Efficacy of Phlebiopsis gigantea against Heterobasidion conidiospore and basidiospore infection in spruce wood
iForest - Biogeosciences and Forestry, Volume 13, Issue 5, Pages 369-375 (2020)
doi: https://doi.org/10.3832/ifor3279-013
Published: Aug 25, 2020 - Copyright © 2020 SISEF
Research Articles
Abstract
Treatment of freshly cut stumps with biological control agents containing Phlebiopsis gigantea spores effectively restricts the spread of new Heterobasidion infections in conifer forests. To test the control efficacy of different P. gigantea strains, conifer stumps or billets cut from tree stems can be artificially infected with asexual Heterobasidion conidiospores or sexual basidiospores or left for natural basidiospore infection. Currently, no information is available about whether the control efficiency of P. gigantea in Norway spruce wood is affected by Heterobasidion spore type. In the present study, the impact of four P. gigantea strains (including the commercial product Rotstop®) on initiation and development of Heterobasidion basidiospore and conidiospore infections as well as the relationship between the area occupied by P. gigantea and control efficacy were analysed in spruce billets. The mean size of the area occupied by P. gigantea was larger, and the efficacy of P. gigantea against Heterobasidion was significantly higher in billets left for natural basidiospore infection compared to treatment with Heterobasidion conidiospore suspension. The control efficacy against Heterobasidion infection was high, although only a small area of the billet surface was occupied by P. gigantea and even when there was no visible discoloration caused by P. gigantea infection on wood surfaces.
Keywords
Introduction
Infection of freshly cut stump surfaces by airborne spores is the most common way of Heterobasidion species establishment into previously uninfected conifer stands. From infected stump roots, the fungus spreads to adjacent healthy trees causing root and butt rot in the residual stand ([31], [46]). An effective way to restrict Heterobasidion spore infections of conifer stumps is to treat the stump surfaces with biological or chemical control agents ([11], [26]). Biological preparations containing asexual spores of Phlebiopsis gigantea - an antagonist of Heterobasidion species - are very effective in pine stumps but can be less effective in spruce stumps ([35] and literature therein). However, good control efficiency can also be achieved in spruce wood when the spore concentration of treatment suspension is high, i.e., 5 million spores L-1 ([14]). A commercial preparation of P. gigantea, Rotstop®, was developed almost 30 years ago in Finland and is the most commonly used biological control agent against Heterobasidion root rot in Europe ([15], [40]). However, the use of a genetically homogenous preparation may negatively affect fungal communities, therefore different studies were carried out to evaluate the impact of the biological control agent on below ground and stump colonizing fungal communities ([11], [42], [33], [44], [45], [19], [37]). Previous studies indicate that local P. gigantea strains can be as effective, or even better, than the Finnish strain used in Rotstop® ([3]). Therefore, studies have been carried out in several countries to identify local P. gigantea strains that could be used for controlling Heterobasidion root rot ([13] and literature therein).
Field testing of the efficiency of P. gigantea strains is usually done in stumps at sites with natural Heterobasidion spore infection ([15], [17], [2], [1], [3], [21], [32], [5], [13]). However, under in vivo conditions, the results can be influenced by several factors such as erratic densities of airborne Heterobasidion spores, as well as variable background levels of natural P. gigantea spore load ([2], [6]). Laborious and long-lasting field experiments may even be inconclusive due to a lack of Heterobasidion infections in control stumps ([15]). The growth rate and efficiency of P. gigantea against Heterobasidion infection is also strongly dependent on the characteristics of individual trees ([35]). In several infection experiments, billets cut from tree stems have been used instead of stumps, or billets are used in conjunction with stumps ([10], [15], [14], [17], [33], [1], [35]). Cutting several billets from each tree reduces the variation due to differences between individual trees and enables the use of more controlled experimental conditions to efficiently acquire information about growth rate of P. gigantea strains and competitive ability against Heterobasidion.
Although both artificial inoculation with conidial suspensions and natural or artificial basidiospore infection have been widely used in several studies testing the efficiency of P. gigantea preparations, only a few studies (and only using stumps of Pinus spp.) have been carried out to compare the efficiency of P. gigantea against both Heterobasidion basidio- and conidiospore infections under the same experimental conditions. Both in Pinus echinata and P. nigra ssp. laricio stumps, P. gigantea proved to be more efficient against infection caused by Heterobasidion conidiospores than by basidiospores ([16], [41]). As the host tree species influences the control effect of P. gigantea as well as susceptibility to Heterobasidion species ([39], [7], [8], [47]), the results obtained in one tree species may not be applicable to others. Besides Scots pine stumps, Norway spruce stumps are the most important targets of stump treatment in northern and most of central Europe. Increased knowledge of interactions between P. gigantea and infection of Norway spruce by Heterobasidion with different spore types would assist in testing the efficiency of novel local P. gigantea strains.
The aim of the present study was: (i) to evaluate the efficacy of different Latvian P. gigantea strains to control Heterobasidion conidio- and basidiospore infections in spruce wood in maximally standardised Norway spruce wood (billets); and (ii) to analyse the relationship between occupied area and control efficacy of P. gigantea against infection caused by both types of Heterobasidion spores.
Materials and methods
Preparation of billets
This experiment was initiated in May 2010. Three trees of Norway spruce (Picea abies [L.] H. Karst; dbh: 13-21 cm) without visual signs of decay at stump level and with long branchless stems were felled in the experimental forests in Kalsnava, eastern Latvia (56°40′ 21″ N, 25°57′ 40″ E). For transport from the forest to the experimental location, the stems were cut into one meter long logs. Immediately before treatment, the logs were cut into 20-30 cm long segments (billets). Billets were numbered starting from the root collar. To avoid the effect of individual wood properties of each tree on the results, billets for each treatment variant were chosen randomly from all trees and also from different heights.
Preparation of treatment suspensions
Three Latvian P. gigantea strains and the biological control agent Rotstop® were used in the experiment. The Latvian strains of P. gigantea were selected based on the characteristics obtained in laboratory (Tab. 1) and field experiments (unpublished data except the data concerning the Latvian strain G1 - [13], [47]). For preparation of treatment suspension, each P. gigantea strain was cultured in six Petri dishes on malt extract agar medium for 3 weeks at 20 °C in dark conditions. Spore suspensions were prepared by washing the spores several times from one Petri dish with unsterilized tap water, agitating the colony gently three times with a glass triangle. Tap water was added to the spore suspension obtained to a final volume of one liter. To count the number of spores in the suspensions, 0.5 mL was transferred to a Petri dish containing malt extract agar medium and spread evenly.
Tab. 1 - Properties of the P. gigantea strains grown on malt extract agar medium at 20 °C. (*): Three repetitions per strain; (**): Two repetitions per strain.
| Strain | Host tree species | Growth rate (mm day-1)* |
Growth rate over Heterobasidion colony (mm day-1)** |
Spore production (million/ Petri dish)* |
|
|---|---|---|---|---|---|
| H. parviporum | H. annosum | ||||
| J4 | Pinus sylvestris | 8.0 | 1.4 | 0.9 | 12.8 |
| Kn107E | Picea abies | 6.5 | 0.8 | 0.7 | 18.5 |
| G1 | P. abies/P. sylvestris | 7.1 | 1.4 | 0.9 | 47.3 |
| Rotstop® | P. abies | 7.8 | 0.9 | 0.8 | 42.9 |
P. gigantea spores were counted under a microscope (magnification 100×) within 30 sight fields distributed systematically over the dish. The total number of spores in suspension was calculated taking into account the number of spores in the sight field, the area of the sight field and the area of the Petri dish. Treatment suspensions were prepared 2-4 hours before the experiment and the spore concentration in suspension was adjusted to ca. 5000 spores mL-1. Suspension of Heterobasidion conidiospores was prepared as a mixture from two heterokaryotic H. annosum (ISm15, VMa15) and two H. parviporum (No.66 and S37-9.8) strains of Latvian origin. Suspensions were prepared by washing spores several times from one Petri dish of each Heterobasidion strain with tap water, agitating the colony gently three times with a glass triangle and creating a mixture of all four strains. Spore concentration in suspension was adjusted to ca. 500 spores mL-1.
Treatments
The upper surface of each billet was divided in two sectors leaving a two cm wide buffer zone between sectors to avoid cross contamination. Half of the billet upper surface was covered with a paper sheet while the other half was treated with a suspension of P. gigantea until the surface was wet, i.e., an approx. 1 mm thick layer. One hour after application of P. gigantea, the entire surface of 28 billets was inoculated with Heterobasidion conidiospore suspension. After treatment, these billets (treated with P. gigantea and Heterobasidion) were placed in the field (56°40′ 51″ N, 25°57′ 53″ E). To avoid contact with soil, folding garden fabric was used and billets were watered regularly to provide appropriate moisture content for fungal growth.
The remaining 32 billets, treated with only P. gigantea strains, were transported to an experimental site (56°41′ 39″ N, 25°54′ 14″ E) - a Norway spruce stand growing on drained peat soil; forest type: Oxalidosa turf. mel.; stand age: 65 years - and exposed to natural infection by Heterobasidion basidiospores. Billets were placed in a radius of 4 metres surrounding spruce logs and stumps with abundant Heterobasidion fruit body development. Development of fruit bodies was favoured by shaded location rich in vegetation. Exposure time in the experimental site was five days. The billets were then transported to the field and placed next to the other billets. In total, billets were incubated in the field for 4 weeks. Mean daily air temperature during incubation was 16 o C. In total, 60 billets (7 repetitions for each P. gigantea strain treated with Heterobasidion conidiospores and 8 repetitions for basidiospore infection of Heterobasidion) were analysed.
Sampling and laboratory analysis
After incubation in the field, one sample disc (3 cm thick) was cut from the top of the billets and a second disc 2-3 cm lower so that the underside of the second disc was 8 cm from the top of the billet. The discs were transported to the laboratory, debarked and washed with a stiff brush under running tap water. After that the discs were placed in loosely closed transparent plastic bags and incubated for 7 days at room temperature in the daylight. The lower side of the discs at depth of 3 cm and 8 cm from the top of the billet were examined. A plastic grid consisting of 0.49 cm2 squares was fixed on each disc with pins. A dissection microscope was used to examine each square for the presence of Heterobasidion conidiophores (area colonised by the fungus was marked on the disc with red dots). The area occupied by P. gigantea was identified by the typical orange brown colouration in wood. The surface area occupied by the fungus was redrawn on transparent paper and measured using a planimeter (PLANIX 10S “Marble”, Tamaya, Japan).
Calculations and statistics
Efficacy of P. gigantea treatment was calculated taking into account the area occupied by Heterobasidion on sectors of the disc treated with P. gigantea (sapwood and heartwood included) and the area of Heterobasidion on the untreated (control) sector. The efficacy was calculated at depths of 3 and 8 cm from the billet surface, and results were obtained from 7 or 8 billet replicates. The following formula was used to calculate the efficacy of different P. gigantea strain treatments (eqn. 1):
where nt and nu represent the percentage of area occupied by Heterobasidion in treated and untreated sectors, respectively.
The correlation between the area occupied by P. gigantea and its control efficacy was calculated. Area occupied and efficacy between different P. gigantea strains were compared using the Mann-Whitney test in R ([27]). Percentages were arcsin transformed before calculations.
Results
Area occupied by P. gigantea
After Heterobasidion conidiospore infection, the mean area occupied by P. gigantea strains varied from 5.8% to 21.6% and from 0.6% to 5.1% at 3 and 8 cm depths, respectively (Tab. 2). All strains occupied a greater proportion of the disc area at a depth of 3 cm compared to 8 cm depth; moreover, the values differed significantly for strains J4, G1 and Rotstop® (p<0.05). Mean area occupied by strain G1 was significantly smaller in both analyzed depths compared to Rotstop® (p<0.05). Differences between strains were not significant at the 8 cm depth.
Tab. 2 - Mean area (%, ± standard error) occupied by P. gigantea after Heterobasidion treatment with conidiospores/basidiospores. (Strain): P. gigantea strain used for treatment.
| Strain | Conidiospore infection | Basidiospore infection | ||
|---|---|---|---|---|
| Depth: 3 cm | Depth: 8 cm | Depth: 3 cm | Depth: 8 cm | |
| J4 | 10.5 ± 4.1 | 1.2 ± 0.7 | 23.6 ± 6.6 | 22.4 ± 7.0 |
| G1 | 5.8 ± 3.0 | 0.6 ± 0.4 | 28.3 ± 7.7 | 20.9 ± 7.7 |
| Kn107E | 10.1 ± 5.5 | 2.3 ± 1.5 | 25.6 ± 6.9 | 18.3 ± 5.7 |
| Rotstop® | 21.6 ± 7.6 | 5.1 ± 1.6 | 31.4 ± 6.3 | 14.3 ± 4.5 |
| Mean | 12.0 ± 3.4 | 2.3 ± 1.0 | 27.2 ± 1.7 | 19.0 ± 1.8 |
After Heterobasidion basidiospore infection, the mean area occupied by P. gigantea strains compared to total disc area varied from 23.6% to 31.4% and from 14.3% to 22.4% at 3 and 8 cm depths, respectively. No significant differences were found in the area occupied between P. gigantea strains at either analysed depth.
Mean area occupied by P. gigantea strains was significantly larger in billets infected by Heterobasidion basidiospores compared to conidiospores at a depth of 3 cm for the strain G1. Correspondingly, at a depth of 8 cm the P. gigantea strains J4, G1 and Kn107E occupied a significantly larger area after Heterobasidion basidiospore infection compared to conidiospore infection.
Area occupied by Heterobasidion
The area occupied by Heterobasidion mycelium after conidiospore inoculation was significantly smaller in sectors treated with P. gigantea compared to sectors without P. gigantea treatment. This applied to all analysed variants at both depths (p<0.05), except for treatment with the P. gigantea strain J4 at a depth of 8 cm (Tab. 3). Mean area occupied by Heterobasidion in control sectors after conidiospore inoculation was significantly larger at a depth of 3 cm compared to 8 cm depth: 52.6% and 28.7%, respectively.
Tab. 3 - Mean area (%, ± standard error) occupied by Heterobasidion in sectors treated with P. gigantea and control sectors, analysed at depths of 3 and 8 cm.
| Infection | Strain | Depth: 3 cm | Depth: 8 cm | ||
|---|---|---|---|---|---|
| Treated | Control | Treated | Control | ||
| Conidiospore | J4 | 22.6 ± 6.4 | 46.6 ± 8.5 | 16.5 ± 7.3 | 19.9 ± 6.9 |
| G1 | 27.4 ± 5.3 | 59.7 ± 5.1 | 9.5 ± 4.3 | 25.3 ± 4.6 | |
| Kn107E | 26.9 ± 5.1 | 54.7 ± 4.0 | 11.8 ± 5.0 | 31.9 ± 3.0 | |
| Rotstop® | 5.6 ± 2.1 | 49.4 ± 5.4 | 12.0 ± 3.1 | 37.7 ± 6.6 | |
| Mean | 20.6 ± 5.1 | 52.6 ± 2.9 | 12.5 ± 1.5 | 28.7 ± 3.9 | |
| Basidiospore | J4 | 3.0 ± 1.0 | 36.9 ± 5.9 | 1.8 ± 0.7 | 35.6 ± 5.1 |
| G1 | 2.9 ± 0.8 | 41.6 ± 4.9 | 2.7 ± 1.1 | 38.3 ± 5.2 | |
| Kn107E | 4.2 ± 2.1 | 33.3 ± 7.0 | 2.9 ± 1.8 | 33.8 ± 6.5 | |
| Rotstop® | 1.2 ± 0.6 | 33.4 ± 6.8 | 1.0 ± 0.4 | 30.6 ± 7.2 | |
| Mean | 2.8 ± 0.6 | 36.3 ± 2.0 | 2.1 ± 0.4 | 34.6 ± 1.6 | |
The area occupied by Heterobasidion mycelium after natural basidiospore infection was significantly smaller in sectors treated with P. gigantea compared to sectors that were untreated in all analysed variants at both depths (p<0.05). Variation of the areas occupied by Heterobasidion in control sectors was quite large. After conidiospore treatment, the area occupied by Heterobasidion ranged from 14.9% to 80.7% (average 52.6%) and from 3.9% to 62.3% (average 28.7%) at a depth of 3 and 8 cm, respectively. After basidiospore infection, the area occupied by Heterobasidion varied from 2.9% to 70.3% (average 36.3%) and from 9.7% to 70.9% (average 34.6%) at a depth of 3 and 8 cm, respectively.
Differences in the area occupied by Heterobasidion (in sectors treated with P. gigantea) at both analysed depths were significant between basidiospore and conidiospore infections. In addition, in the control sector (without P. gigantea treatment) at a depth of 3 cm, the difference in area occupied by Heterobasidion was significant: 52.6% after conidiospore and 36.3% after basidiospore infection. In the control sector at a depth of 8 cm, there were no significant differences between basidiospore and conidiospore infection in relation to the size of area colonized by Heterobasidion.
Efficacy of P. gigantea against Heterobasidion
Mean efficacy of native P. gigantea strains and Rotstop® against conidiospore infection of Heterobasidion varied from 47% to 89% (Tab. 4). Efficacy of Latvian strains of P. gigantea was significantly lower compared to Rotstop® at a depth of 3 cm (p<0.05). At a depth of 8 cm, however, there were no statistical differences between P. gigantea strains (p>0.05). The efficacy at a depth of 8 cm was lower for P. gigantea strains J4 and Rotstop® but higher for G1 and Kn107E compared to a depth of 3 cm.
Tab. 4 - Mean efficacy of P. gigantea strains at a depth of 3 and 8 cm in Norway spruce billets.
| Infection | Depth | Strain Efficacy (%) | |||
|---|---|---|---|---|---|
| J4 | G1 | Kn107E | Rotstop® | ||
| Conidiospore | 3 cm | 51.6 | 54.1 | 50.8 | 88.7 |
| 8 cm | 47.1 | 62.5 | 63.1 | 68.3 | |
| Basidiospore | 3 cm | 91.8 | 93.0 | 87.2 | 96.4 |
| 8 cm | 94.8 | 93.0 | 91.3 | 96.9 | |
Mean efficacy of P. gigantea strains against basidiospore infection of Heterobasidion varied from 87% to 96% and from 91% to 97% in a depth of 3 and 8 cm, respectively. Rotstop® showed the highest efficacy at both analysed depths, but differences between the strains were not significant at either of the depths analysed. Mean efficacy of the three Latvian P. gigantea strains against conidiospore infection of Heterobasidion at a depth of 3 and 8 cm combined was 54.9% and for the Rotstop® strain 78.5%. Mean efficacy of the Latvian P. gigantea strains against basidiospore infection of Heterobasidion (depths of 3 and 8 cm combined) was 91.9% compared to 96.7% for the Rotstop® strain.
Mean efficacy (combined data from both analysed depths) of P. gigantea was significantly higher in variants after Heterobasidion natural basidiospore infection instead to artificial conidiospore infection (p<0.05).
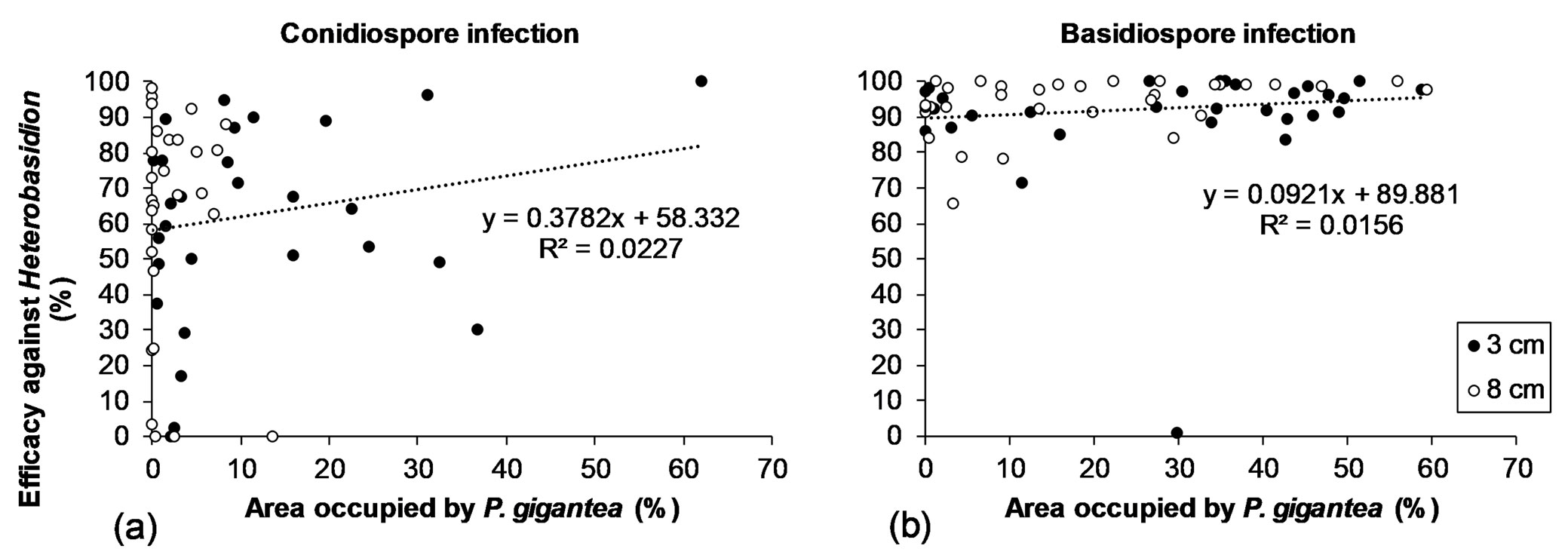
Relationship between the area occupied by P. gigantea and efficacy against Heterobasidion (combined data of both analysed depths: 3 and 8 cm) was not significant (p>0.05) in either conidiospore or in basidiospore infection (Fig. 1a, Fig. 1b).
Fig. 1 - Relationship between area occupied by P. gigantea and efficacy against conidiospore (a) and basidiospore (b) infection of Heterobasidion in billets at a depth of 3 and 8 cm.
Discussion
In this study, the efficacy of three Latvian P. gigantea strains and Rotstop® (commercial preparation of P. gigantea) against Heterobasidion infections caused naturally by basidiospores and artificially by conidiospores was evaluated in Norway spruce billets. In several studies analyzing the efficacy of chemical and biological control agents against Heterobasidion infection, conifer stumps and logs were treated with conidio- or basidiospores of the pathogen or left for airborne Heterobasidion inoculation ([22], [14], [1], [21], [3], [32], [41], [18], [13], [24], [8]). To our knowledge, our experiment is the first study where treatment efficacy of a P. gigantea isolate against Heterobasidion conidiospores (artificial infection) and basidiospore (natural) infection was characterized in a maximally standardized substrate such as spruce billets from the same trees. Four weeks after treatment, the area occupied by P. gigantea strains was larger in billets exposed to natural infection of Heterobasidion basidiospores than in billets artificially inoculated with Heterobasidion conidiospores. Because our experiment only lasted four weeks, it is possible that in the longer term the relationship regarding the areas occupied by P. gigantea and Heterobasidion may change.
As shown in earlier studies, number of both P. gigantea and Heterobasidion spores may be critical to the infection success and the following wood occupation ([15], [3]). In our study, Heterobasidion occupied a larger area on average after conidiospore infection in sectors treated with P. gigantea than after natural basidiospore infection. The advantages of artificial inoculation with Heterobasidion have been indicated in several studies ([38], [2], [41]). The advantage of artificial infection compared to natural spore infection was also demonstrated for P. gigantea in experiments with Pinus resinosa logs ([33]). In the previously mentioned study, two months after artificial infection with P. gigantea, the mycelium was found at a depth of 5 cm but at the depth of only one centimeter after natural infection. In our experiment, the billet surface was completely covered by P. gigantea suspension. Also the amount of spores in the treatment suspension was sufficiently high (ca. 5000 spores mL-1) to effectively prevent Heterobasidion infections in spruce wood ([14]). For natural Heterobasidion basidiospore infection, the billets were placed close to fruit bodies to ensure sufficient spore load. The vast majority of the released basidiospores are deposited within a distance of a few meters from the fruit body ([12], [34]). The method used in our experiment ensured a high Heterobasidion infection rate (area occupied by Heterobasidion was on average 36.3% at a depth of 3 cm) in analysed control sector of billets, whereas in similar studies in Sweden average Heterobasidion occupied area in control Picea abies stumps ranged from 0.70% to 2.12% ([2], [32]). Consequently, it is unlikely that a low density of basidiospores or conidiospores (i.e., 500 spores mL-1) had limited Heterobasidion infection in the present study. Thus, taking in account the area occupied by Heterobasidion in control sector, better efficiency of P. gigantea treatment against natural basidiospore than conidiospore infections may not be due to inadequate level of basidiospore inoculum. On the other hand, very high natural infection rate of Heterobasidion spores ([2], [3], [13]) as well as season of application ([8]) can negatively affect the efficacy of biological control agents.
Norway spruce can be infected by both H. parviporum and H. annosum. However, H. parviporum is better adapted for spruce wood ([43], [23]). As shown in Fig. 1, a greater variance of P. gigantea efficacy was unexpectedly observed after artificial treatment with Heterobasidion conidiospores, even though Heterobasidion basidiospore development may be more affected by other wood colonizing fungi in comparison to conidiospore development ([41], [23]). Moreover, physical conditions, especially moisture, may have a greater impact on natural Heterobasidion basidiospore infection in the upper layers of stumps ([28], [29]). In our experiment, conidiospore suspension contained both H. annosum and H. parviporum strains. The main reason for the high variance might probably be interspecies competition between different Heterobasidion genotypes ([30]).
For the natural basidiospore infection the billets were exposed near H. parviporum fruit bodies (unpublished data). Therefore, the majority of basidiospores causing natural infection were probably derived from H. parviporum. Inoculation experiments carried out by Gunulf et al. ([9]) showed the competitive advantage of H. parviporum over H. annosum: H. parviporum totally replaced H. annosum in Norway spruce billets inoculated with a mixture of homokaryotic conidiospores of both species. Moreover, as also demonstrated in a study by Gunulf et al. ([9]), H. parviporum grows successfully deeper in spruce wood compared to H. annosum. Dominance of H. parviporum may partly explain the result of our study indicating that infections caused by basidiospores were larger in area in the lower part of the control sector in billets.
In our experiment, we used billets cut from three individual trees instead of stumps in order to decrease variability due to differences between wood characteristics and of the individual trees. Despite maximizing the homogeneity of wood material, the variation in fungal colonization was high. The area occupied by Heterobasidion varied greatly both after conidiospore (4%-81%) and natural basidiospore (3%-71%) infections. A similar range in area occupied by Heterobasidion in Sitka spruce stumps after infection with Heterobasidion basidiospores (0.02%-56.6%), was found in a study by Morrison & Redfern ([20]). In our study with spruce billets, the area occupied by Rotstop® was on average 22.8%. Whereas, a study in Sweden showed that area occupied by P. gigantea after treatment with Rotstop® in spruce stumps at depths of 2-12 cm was 5.9% ([2]). Stumps can remain alive at least for 10 years after cutting if they have root contact with neighbouring trees ([29]). Unlike Heterobasidion, P. gigantea colonizes only deadwood, thus growth of P. gigantea is more likely to be inhibited in stumps than in billets ([42] and literature therein, [41]). Thus, our results indicate that P. gigantea grows faster and may be more efficient against Heterobasidion infections in Norway spruce billets than in Norway spruce stumps.
In several studies P. gigantea efficacy is related to its occupied area ([14], [2], [41]). An earlier Latvian study indicated that when the area occupied by P. gigantea exceeds 10% of stump surface area, occurrence of Heterobasidion is significantly decreased ([13]). Interestingly, in the present study, the efficacy of P. gigantea against conidiospore infection has been demonstrated even when P. gigantea occupied a relatively small area or when there was no discoloration in wood. Similar data was obtained in research carried out in Finland ([36]). It is possible that P. gigantea mycelium can be present in the wood and affect growth of Heterobasidion, but wood discoloration by P. gigantea appears only after longer period of incubation (K. Korhonen personal communication). This is supported by the results obtained by Oliva et al. ([24]). By quantifying the biomass of H. annosum and P. gigantea in Norway spruce stumps, they reported that visual assessment after incubation may be a poor measure of presence or absence of both fungi. In Rotstop® treated stumps, no differences in biomass of P. gigantea could be found between areas with visual presence or absence of P. gigantea after incubation. For Heterobasidion, a significant difference in Heterobasidion biomass between areas with or without growth of Heterobasidion after incubation was found in stumps artificially inoculated with conidia suspension but not in naturally infected stumps ([24]).
In several studies, the efficacy of the biological control agent Rotstop® varies from 50 to 100% ([14], [2], [3], [21], [32], [4], [13]). In our study, the average efficacy of Rotstop® was 55% to 96 %. The average area occupied by the three Latvian P. gigantea strains after treatment with Heterobasidion conidiospores was smaller than after treatment with Rotstop®. However, indigenous P. gigantea strains showed slightly higher occupation areas at a depth of 8 cm after Heterobasidion basidiospore infection. Efficacy of the local P. gigantea strain G1 used in our experiment has also been demonstrated in previous studies ([13]). To limit the spread of Heterobasidion in the long term (via secondary infection) at a stand level, it is critical to restrict the growth of Heterobasidion mycelium deeper in the wood ([15], [25], [2]). Therefore, the obtained results demonstrate the potential of local P. gigantea strains to limit Heterobasidion infection in long term. Although in our experiment with Heterobasidion conidiospores local P. gigantea strains showed lower efficacy than Rotstop®, with high ambient Heterobasidion natural basidiospore level, efficacy of the same P. gigantea strains was high.
Conclusions
Efficacy of P. gigantea treatment in a representative site was lower in Norway spruce billets after inoculation with conidiospores from four Heterobasidion strains belonging to two species than after natural infection through Heterobasidion basidiospores. Therefore, spruce billets artificially inoculated with conidiospore suspension provide a reliable method for screening effective P. gigantea strains for controlling Heterobasidion spore infections on Norway spruce stumps. The commercial biological control agent Rotstop® was more effective against Heterobasidion conidiospore and natural basidiospore infection in spruce wood compared to the local P. gigantea strains used in the study. However, local P. gigantea strains have the potential to effectively limit advance of Heterobasidion infection deeper in the wood, thereby decreasing vegetative spread of Heterobasidion.
Acknowledgements
The authors thank Kari Korhonen for valuable comments, and Dainis Edgars Rungis for language revision. The authors gratefully thank to three anonymous referees for their suggestions to improve manuscript quality.
Study was financially supported by Joint stock company “Latvia’s State Forests” project No. 5-5.5_0004_101_16_4 “Investigation of the factors limiting the spread of root rot”, Latvian Council of Science fundamental and applied research project No. lzp-2018/1-0431 “Investigations on the role of Phlebiopsis gigantea in restricting vegetative spread of Heterobasidion spp. in stumps of Norway spruce and Scots pine”, and in accordance with the contract No. 1.2.1.1/18/A/004 between Forest Sector Competence Centre of Latvia Ltd. and the Central Finance and Contracting Agency, the study “Development of biological preparation for reducing root rot caused losses in conifer stands” is conducted by LSFRI Silava with support from the ERDF within the framework of the project “Forest Sector Competence Centre of Latvia”.
References
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Online | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Darta Klavina 0000-0002-1455-9062
Astra Zaluma 0000-0003-2980-4435
Kristine Kenigsvalde 0000-0002-2761-2651
Natalija Burneviča
Talis Gaitnieks 0000-0003-1951-4426
Latvian State Forest Research Institute Silava, Riga street 111, Salaspils, LV-2169 (Latvia)
University of Latvia, Raina boulevard 19, Riga, LV-1050 (Latvia)
Natural Resources Institute Finland LUKE, Latokartanonkaari 9, 00790 Helsinki (Finland)
Corresponding author
Paper Info
Citation
Bruna L, Klavina D, Zaluma A, Kenigsvalde K, Burneviča N, Nikolajeva V, Gaitnieks T, Piri T (2020). Efficacy of Phlebiopsis gigantea against Heterobasidion conidiospore and basidiospore infection in spruce wood. iForest 13: 369-375. - doi: 10.3832/ifor3279-013
Academic Editor
Alberto Santini
Paper history
Received: Nov 01, 2019
Accepted: Jun 17, 2020
First online: Aug 25, 2020
Publication Date: Oct 31, 2020
Publication Time: 2.30 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2020
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 39180
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 33412
Abstract Page Views: 3107
PDF Downloads: 2004
Citation/Reference Downloads: 5
XML Downloads: 652
Web Metrics
Days since publication: 2016
Overall contacts: 39180
Avg. contacts per week: 136.04
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2020): 6
Average cites per year: 1.00
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Effects of substrate and ectomycorrhizal inoculation on the development of two-years-old container-grown Norway spruce (Picea abies Karst.) seedlings
vol. 8, pp. 487-496 (online: 10 November 2014)
Research Articles
Dynamics of soil organic carbon (SOC) content in stands of Norway spruce (Picea abies) in central Europe
vol. 11, pp. 734-742 (online: 06 November 2018)
Short Communications
Culturable fungi associated with wood decay of Picea abies in subalpine forest soils: a field-mesocosm case study
vol. 11, pp. 781-785 (online: 28 November 2018)
Research Articles
Short- and long-term natural regeneration after windthrow disturbances in Norway spruce forests in Bulgaria
vol. 11, pp. 675-684 (online: 23 October 2018)
Research Articles
Assessment of presence and distribution of Armillaria and Heterobasidion root rot fungi in the forest of Vallombrosa (Apennines Mountains, Italy) after severe windstorm damage
vol. 12, pp. 118-124 (online: 11 February 2019)
Review Papers
Dutch elm disease and elm bark beetles: a century of association
vol. 8, pp. 126-134 (online: 07 August 2014)
Research Articles
A rapid method for estimating the median diameter of the stem profile of Norway spruce (Picea abies Karst) trees
vol. 10, pp. 328-333 (online: 11 February 2017)
Research Articles
The effect of soil conditions on submountain site suitability for Norway spruce (Picea abies Karst.) in Central Europe
vol. 16, pp. 210-217 (online: 31 July 2023)
Research Articles
Concordance between vascular plant and macrofungal community composition in broadleaf deciduous forests in central Italy
vol. 8, pp. 279-286 (online: 22 August 2014)
Research Articles
Ozone fumigation effects on the morphology and biomass of Norway spruce (Picea abies L.) saplings
vol. 2, pp. 15-18 (online: 21 January 2009)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword