic diversity are not clear in the published literature. In general, responses of species diversity to forest management are often assumed to be negative ([24]). Specific responses to PCT treatments are inconsistent, and range from increased diversity ([40], [73]) to decreased diversity ([71], [31], [28], [22]), to similar levels of diversity ([48], [34], [29], [13], [72]), compared with unmanaged stands. Many of these studies are based on relatively short-term observations (e.g., < 20 years after thinning), or larger than typically applied spacings ([50]). It is not clear whether these effects would still be evident in the longer term (e.g., after treated stands enter the stem exclusion stage - [29], [40]), or if operational spacings (1.8 to 2.4 m) had been used. Further research is needed to clarify the numerous conflicting claims in the literature.
There are few long term research (LTR) spacing trials in North America which could provide an opportunity to test the restoration of native forest biodiversity. The Green River spacing trials, installed between 1959 and 1961 ([7], [8], [1]) in northwestern New Brunswick by the Canadian Forest Service (previously the Department of Forestry), represent the oldest known, replicated PCT experiments in eastern North America. The Green River trials are particularly relevant to today’s growth and yield needs because they encompass typical operational thinning intensities and intervention times ([55]). Although the effects of the Green River experiment on growth and yield have been published ([32], [33], [55]), the response of understory plant species to the treatments has received no attention to date. The Green River thinning trials therefore provide a unique opportunity for the study of long term effects on plant diversity.
Our study examines the response of floristic plant diversity to the PCT treatments of the Green River LTR trial four decades after thinning, or approximately five decades post-harvest. Floristic diversity was assessed by comparing the thinning treatments (unthinned control, 1.2 m, 1.8 m, and 2.4 m spacing) for alpha diversity (richness, abundance, Simpson’s index, Shannon’s index, Brillouin’s index and Pielou’s index) and beta diversity (community ordination analyses). Specific a priori contrasts compared plant diversity among a) control and average of the wider spacings (1.8 m and 2.4 m), b) control and the narrowest spacing (1.2 m), and c) the narrowest spacing and the widest spacing.
Methods
Study area
The study area is located in the Green River watershed of northwestern New Brunswick, approximately 48 km north of the town of Edmundston ([10]). This region is classified by Rowe ([62]) as the Gaspé section (B.2) of the Boreal Forest Region, and by Loucks ([42]) as the Green River Site District of the Gaspé-Cape Breton Ecoregion. Topography is strongly rolling, with occasional steep areas. Elevations range between 300 and 450 m. Soils are predominantly stony loams and silt-loams derived from underlying Paleozoic slates and argillites ([42]). The area receives approximately 1000 mm of precipitation annually, nearly half of which falls between June and September. The annual frost-free period is 110 days, with a mean monthly summer temperature of 15 °C.
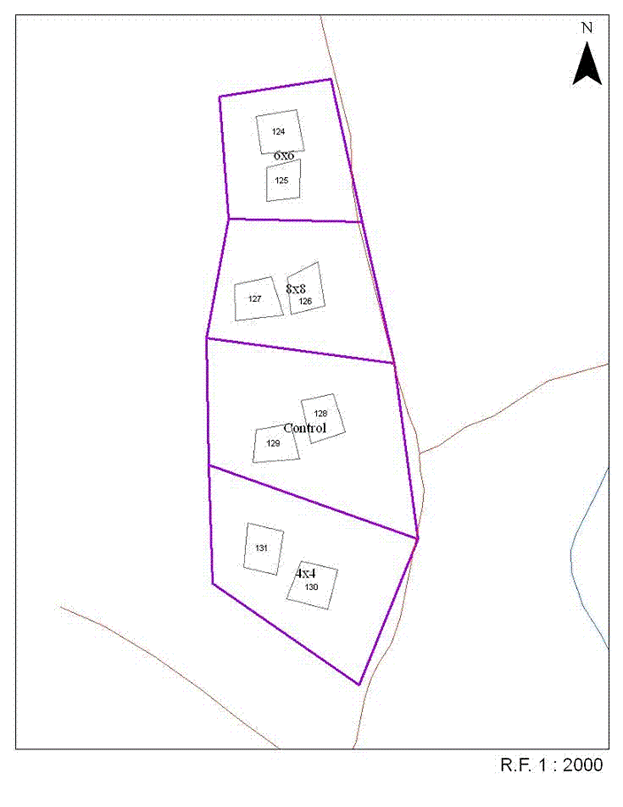
Five blocks that had been clearcut harvested for softwood pulpwood between 1946 and 1955 were selected for the study between 1959 and 1961 ([55]). Prior to harvest, these areas were dominated by balsam fir (Abies balsamea (L.) Mill.) with minor components of red spruce (Picea rubens Sarg.) and white birch (Betula papyrifera Marsh.). At the time of site selection, they contained abundant natural regeneration of balsam fir and red spruce, with minor components of white birch and shrub species, including pin cherry (Prunus pensylvanica L.f.), mountain ash (Sorbus decora (Sarg.) C.K. Schneid.), mountain maple (Acer spicatum Lam.), elderberry (Sambucus racemosa L.), and service berry (Amelanchier spp.). Each of the five blocks was divided into four approximately equal-sized treatment plots of at least 2 ha. One of the blocks (Upper Belone Bk.) was large enough (>16 ha) to accommodate eight treatment plots. Within the approximate center of each half of each treatment plot, a 28.5 m x 28.5 m (0.081 ha) permanent sample plot (PSP) was located (total of 48 PSPs - Fig. 1). The PSPs typically encompassed representative portions of the harvest area and extraction trails characteristic of the harvest method used. Although not specifically protected, these plots were part of large-scale spruce budworm (Christoneura fumiferana Clem.) aerial spray programs conducted throughout the region between 1953 and 1984 (Wayne MacKinnon, Canadian Forest Service, pers. comm.).
Fig. 1 - One block (Summit Block) showing the set-up of the four treatments (Unthinned control, 1.2 m (4x4), 1.8 m (6x6), and 2.4 m (8x8) spacings) and position of the permanent sample plots.
As of 2004, treatment plots and PSPs of the Green River study were generally in good condition; plots and monumentation still intact; no excessive blowdowns present; and trees generally free of major insect, disease, and weather stresses ([55] - Fig. 2). All standing trees were numbered and tagged and the PSP corners well marked. An area immediately to the north of one study block (Summit Rd. site) was harvested following the year-30 assessment and considerable blowdown ensued in the 1.8 m spacing treatment nearest the cut boundary. As a result of this anomaly, the data from the two PSPs in this plot were excluded from all analyses.
Thinning treatments
Three nominal spacing treatments were tested in this study: 4’ (1.2 m, 6727 stems per ha - henceforth: sph), 6’ (1.8 m, 2990 sph), and 8’ (2.4 m, 1682 sph). Each of these three spacings and an unthinned control were assigned at random to the treatment plots in each of the five blocks. Thinning took place on the blocks late in the growing season, immediately after plot establishment ([55]). The same Fraser Paper Co. thinning crews were used each year in the study, equipped with axes and McCulloch Brushmaster circular saws. The objective of thinning was to favor uniform spacing by leaving the best spruce or fir tree at or near each spacing coordinate ([7]). Shrubs were cleared from a 1 m radius around each potential crop tree. No attempts were made to compensate for the area occupied by extraction trails or natural openings within the plots. In 1972, the thinned plots were cleaned to remove trees that were, at the time of thinning, lost in the slash ([9]) and beginning to form a potentially competitive understory in the main stand. Tab. 1 presents summary stand characteristics of the treatments since thinning.
Tab. 1 - Treatment means of stand characteristics since thinning for the unthinned control and three spacing treatments.
| Years Since Thinning |
Stems Per Ha (x 1000) (> 1.3 m height) |
Dominant Height (m) |
Gross Total Volume (m3/ha) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1.2m | 1.8m | 2.4m | Control | 1.2m | 1.8m | 2.4m | Control | 1.2m | 1.8m | 2.4m | |
| 0 | 7465 | 2415 | 1797 | 1025 | 4.6 | 5.5 | 5.9 | 5.2 | 12 | 10 | 10 | 6 |
| 10 | 7989 | 3462 | 2258 | 1538 | 10.6 | 10.3 | 10.8 | 10.3 | 95 | 84 | 85 | 63 |
| 20 | 5825 | 3209 | 2151 | 1549 | 13.9 | 14.1 | 14.7 | 14.2 | 181 | 185 | 185 | 155 |
| 30 | 2840 | 2084 | 1631 | 1320 | 16.5 | 16.6 | 17.1 | 17.2 | 222 | 239 | 257 | 242 |
| 43 | 1834 | 1626 | 1387 | 1143 | 20.3 | 20.1 | 20.7 | 20.9 | 298 | 327 | 354 | 341 |
Subplot layout and data collection
For the purposes of our investigation, two subplots were systematically established within each PSP between July 1 and 28, 2004, as late June to early September is the appropriate time to collect floristic data in New Brunswick ([30]). These circular (4 m radius) subplots were centered at 9 and 18 meters along a transect run diagonally through the PSP. Species-area curves leveled off in the unthinned control plots and in each of the treatment plots, indicating adequate subplot sampling among the treatments. Two field technicians completed botanical surveys in each subplot (Appendix 1). Each plant species identified was given a percent cover estimate for each layer in which it occurred (ocular estimate) following the protocols of the Forest Ecosystem Classification system ([64]). Layers were (i) canopy trees; (ii) sub-canopy trees; (iii) woody species 2-10m height; (iv) woody species 0.5-2m height; (v) woody species <0.5m height; (vi) non-woody species including herbs, grasses, sedges, ferns, and fern allies; (vii) mosses (including liverworts), and lichens. Percent cover for all layers is defined as the vertical projection onto the ground of the aerial canopy of a given species. Common species were identified in the field; others were collected as vouchers for laboratory identification.
Diversity Analyses
Alpha diversity
Diversity was measured using richness (number of species), Simpson’s index, Shannon’s index, Brillouin’s index and Pielou’s index and abundance. Simpson’s index provides the probability that any two individuals selected at random from an infinitely large community are different species. Simpson’s index (eqn. 1):
where 1- λ is Simpson’s index of diversity and Ni is total proportion of the i-th species. This index ranges from zero, indicating low diversity, to almost 1, indicating high diversity ([35]). The Shannon-Weiner function (eqn. 2):
where H’ is the Shannon-Weiner index of species diversity, s is the total number of species, Pi is the proportion of the total sampled belonging to the i-th species. The Shannon-Weiner function typically ranges from zero to five when examining biological communities, with increasing values indicating higher species diversity ([35]). The Brillouin diversity measure (eqn. 3):
where H is Brillouin’s index, N is the total number of individual’s in entire collection, and ni is the total number of individual’s belonging to i-th species ([35]).
For proportion values in both the Simpson’s and Shannon-Weiner indexes, and the number of individuals in Brillouin’s index, percent-cover was used in the calculations. Finally, evenness, the relative equitability of species within a community, was estimated using Pielou’s Evenness (eqn. 4):
where J’ is Pielou’s measure of Evenness, H’ is the Shannon-Wiener index of diversity and S is total number of species. Pielou’s measure of evenness describes the degree to which species are evenly distributed within a community ([54]). All of the estimated parameters for diversity and abundance measures were grouped into the seven layers defined above, based on 4 subplots per treatment plot and diversity indices were calculated for each layer. The treatment-plot parameter estimates for each of the seven layers were then used as raw data in analyses of variance (ANOVA) that incorporated the underlying randomized complete block experimental design (4 treatments x 5 blocks). PROC MIXED of the SAS® System ([41]) was used to accommodate the additional 4 treatment plots at Upper Belone Bk. in the analysis and compute the correct least squares treatment means and standard errors. A priori contrasts were used to specifically compare the parameter estimates of a) unthinned plots (control) to the average of the wider spaced plots (1.8 m and 2.4 m), b) control to the narrowest spaced plots (1.2 m), and c) the narrowest (1.2 m) and widest (2.4 m) spaced plots. In these analyses, model residuals were examined to ensure that the assumptions of homogeneity of variance and normality were met. Data transformations were not necessary.
Beta Diversity
Multivariate analyses were then used to compare community composition among the treatment and control plots. Indirect ordination (detrended correspondence analysis, DCA - [70]) was used to identify the length of the gradients in standard deviations. Non-metric multidimensional scaling (NMS - [37], [43]) was used to ordinate the plot data. In NMS, the Bray Curtis distance measure was used because of its robustness for both large and small ecological gradients ([46]). Data were standardized by species maxima, and two-dimensional solutions were appropriately chosen based on plotting a measure of fit (“stress”) to the number of dimensions. One hundred iterations were used for each NMS run, using random start coordinates. The first two ordination axes were rotated to enhance interpretability (greatest spread in the clusters).
The object of discriminant function analysis is to predict multivariate responses that best discriminate the subjects in different groups ([58]). Discriminant analyses were used to classify the treatment plots using the site scores from the DCA analysis, while considering any variation in the five experimental blocks. The cluster groups from the DCA site scores and the four treatments were, for each experimental unit, used as input in a discriminant analysis which 1) determined if the classification was accurate, 2) provided discriminant functions for the classification of microhabitat types and, 3) indicated if DCA site scores or experimental units were important variables for defining treatment clusters. This provided an independent check of the clusters identified in the DCA ordination.
Results
Diversity Analyses
Alpha Diversity
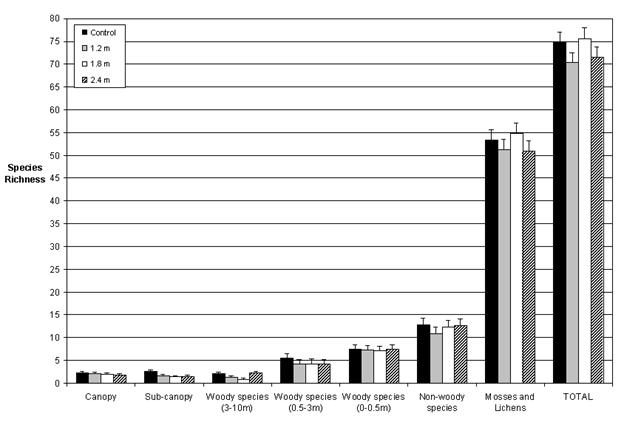
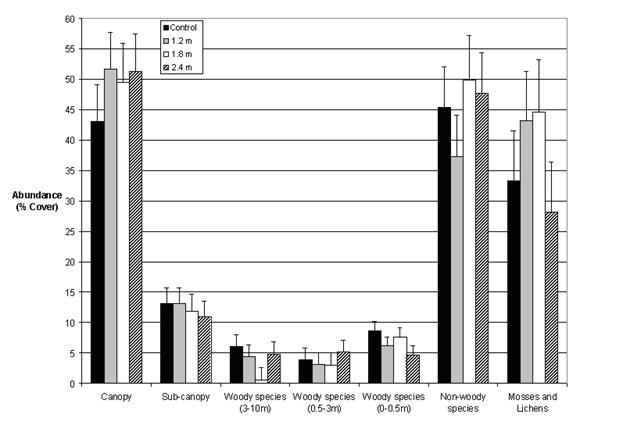
Our data indicate that the floristic diversity of PCT plots was largely restored to that of the unthinned plots four decades after treatment, or five decades after harvest. Precommercial thinning therefore appeared to have little long term effect on plant structural diversity. We observed no differences (p > 0.05) in the richness of any of the layers except the sub-canopy, where the thinned plots had somewhat lower richness than the unthinned (p = 0.04; Fig. 3). Similarly, orthogonal contrasts revealed no differences (p > 0.05) related to the abundance of the seven vegetation layers, except in the case of non-woody abundance, which was found to be lower (p = 0.04) in the larger spacings (1.8 m and 2.4 m) than in the control (Fig. 4). Moreover, the diversity indices measured (Simpson’s diversity, Shannon’s diversity, Brillouin’s diversity, and Pielou’s Eveness) revealed no differences (p > 0.05) among the treatments for any of the seven vegetation layers studied (Tab. 2).
Fig. 3 - Least squares means and their standard errors for total species richness in each forest layer and the seven forest layers combined (total). Plotted values represent the averages of five blocks.
Fig. 4 - Least squares means and their standard error of total abundance (mean % cover) of species in each forest layer. Plotted values represent the averages of five blocks.
Tab. 2 - Least squares means (M) and their standard errors (SE) of four species diversity indices for the unthinned control and three spacing treatments.
| Treatment | Simpson’s Diversity |
Shannon’s Diversity |
Brillouin’s Diversity |
Pielou’s Eveness |
|---|---|---|---|---|
| M ± SE | M ± SE | M ± SE | M ± SE | |
| Control | 0.7133 ± 0.03035 | 1.6400 ± 0.08091 | 1.4017 ± 0.07724 | 0.4233 ± 0.02060 |
| 1.2 m | 0.6983 ± 0.03035 | 1.5467 ± 0.08091 | 1.3517 ± 0.07724 | 0.3983 ± 0.02060 |
| 1.8 m | 0.6952 ± 0.03160 | 1.5452 ± 0.08540 | 1.3643 ± 0.08000 | 0.3903 ± 0.21680 |
| 2.4 m | 0.6683 ± 0.03035 | 1.5433 ± 0.08091 | 1.3200 ± 0.07724 | 0.3950 ± 0.02060 |
Beta Diversity
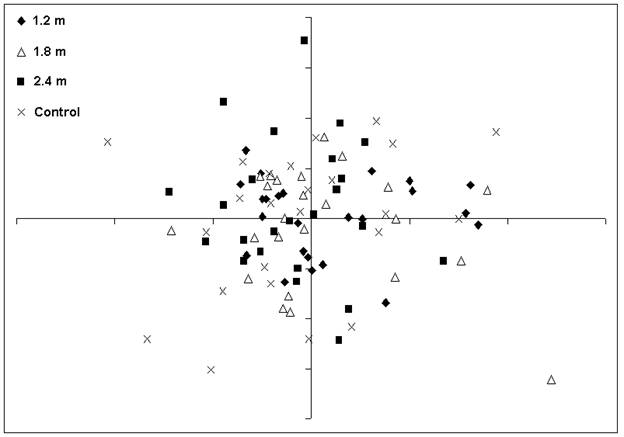
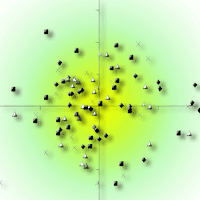
Ordination analyses indicated that plant community composition and structure were similar among the PCT treatments and the unthinned control. Non-metric multidimensional scaling (NMS) and Detrended Correspondence Analysis (DCA) analyses of 96 plots and 168 species resulted in ordinations with overlapping stand groups (representing treatment plots) indicated by low eigenvalues (first axis 0.248, gradient length 2.239 standard deviations - Fig. 5). The discriminant analysis did not classify the PCT treatments based on the heterogeneity in community composition in all plants or any specific plant groups. The canonical correlation from the discriminant functions is the ratio of the between-groups sums of squares to the total sums of squares. These discriminant functions were very low (< 3%). Wilk’s Lambda was used to test the hypothesis that, in the population, there are no differences between the groups ([67]). There were no differences (p > 0.05) for the first two DCA axes scores attributed to the experimental units (blocks).
Fig. 5 - NMS ordination (stress = 0.21) of 96 plots and 168 species in the control and three spacing treatments.
Discussion
Some researchers predict that thinning of the forest overstory may result in increased light penetration into the understory, which will favor the development of forest floor vegetation ([6], [71], [29], [40]). However, there appears to be a paradox in the results of several thinning research trials that suggest the dynamic nature of forest plant communities may override the influence of a particular limiting resource such as light.
The results are contradictory, ranging from no differences ([48], [34], [29], [13], [72]), to diversity going significantly higher ([40], [20], [6], [73], [11]), or lower ([71], [31], [28], [22]) following canopy thinning. Jobidon et al. ([31]) investigated the influence of thinning and found that large increases in hardwood productivity occurred at the expense of species richness and diversity of the understory stratum, which could not be explained by variability in canopy light. Much of the discrepancies in these research results can be attributed to different thinning intensities, time since thinning, or the type of forest ecosystem.
Thinning intensity imposes different effects on plant diversity. Below a certain level of thinning intensity, species richness increases with the intensity of thinning ([50], [20]). Moderate thinning (e.g., up to two thirds of basal area removed) is expected to promote understory species diversity, which was found to decrease under the heavy thinning performed in many types of forest ([2], [59]).
Studies in which thinning treatments appear to have had an effect on understory vegetation have largely involved early successional forests (2-20 years post thinning). Otsamo ([52]) found dramatic changes in understory vegetation in a spacing trial, which he attributed to differences in light intensity under different canopies, including a canopy species × spacing interaction. Researchers need to consider the importance of time since thinning and the consequences of natural recovery of plant diversity.
Long-term thinning studies indicate recovery of understory vegetation. There are relatively few long term thinning trials (>27 years) and surprisingly all of these studies report the recovery of understory vegetation. He & Barclay ([29]) found that, after 27 years thinning and fertilization in conifer stands had little effect on understory vegetation richness or vegetation cover. Other studies have recorded the effects at longer times since thinning, suggesting that recovery of understory species requires at least 50 years according to Metzger & Schultz ([45]) and at least 80 years according to Duffy & Meier ([21]). Kern et al. ([34]) reported that ground-layer plant communities in northern ecosystems are either resistant to change, or have recovered within the 40 years since disturbance in the even-age treatments, and within 10 years since disturbance in the uneven-age treatments. Unfortunately, none of these studies have recorded vegetation in regular intervals beyond 20 years; they only provide a single long term (usually > 40 years) assessment of recovery.
Explanation for effects observed at Green River
The Green River LTR provides an example of understory regeneration within forty years post-precommercial thinning. Our results are supported by other LTR thinning trials in both conifer and hardwood stands ([45], [34], [29]). We sampled at a single point in time 4 decades after thinning and found no differences in the floristic diversity between our unthinned controls and nominal thinning spacings between 1.2 and 2.4 m. However, we cannot discount the possibility of earlier (< 20years) differences among these treatments, which could be consistent with the findings at other thinning trials ([25], [27]). Full crown closure and subsequent intraspecific competition took place on the Green River plots about 15 years after PCT ([55]). We suspect that any differences that existed between thinned and unthinned understories would have attenuated subsequent to this point in time.
Authors of most of the thinning research trials have commented on the dynamic nature of forest plant communities ([2], [29]). A detailed look at the species reveal that some forest generalist species appear to tolerate treatments such as canopy spacing through the allocation of resources to growth, reproduction, or survival ([23], [19]). Other forest species are absent because of the lack of forest structure including microhabitats ([15], [12]). One might expect that major changes in understory vegetation occur between 10-30 years. McWilliams & Thérien ([44]) reported that differences between thinning treatments and controls were narrowing considerably over time (27-year study). It is crucial that the short term research trials continue to gather data in order to determine if the understory vegetation recovers and if so at what temporal threshold following PCT.
Alternatively, the intensity of canopy thinning at the Green River trial may have been too low to influence understory vegetation. As stated earlier, thinning intensity imposes different effects on plant diversity ([50], [20], [2], [59]). In a mature forest there is often no difference in understory communities between small gaps (33 m2) and closed canopy; higher understory richness is associated with mid (60-85 m2) to large (120-190 m2) gaps ([3]). He & Barclay ([29]) suggested that the marginal differences in canopy opening from thinning treatments were not big enough to have discernible effects on the majority of understory species. In another study of restoration in conifer plantations, Newmaster et al. ([50]) found that wider spacings (2.7 m and 3.6 m) resulted in higher richness, lower woody plant abundance, slightly higher cover of herbaceous plants, and large increases in cryptogam cover. The Green River trial spacing was narrower (1.2 m, 1.8 m, 2.4 m) and, as such, we cannot discount the possibility that these narrow tree spacings do not change the light resources sufficiently to affect plant community structure and diversity - at least over the longer-term.
Forest Diversity
Silvicultural systems could adopt restoration goals that aim to recover or sustain native and late-successional species diversity, while also meeting goals for timber production. Contemporary silvicultural systems have been moving in this direction for a wide variety of forest ecosystems (e.g., [49], [63], [53], [5], [34]). Goals need to sustain the plant species that coexist in different forest ecosystems along site quality gradients ([60], [39], [4]). For example, Jobidon et al. ([31]) has suggested a strategy aiming to maximise productivity by means of precommercial thinning treatments that will maintain or even increase stand structural diversity, which helps protect biodiversity, without affecting understory plant species diversity.
Precommercial thinning is a variable silvicultural tool that could be used to attain both economic productivity and biodiversity restoration goals. In general, thinning stands leads to positive responses in biomass ([71], [68]), with the added benefits of control over forest species composition. For example, in eastern Canada, PCT treatments are used to maintain a proportion of deciduous tree species within canopy of spruce spacing trials ([47], [31]). Conventional PCT prescribes a single target density to an entire stand. Forest managers could, however, implement restoration goals that compensate for the strong early successional response of understory vegetation to thinning by varying the intensity of thinning within and among stands, leaving a mixture of open and dense canopies. Although we have shown that after forty years, plant diversity was comparable between thinned and unthinned stands, in other forest ecosystems, the limited dispersal and slow rate of growth of some native species may prevent their recovery ([14], [61]). Further research is needed to investigate the habitat and dispersal requirements of native species with respect to the size of fragmented forest ([56], [50]). Further research on understory diversity consequences of very high thinning intensities is needed, and would be of direct relevance to “new forestry” methods such as green tree retention ([69], [26], [71]). We recommend a shift away from focusing on the effects of forest management on biodiversity, and instead to try to better understand the underlying mechanisms in ecology that will serve forest managers and conservationists as tools in the preservation of biodiversity.
Acknowledgements
We are greatly indebted to Dr. Gordon Baskerville for his foresight and early efforts in the sound establishment of the Green River thinning trials. The long and continued efforts of a number of field and office personnel over the years have kept this study “alive”. At the risk of missing some of these folks, they include B.J. Akerly, Rodney Foster, Mike Ker, Mike Lavigne, Wayne Malloy, Gerrit van Raalte, and Ben Weale. To the credit of Nexfor Fraser Papers Inc., now Acadian Timber, (Kevin Topolniski, Luc Ouellette, and Gordon Whitmore) and N.B. Department of Natural Resources and Energy, these plots have been carefully protected through the years and remain relatively undisturbed. We would also like to thank Tara Stephens, Chris Roosenboom, and Trevor Wilson for their assistance in the field and at the herbarium. Aron Fazekas and Candice Newmaster provided editorial support for which we a grateful. Funding for the 2004 measurements and analysis was provided by the Canadian Forest Service, Canadian Ecology Centre - Forestry Research Partnership, and the New Brunswick Growth and Yield Committee.
Box 1 - Abundance (% cover by ocular estimate) and frequency (%) of species occurrence in subplots (total = 24) for control and three spacing treatments.
| Layer | Species | TREATMENTS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 1.2 m | 1.8 m | 2.4 m | ||||||
| Abun. | Freq. | Abun. | Freq. | Abun. | Freq. | Abun. | Freq. | ||
| Canopy | Abies balsamea | 36 | 100 | 47 | 100 | 46 | 100 | 48 | 100 |
| Acer saccharum | 0 | 0 | 0.1 | 4 | 0 | 0 | 0 | 0 | |
| Betula alleghaniensis | 10 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Betula papyrifera | 10 | 8 | 20 | 4 | 0 | 0 | 0 | 0 | |
| Picea rubens | 8 | 71 | 8 | 46 | 7 | 58 | 8 | 42 | |
| Sub-Canopy | Abies balsamea | 10 | 88 | 14 | 88 | 13 | 75 | 10 | 96 |
| Betula alleghaniensis | 5 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Betula papyrifera | 7 | 38 | 0.1 | 4 | 10 | 4 | 3 | 8 | |
| Picea rubens | 8 | 17 | 2 | 21 | 1 | 21 | 8 | 17 | |
| Sorbus decora | 5 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Woody Species (2-10m) |
Abies balsamea | 6 | 63 | 8 | 50 | 2 | 38 | 18 | 25 |
| Acer saccharum | 0 | 0 | 0 | 0 | 0.1 | 4 | 1 | 4 | |
| Acer spicatum | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Amelanchier spp. | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 4 | |
| Betula alleghaniensis | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 4 | |
| Betula papyrifera | 4 | 54 | 0 | 0 | 0 | 0 | 0.7 | 13 | |
| Picea rubens | 0 | 0 | 3 | 8 | 0 | 0 | 0.7 | 13 | |
| Woody Species (0.5-2m) |
Abies balsamea | 3 | 58 | 4 | 42 | 0 | 0 | 0.1 | 4 |
| Acer pensylvanicum | 1 | 4 | 0.1 | 8 | 0 | 0 | 0.1 | 4 | |
| Acer rubrum | 0 | 0 | 0.1 | 4 | 6 | 50 | 6 | 50 | |
| Acer pensylvanicum | 0 | 0 | 0 | 0 | 0.1 | 4 | 0.1 | 4 | |
| Acer rubrum | 0 | 0 | 0 | 0 | 0.1 | 4 | 0.4 | 13 | |
| Acer saccharum | 0 | 0 | 0 | 0 | 0.6 | 21 | 0.6 | 8 | |
| Acer spicatum | 8 | 8 | 1 | 4 | 0.4 | 13 | 0.4 | 13 | |
| Amelanchier spp. | 0.1 | 13 | 0.1 | 13 | 0.3 | 17 | 1 | 25 | |
| Betula alleghaniensis | 0.8 | 42 | 1 | 29 | 2 | 46 | 5 | 25 | |
| Betula papyrifera | 1 | 75 | 1 | 63 | 0.2 | 63 | 0.2 | 50 | |
| Corylus cornuta ssp. cornuta | 0 | 0 | 0.1 | 4 | 0 | 0 | 1 | 4 | |
| Lonicera canadensis | 1 | 4 | 0.1 | 4 | 0.1 | 13 | 0 | 0 | |
| Picea rubens | 0.6 | 8 | 10 | 4 | 0.1 | 4 | 0.1 | 4 | |
| Prunus pensylvanica | 0.4 | 13 | 0 | 0 | 0 | 0 | 0.1 | 4 | |
| Ribes lacustre | 0 | 0 | 0 | 0 | 0.1 | 4 | 0.1 | 4 | |
| Rubus idaeus ssp. idaeus | 0 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | |
| Sambucus racemosa var. racemosa | 0 | 0 | 0 | 0 | 0.1 | 8 | 0 | 0 | |
| Sorbus decora | 0.1 | 29 | 0.1 | 21 | 0.1 | 13 | 0.1 | 8 | |
| Viburnum lantanoides | 0.1 | 4 | 0 | 0 | 0 | 0 | 0.1 | 4 | |
| Woody Species (<0.5m) |
Abies balsamea | 8 | 100 | 5 | 100 | 7 | 100 | 4 | 100 |
| Acer pensylvanicum | 0.1 | 4 | 0.1 | 13 | 0.1 | 8 | 0.1 | 17 | |
| Acer rubrum | 0.1 | 4 | 0.1 | 17 | 0.1 | 13 | 0.1 | 21 | |
| Acer saccharum | 0 | 0 | 0 | 0 | 0.3 | 17 | 0.1 | 4 | |
| Acer spicatum | 0.1 | 25 | 0.1 | 33 | 0.2 | 33 | 0.1 | 33 | |
| Amelanchier laevis | 0 | 0 | 0 | 0 | 0.1 | 4 | 0 | 0 | |
| Amelanchier spp. | 0.1 | 8 | 0.1 | 4 | 0.1 | 8 | 0.1 | 4 | |
| Betula alleghaniensis | 0.2 | 54 | 0.2 | 54 | 0.3 | 42 | 0.2 | 58 | |
| Betula papyrifera | 0.2 | 83 | 0.2 | 75 | 0.2 | 75 | 0.1 | 67 | |
| Corylus cornuta ssp. cornuta | 0 | 0 | 0.1 | 4 | 0 | 0 | 0.1 | 4 | |
| Linnaea borealis ssp. longiflora | 0.1 | 8 | 0.5 | 21 | 0.6 | 17 | 0.1 | 13 | |
| Lonicera canadensis | 0 | 0 | 0.1 | 4 | 0.1 | 8 | 0.1 | 4 | |
| Picea glauca | 0 | 0 | 0.1 | 8 | 0 | 0 | 0 | 0 | |
| Picea rubens | 0.1 | 88 | 0.1 | 79 | 0.2 | 71 | 0.1 | 75 | |
| Prunus pensylvanica | 0.1 | 13 | 0 | 0 | 0.1 | 17 | 0.1 | 4 | |
| Ribes lacustre | 0.1 | 13 | 0 | 0 | 0 | 0 | 0.1 | 4 | |
| Rubus idaeus ssp. idaeus | 0.1 | 8 | 0 | 0 | 0.1 | 4 | 0 | 0 | |
| Rubus pubescens | 1 | 4 | 0 | 0 | 0.1 | 4 | 0 | 0 | |
| Sambucus racemosa var. racemosa | 0 | 0 | 0.1 | 4 | 0 | 0 | 0.1 | 4 | |
| Sorbus decora | 0.2 | 83 | 0.1 | 67 | 0.1 | 50 | 0.1 | 63 | |
| Gaultheria hispidula | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 4 | |
| Picea rubens | 0.1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Viburnum lantanoides | 0.1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Non-Woody Species | Poa spp. | 0.1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aralia nudicaulis | 0.5 | 21 | 0.6 | 42 | 2 | 42 | 3 | 71 | |
| Symphyotrichum ciliolatum | 0.1 | 8 | 0 | 0 | 0.1 | 8 | 0.1 | 8 | |
| Circaea alpina | 0.1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Clintonia borealis | 2 | 88 | 2 | 92 | 2.2 | 96 | 3 | 96 | |
| Coptis trifolia | 0.1 | 67 | 0.4 | 79 | 1 | 58 | 0.2 | 54 | |
| Cornus canadensis | 0.3 | 88 | 2 | 100 | 3 | 100 | 1 | 100 | |
| Galium triflorum | 0.1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Goodyera repens | 0.1 | 25 | 0.1 | 46 | 0.1 | 54 | 0.1 | 46 | |
| Goodyera tesselate | 0.1 | 17 | 0.1 | 17 | 0.1 | 42 | 0.1 | 13 | |
| Goodyera spp. | 0 | 0 | 0.1 | 4 | 0 | 0 | 0 | 0 | |
| Maianthemum canadense | 0.5 | 79 | 0.4 | 67 | 0.5 | 75 | 0.1 | 75 | |
| Mitella nuda | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Monotropa uniflora | 0.1 | 42 | 0.1 | 33 | 0.1 | 46 | 0.1 | 13 | |
| Orthilia secunda | 0.6 | 8 | 0.1 | 8 | 0 | 0 | 0.1 | 4 | |
| Oxalis montana | 0.5 | 83 | 36 | 83 | 40 | 92 | 42 | 88 | |
| Streptopus amplexifolius | 0.1 | 4 | 0 | 0 | 0 | 0 | 0.1 | 4 | |
| Trientalis borealis ssp. borealis | 0.1 | 75 | 0.1 | 83 | 0.1 | 8 | 0.1 | 17 | |
| Trillium spp. | 0.1 | 4 | 0 | 0 | 0.1 | 92 | 0.1 | 88 | |
| Viola renifolia | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 4 | |
| Viola spp. | 0.1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Athyrium filix-femina var. angustu | 1 | 4 | 0 | 0 | 0.1 | 4 | 0 | 0 | |
| Dryopteris carthusiana | 0.1 | 4 | 0 | 0 | 2 | 21 | 1 | 4 | |
| Dryopteris expansa | 5 | 88 | 2 | 92 | 3 | 71 | 3 | 100 | |
| Dryopteris intermedia | 0.4 | 46 | 0.4 | 46 | 2 | 42 | 0.5 | 42 | |
| Dryopteris spp. | 0.1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Lycopodium clavatum | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 4 | |
| Lycopodium dendroideum | 0.1 | 8 | 0.1 | 21 | 0.1 | 17 | 0.1 | 17 | |
| Phegopteris connectilis | 0.1 | 4 | 0 | 0 | 0 | 0 | 0.1 | 4 | |
| Polystichum braunii | 0 | 0 | 0 | 0 | 0.1 | 4 | 0 | 0 | |
| Pteridium aquilinum var. latiuscul | 0 | 0 | 0 | 0 | 0.1 | 8 | 3 | 21 | |
| Thelypteris noveboracensis | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 8 | |
| Carex sp.1 | 0.1 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Carex sp.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 4 | |
| Mosses | Anomodon attenuatus | 0 | 0 | 0 | 0 | 0.1 | 4 | 0 | 0 |
| Aulacomnium palustre | 0.1 | 4 | 0 | 0 | 0 | 0 | 0.1 | 4 | |
| Bazzania trilobata var. trilobata | 0.2 | 83 | 0.1 | 92 | 0.2 | 75 | 0.2 | 79 | |
| Blepharostoma trichophyllum ssp. t | 0.1 | 25 | 0.1 | 17 | 0.1 | 29 | 0.1 | 13 | |
| Brachythecium acuminatum | 0.1 | 83 | 0.1 | 92 | 0.2 | 96 | 0.1 | 83 | |
| Brachythecium oedipodium | 0.1 | 17 | 0.1 | 38 | 0.1 | 25 | 0.1 | 25 | |
| Brachythecium reflexum var. reflex | 0.1 | 4 | 0.1 | 4 | 0 | 0 | 0 | 0 | |
| Brachythecium salebrosum var. sale | 0.1 | 13 | 0.1 | 17 | 0.1 | 4 | 0.1 | 8 | |
| Callicladium haldanianum | 0.1 | 42 | 0.1 | 29 | 0.1 | 21 | 0.1 | 38 | |
| Campylium chrysophyllum | 0.1 | 17 | 0.1 | 8 | 0.1 | 13 | 0.1 | 21 | |
| Cephalozia bicuspidata ssp.bicusp | 0.1 | 96 | 0.1 | 100 | 0.1 | 92 | 0.1 | 96 | |
| Cephalozia connivens var. conniven | 0.1 | 38 | 0.1 | 33 | 0.1 | 38 | 0.1 | 50 | |
| Cephalozia lunulifolia | 0.1 | 88 | 0.1 | 88 | 0.1 | 92 | 0.1 | 92 | |
| Cephaloziella rubella var. rubella | 0 | 0 | 0 | 0 | 0.1 | 4 | 0 | 0 | |
| Dicranum flagellare | 0.1 | 92 | 0.1 | 88 | 0.1 | 88 | 0.1 | 100 | |
| Dicranum fuscescens var. fuscescen | 0.1 | 100 | 0.1 | 100 | 0.1 | 100 | 0.1 | 100 | |
| Dicranella heteromalla | 0.1 | 92 | 0.1 | 79 | 0.1 | 92 | 0.1 | 83 | |
| Dicranum montanum | 0.1 | 54 | 0.1 | 42 | 0.1 | 46 | 0.1 | 63 | |
| Dicranum ontariense | 0.1 | 25 | 0.1 | 13 | 0.1 | 13 | 0.1 | 38 | |
| Dicranum polysetum | 0.2 | 79 | 0.3 | 88 | 0.2 | 83 | 0.2 | 83 | |
| Eurhynchium pulchellum var. pulche | 0.1 | 21 | 0.1 | 17 | 0.1 | 21 | 0.1 | 13 | |
| Frullania spp. | 0.1 | 4 | 0 | 0 | 0.1 | 4 | 0 | 0 | |
| Geocalyx graveolens | 0.1 | 33 | 0.1 | 25 | 0.1 | 38 | 0.1 | 29 | |
| Harpanthus drummondii | 0.1 | 4 | 0.1 | 4 | 0.1 | 4 | 0 | 0 | |
| Heterocladium dimorphum | 0.1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hylocomium splendens | 13 | 79 | 14 | 83 | 16 | 79 | 9 | 75 | |
| Hypnum cupressiforme var. cupressi | 0 | 0 | 0 | 0 | 0.1 | 4 | 0 | 0 | |
| Hypnum imponens | 0.1 | 88 | 0.1 | 100 | 0.1 | 83 | 0.1 | 79 | |
| Hypnum pallescens var. pallescens | 0.1 | 96 | 0.1 | 92 | 0.1 | 75 | 0.1 | 88 | |
| Hypnum pratense | 0.1 | 33 | 0.1 | 33 | 0.1 | 42 | 0.1 | 25 | |
| Jamesoniella autumnalis var. autum | 0.1 | 46 | 0.1 | 50 | 0.1 | 67 | 0.1 | 71 | |
| Lepidozia reptans | 0 | 0 | 0.1 | 4 | 0 | 0 | 0 | 0 | |
| Loeskeobryum brevirostre | 1 | 67 | 3 | 50 | 0.7 | 75 | 1 | 88 | |
| Lophozia badensis var. badensis | 0 | 0 | 0 | 0 | 0.1 | 4 | 0 | 0 | |
| Lophozia bicrenata | 0.1 | 63 | 0.1 | 63 | 0.1 | 58 | 0.1 | 71 | |
| Lophozia capitata | 0.1 | 8 | 0.1 | 17 | 0.1 | 8 | 0.1 | 4 | |
| Lophozia heterocolpos var. heteroc | 0.1 | 13 | 0.1 | 8 | 0.1 | 25 | 0 | 0 | |
| Lophocolea heterophylla | 0.1 | 79 | 0.1 | 100 | 0.1 | 83 | 0.1 | 92 | |
| Nowellia curvifolia | 0.1 | 29 | 0.1 | 38 | 0.1 | 17 | 0.1 | 17 | |
| Oncophorus wahlenbergii | 0 | 0 | 0.1 | 4 | 0 | 0 | 0 | 0 | |
| Plagiothecium denticulatum | 0.1 | 4 | 0.1 | 4 | 0.1 | 17 | 0.1 | 8 | |
| Plagiothecium laetum | 0.1 | 100 | 0.1 | 100 | 0.1 | 100 | 0.1 | 100 | |
| Plagiomnium medium var. medium | 0 | 0 | 0 | 0 | 0.1 | 4 | 0.1 | 4 | |
| Platygyrium repens | 0.1 | 88 | 0.1 | 79 | 0.1 | 88 | 0.1 | 92 | |
| Pleurozium schreberi | 17 | 100 | 26 | 96 | 23 | 100 | 16 | 96 | |
| Pohlia nutans | 0.1 | 33 | 0.1 | 67 | 0.1 | 58 | 0.1 | 54 | |
| Polytrichum commune var. commune | 0.1 | 17 | 0.1 | 25 | 0.1 | 25 | 0.1 | 21 | |
| Polytrichum formosum | 0 | 0 | 0.1 | 4 | 0 | 0 | 0 | 0 | |
| Polytrichum juniperinum | 0.1 | 17 | 0.1 | 8 | 0.1 | 17 | 0.1 | 17 | |
| Polytrichum ohioense | 0.1 | 42 | 0.1 | 25 | 0.1 | 38 | 0.1 | 58 | |
| Ptilidium ciliare | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 4 | |
| Ptilium crista-castrensis | 0.2 | 100 | 0.3 | 100 | 0.2 | 92 | 0.2 | 96 | |
| Ptilidium pulcherrimum | 0.1 | 100 | 0.1 | 100 | 0.1 | 100 | 0.1 | 100 | |
| Radula complanata | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 4 | |
| Sanionia uncinata | 0.1 | 75 | 0.1 | 50 | 0.1 | 75 | 0.1 | 75 | |
| Sphagnum capillifolium | 0.1 | 13 | 0.6 | 8 | 0.1 | 17 | 0 | 0 | |
| Sphagnum girgensohnii | 0.1 | 13 | 0.1 | 13 | 0.1 | 4 | 0.1 | 4 | |
| Sphagnum magellanicum | 0 | 0 | 0.1 | 4 | 0 | 0 | 0 | 0 | |
| Steerecleus serrulatus | 0.1 | 17 | 0 | 0 | 0.1 | 21 | 0.1 | 21 | |
| Tetraphis pellucida | 0.1 | 96 | 0.1 | 100 | 0.1 | 96 | 0.1 | 100 | |
| Thuidium delicatulum | 0.1 | 21 | 0 | 0 | 0.1 | 8 | 0 | 0 | |
| Lichens | Biatora vernalis | 0.1 | 13 | 0.1 | 17 | 0.1 | 13 | 0.1 | 13 |
| Candelaria concolor | 0.1 | 83 | 0.1 | 75 | 0.1 | 83 | 0.1 | 71 | |
| Cladonia cenotea | 0.1 | 33 | 0.1 | 29 | 0.1 | 46 | 0.1 | 38 | |
| Cladonia chlorophaea | 0.1 | 17 | 0.1 | 21 | 0.1 | 21 | 0.1 | 21 | |
| Cladonia coniocraea | 0.1 | 100 | 0.1 | 96 | 0.1 | 96 | 0.1 | 96 | |
| Cladonia cornuta | 0.1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cladonia crispata var.crispata | 0.1 | 8 | 0.1 | 8 | 0.1 | 8 | 0.1 | 25 | |
| Cladonia cristatella | 0 | 0 | 0 | 0 | 0.1 | 8 | 0.1 | 4 | |
| Cladonia deformis | 0.1 | 4 | 0 | 0 | 0.1 | 4 | 0 | 0 | |
| Cladonia digitata | 0 | 0 | 0.1 | 4 | 0 | 0 | 0 | 0 | |
| Cladonia fimbriata | 0.1 | 79 | 0.1 | 79 | 0.1 | 92 | 0.1 | 92 | |
| Cladonia gracilis ssp. turbinata | 0.1 | 4 | 0 | 0 | 0 | 0 | 0.1 | 8 | |
| Cladina mitis | 0 | 0 | 0.1 | 4 | 0 | 0 | 0 | 0 | |
| Cladonia multiformis | 0 | 0 | 0.1 | 4 | 0.1 | 8 | 0.1 | 4 | |
| Cladonia pleurota | 0.1 | 21 | 0.1 | 38 | 0.1 | 29 | 0.1 | 8 | |
| Cladonia pyxidata | 0.1 | 4 | 0.1 | 4 | 0.1 | 4 | 0 | 0 | |
| Cladina rangiferina | 0.1 | 25 | 0.1 | 42 | 0.1 | 21 | 0.1 | 29 | |
| Cladonia squamosa | 0.1 | 21 | 0.1 | 29 | 0.1 | 29 | 0.1 | 21 | |
| Leproloma membranaceum | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 8 | |
| Evernia mesomorpha | 0.1 | 42 | 0.1 | 54 | 0.1 | 38 | 0.1 | 25 | |
| Hypogymnia bitteri | 0.1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hypgymnia krogiae | 0 | 0 | 0 | 0 | 0.1 | 4 | 0 | 0 | |
| Hypogymnia physodes | 0.1 | 79 | 0.1 | 67 | 0.1 | 83 | 0.1 | 100 | |
| Icmadophila ericetorum | 0.1 | 4 | 0.1 | 13 | 0.1 | 8 | 0.1 | 8 | |
| Lecanora hybocarpa | 0 | 0 | 0.1 | 4 | 0 | 0 | 0 | 0 | |
| Lepraria lobificans | 0.1 | 83 | 0.1 | 88 | 0.1 | 88 | 0.1 | 79 | |
| Loxospora pustulata | 0.1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mycoblastus sanguinarius | 0.1 | 88 | 0.1 | 96 | 0.1 | 92 | 0.1 | 100 | |
| Pannaria rubiginosa | 0 | 0 | 0 | 0 | 0.1 | 4 | 0 | 0 | |
| Parmelia saxatilis | 0.1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Parmelia sulcata | 0.1 | 63 | 0.1 | 67 | 0.1 | 75 | 0.1 | 71 | |
| Peltigera neopolydactyla | 0.1 | 25 | 0.1 | 8 | 0.1 | 25 | 0.1 | 29 | |
| Pertusaria amara | 0.1 | 21 | 0.1 | 25 | 0.1 | 4 | 0.1 | 4 | |
| Phaeophyscia adiastola | 0 | 0 | 0 | 0 | 0.1 | 4 | 0 | 0 | |
| Phaeophyscia ciliata | 0.1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Platismatia glauca | 0.1 | 92 | 0.1 | 96 | 0.1 | 92 | 0.1 | 96 | |
| Phlyctis argena | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 4 | |
| Physconia muscigena | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 13 | |
| Ramalina americana | 0 | 0 | 0 | 0 | 0.1 | 4 | 0.1 | 8 | |
| Ramalina dilacerata | 0.1 | 8 | 0.1 | 8 | 0.1 | 4 | 0.1 | 4 | |
| Trapeliopsis granulosa | 0.1 | 4 | 0.1 | 4 | 0 | 0 | 0 | 0 | |
| Tuckermannopsis americana | 0.1 | 8 | 0 | 0 | 0.1 | 17 | 0.1 | 17 | |
| Tuckermannopsis orbata | 0.1 | 4 | 0.1 | 33 | 0.1 | 33 | 0.1 | 38 | |
| Tuckermannopsis spp. | 0 | 0 | 0.1 | 4 | 0.1 | 4 | 0.1 | 4 | |
| Usnea filipendula | 0.1 | 88 | 0.1 | 92 | 0.1 | 100 | 0.1 | 96 | |
| Usnea lapponica | 0 | 0 | 0 | 0 | 0.1 | 4 | 0 | 0 | |
| Usnea spp. | 0 | 0 | 0.1 | 4 | 0 | 0 | 0 | 0 | |
| Xanthoparmelia somloënsis | 0 | 0 | 0.1 | 4 | 0 | 0 | 0 | 0 | |
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Swiss Federal Research Institute WSL, Biodiversity and Conservation Biology, Zürcherstr. 111, CH-8903, Birmensdorf (Switzerland)
Floristic Diversity Research Group, OAC Herbarium, University of Guelph, Guelph, N1G 2W1 - Ontario, (Canada)
Canadian Forest Service, Atlantic Forestry Centre, Hugh John Fleming Forestry Centre, P.O. Box 400, E3B 5P7 - Fredericton, New Brunswick (Canada)
Canadian Forest Service, Great Lakes Forestry Centre, 1219 Queen St. E., Sault Ste. Marie, P6A 5M7 - Ontario (Canada)
Corresponding author
Paper Info
Citation
Cole HA, Newmaster SG, Lanteigne L, Pitt D (2008). Long-term outcome of precommercial thinning on floristic diversity in north western New Brunswick, Canada. iForest 1: 145-156. - doi: 10.3832/ifor0470-0010145
Paper history
Received: Oct 02, 2008
Accepted: Nov 13, 2008
First online: Nov 25, 2008
Publication Date: Nov 25, 2008
Publication Time: 0.40 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2008
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 58748
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 49694
Abstract Page Views: 3306
PDF Downloads: 4468
Citation/Reference Downloads: 97
XML Downloads: 1183
Web Metrics
Days since publication: 6246
Overall contacts: 58748
Avg. contacts per week: 65.84
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2008): 10
Average cites per year: 0.56
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Modeling of time consumption for selective and situational precommercial thinning in mountain beech forest stands
vol. 14, pp. 137-143 (online: 16 March 2021)
Research Articles
Tree-oriented silviculture: a new approach for coppice stands
vol. 9, pp. 791-800 (online: 04 August 2016)
Research Articles
Interactions between thinning and bear damage complicate restoration in coast redwood forests
vol. 13, pp. 1-8 (online: 08 January 2020)
Research Articles
The conversion into high forest of Turkey oak coppice stands: methods, silviculture and perspectives
vol. 13, pp. 309-317 (online: 10 July 2020)
Review Papers
Opportunities for coppice management at the landscape level: the Italian experience
vol. 9, pp. 775-782 (online: 04 August 2016)
Research Articles
Effects of different silvicultural measures on plant diversity - the case of the Illyrian Fagus sylvatica habitat type (Natura 2000)
vol. 9, pp. 318-324 (online: 22 October 2015)
Review Papers
Biodiversity assessment in forests - from genetic diversity to landscape diversity
vol. 2, pp. 1-3 (online: 21 January 2009)
Research Articles
Early responses of biodiversity indicators to various thinning treatments in mountain beech forests
vol. 11, pp. 609-618 (online: 25 September 2018)
Review Papers
The forest biodiversity artery: towards forest management for saproxylic conservation
vol. 9, pp. 205-216 (online: 26 October 2015)
Research Articles
Scale dependency of the effects of landscape structure and stand age on species richness and aboveground biomass of tropical dry forests
vol. 16, pp. 234-242 (online: 23 August 2023)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword