Ozone fumigation effects on the morphology and biomass of Norway spruce (Picea abies L.) saplings
iForest - Biogeosciences and Forestry, Volume 2, Issue 1, Pages 15-18 (2009)
doi: https://doi.org/10.3832/ifor0483-002
Published: Jan 21, 2009 - Copyright © 2009 SISEF
Research Articles
Collection/Special Issue: Cost Action E29 Meeting 2008 - Istanbul (Turkey)
Future Monitoring and Research Needs for Forest Ecosystems
Guest Editors: Marcus Schaub (WSL, Birmensdorf, CH)
Abstract
The study examined Norway spruce (Picea abies) saplings morphological and biomass sensitivity to ozone fumigation using closed indoor chambers of controlled environment. 4-year-old potted saplings were exposed to three different ozone concentrations: 80 µg/m3, 160 µg/m3, and 240 µg/m3 (7 h/day, 5 days/week). Half of the saplings were harvested after the exposure, and the second half of the saplings were left in the pots in open field until next autumn. The reaction of the saplings of different timing of bud burst was also investigated. The terminal shoot length and the total current year shoot length of Norway spruce saplings after one month of ozone fumigation were significantly (p<0.05) suppressed in all the treatments comparing to the control saplings. The needles of saplings fumigated with ozone were smaller than the needles of control saplings. No significant changes of the biomass of different fractions of Norway spruce saplings were determined right after the fumigation, as well as, in 14 month after the cessation of the fumigation. Norway spruce saplings of early and late timing of bud burst reacted differently to ozone fumigation during the active growth period. The formation of new needles and shoots of the saplings of late bud burst stage was more suppressed comparing to the saplings of the early bud burst stage. The results suggest that the generatively younger organs during their formation are more susceptible to ozone stress. The differences of the needle age between ozone affected and control saplings decreased in one year after the end of fumigation keeping affected Norway spruce saplings in the open field and thus indicating the start of the recovery process.
Keywords
Ozone fumigation, Norway spruce saplings, Shoot morphology, Biomass
Introduction
In Lithuania the upward trend of 0.93 μg/m3 ozone concentration per year was established during 1981-1999 ([10]). This reflects the increase of ozone concentration in the global background average ([7]). The 1-h ozone concentration in forested areas now varies between 50-80 µg/m3, and in 2006 the highest 1-h ozone concentration in Lithuania was 181.3 µg/m3 ([1]). Even though the critical levels of ozone are not frequently exceeded in Eastern Europe, it is expected that transboundary pollution as well as the local pollution sources may lead to the rapid increase of ozone concentration in this region ([19]).
The results of many different experiments show that the high ambient ozone concentrations induce oxidative stress in plants. First of all the photosynthetic apparatus of plants is being affected. Ozone exposure causes injuries of cell membranes and plasma, disturbs the metabolism, subsequently carbon allocation alters. This in turn has negative effect on the plant growth. Although there are data showing that ozone induced negative changes of tree condition can be permanent ([15]), the experiments on the persistent effects of ozone fumigation are scarce ([8]).
Plant species, populations and individual plants within species vary greatly in their ozone tolerance due to the genetic and phenologic differences ([17], [12], [11]). Norway spruce trees even from the same population are not homogeneous in timing of bud burst. Trees of different bud burst stages are also known to have different sensitive to the environmental stress factors, e.g., frosts ([5]), and are characterized also by different annual increment ([9]). However, it is not known how the trees with different timing of bud burst react to ozone.
Norway spruce (Picea abies L.) is one of the main tree species in Lithuania that compose 22.4% of the forested area in the country ([14]). The ratio of young trees of early, intermediate, and late timing of bud burst in the forests is 1:2:1 ([9]). Even though ozone effects in Norway spruce have been studied using diverse experimental settings or material sampled in the field for nearly 40 years intensively, the importance to study the responses of natively growing species with realistic settings for a particular region remains. There are evidences that trees of the same species growing in different geographical regions react differently to ozone stress ([21], [4]).
The objective of the study was to estimate the short-term ozone fumigation effects on the Norway spruce needle age, shoot growth, and biomass under controlled environmental conditions and also to investigate how persistent can be ozone induced injuries. The aspect of the different timing of bud burst of Norway spruce was also analyzed in this study.
Materials and methods
Eighty Norway spruce (Picea Abies L.) 4-year-old experimental saplings were obtained from the nursery, situated in the middle of Lithuania (Vaišvydava, Kaunas district). The saplings from this nursery are used for reforestation. The saplings were replanted in the 5 L pots (one sapling per pot) of a peat substrate (Klasman KTS-1, pH 5.5-6.0, N - 140 mg/l, P2O5 - 160 mg/l, K2O - 18 mg/l, Mg - 85 mg/l). No additional fertilizers were added during the study. The saplings were watered when necessary throughout the experiment.
The potted saplings were kept in the open field for 1.5 month until the start of the experiment. The active growth period started before the fumigation experiment, and saplings of different timing of bud burst became apparent. The Norway spruce saplings of early, intermediate, and late bud burst stages were distinguished and distributed among the treatments so that there were 6-8 saplings of all three bud burst stages in every treatment. The buds of the saplings of early timing of bud burst were already developed when the fumigation was started. It is known that the difference between the early and late bud burst stages varies from 1 to 3 weeks ([23]).
The fumigation was carried out beginning June 1 through June 28, 2005 using four closed walk-in indoor chambers of controlled environment (40 m3 each) located in the Lithuanian Institute of Horticulture. The photoperiod in all the chambers was ~16 h (from 6:00 to 22:00), air temperature during light period was 21oC and 17oC during dark period, and air humidity was 75%. The light was provided by Son-T-Agro (Philips) lamps. The potted saplings were exposed to ozone concentrations of 0 µg/m3 (control), 80 µg/m3, 160 µg/m3, and 240 µg/m3 for 7 h/day, 5 days/week. The ozone concentrations were generated using ozone generator OSR-8 and the concentrations were measured using Portable Ozone Monitor OMC-1108 (Ozone Solutions, Inc.). The AOT40 in the 160 µg/m3 ozone treatment reached 12460 μg/m3 · h and in the 240 µg/m3 treatment 27200 μg/m3 · h by the end of fumigation.
There were 20 saplings per treatment and control. One half of the saplings were harvested after the exposure. The second half of the saplings were transferred from indoors to outdoors settings to the nursery (54°50’ N, 24°03’ E, 75 m a.s.l.) and left in the pots under open field conditions until September 2006.
During March 2005 - September 2006 no extreme climatic conditions were observed in the vicinity of the nursery. The average monthly temperature varied from -7.0ºC to 20.6ºC with the differences from the 30 year average varying from 0.1 ºC to 3.9 ºC. The amount of precipitation during the same period was 20.0-143.9 mm. In 2006 (April-September) the reported AOT40 in Lithuania reached 21555 μg/m3 · h ([1]).
The upper shoot length was measured before and after the fumigation. The dry biomass of foliage, shoot, total above-ground part and root fractions as well as the total current year shoot length was determined after the fumigation and also in September 2006, 14 month after the fumigation. The foliage and shoot fractions were separated into current year and older (current+n) needles and shoots. The dry biomass of 100 randomly selected current year needles was determined right after the fumigation, as this parameter allows getting the information on the average needle size. The needle age was evaluated in spring 2006, 10 month after the fumigation, before the active growth period started, and in autumn 2006, 14 month after the fumigation. All the assessments were based on the EU/ICP Forests methods ([20]), where applicable.
The differences between mean parameters of control saplings and ozone-exposed saplings were tested using ANOVA and t-tests. All the differences are reported here as statistically significant at the level p<0.05.
Results and discussion
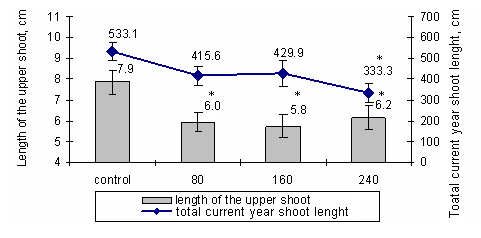
After the fumigation by different ozone concentrations it was detected that the terminal shoot length and the total current year shoot length of Norway spruce saplings were significantly (p<0.05) shorter compared to the control saplings (Fig. 1).
Fig. 1 - The length of the terminal shoot and the total current shoot length of Norway spruce saplings for one month fumigated with 80 µg/m3, 160 µg/m3, and 240 µg/m3 ozone concentrations (July, 2005). (*): Significant differences from control (p< 0.05).
The negative effect of high ozone concentrations on the morphology of Norway spruce saplings was also manifested on the needle age. The decreased needle age of the ozone affected saplings was apparent in 10 months after the fumigation, even though the saplings were kept for one vegetation period in open field conditions (Tab. 1). These differences also remained in September 2006, after one more active growth period. However, the start of the recovery process of the overall condition of affected saplings was noticed as the differences of the needle age between ozone affected and control saplings decreased (Tab. 1).
Tab. 1 - The changes of the needle age of Norway spruce saplings fumigated for one month with 80 µg/m3, 160 µg/m3, and 240 µg/m3 ozone concentrations after 10 month (spring 2006) and 14 month (autumn 2006) after the fumigation. (*): Significant differences from control saplings (p< 0.05).
| Parameter | Treatment (ozone concentration, μg/m3) | |||
|---|---|---|---|---|
| 0 (control) |
80 | 160 | 240 | |
| Needle age (spring, 2006), years | 3.55 ± 0.10 | 3.50 ± 0.13 | 2.58 ± 0.19 * | 2.33 ± 0.14 * |
| The difference from control, % | - | 3 | 28 | 36 |
| Needle age (autumn 2006), years | 3.15 ± 0.10 | 3.14 ± 0.09 | 3.00 ± 0.17 | 2.73 ± 0.12 * |
| The difference from control, % | - | 3 | 6 | 16 |
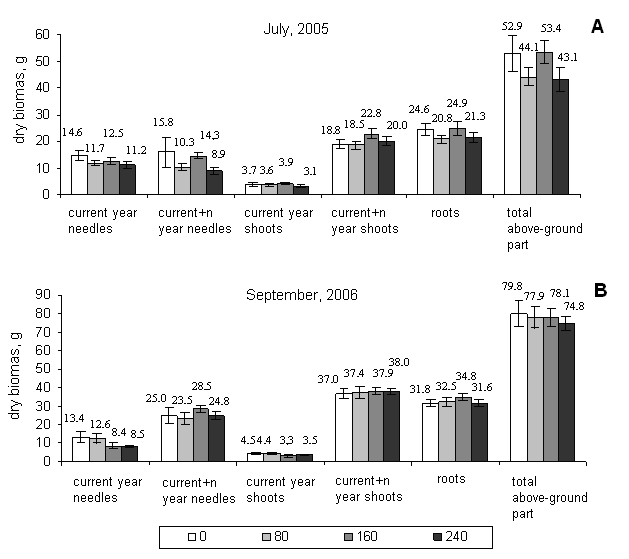
No significant changes of the biomass of different fractions of Norway spruce saplings were determined right after the fumigation, as well as, in 14 month after the end of fumigation (Fig. 2). However, a tendency of decreasing biomass with increasing ozone concentration was observed in the fractions of current year needles and shoots and total above ground part. This is partly in agreement with results of Landolt et al. ([13]), who showed that biomass of Norway spruce saplings in the open top chambers (OTC) was decreasing with increasing ozone exposure. Skre & Mortensen ([18]) found that ozone fumigation with 160 µg/m3 and 320 µg/m3 concentrations significantly decreased all the growth parameters of 3-year-old Norway spruce saplings, and the treatment with 80 µg/m3 concentration stimulated shoots growth. In our case the concentration of 160 µg/m3 tended to act as a stimulator. The biomass of all the fractions of affected saplings was equal to the biomass of control saplings or even greater (Fig. 2). However, Skre & Mortensen ([18]) were fumigating the saplings for longer period (10 h/day, 2 month). Other authors ([22]) found only a slight decrease in current year shoot growth and slight increase in the second year needle loss.
Fig. 2 - The biomasses of different fractions of Norway spruce saplings fumigated with 80 µg/m3, 160 µg/m3, and 240 µg/m3 ozone concentrations for one month: (A) right after the fumigation (July 2005); (B) 14 month after the fumigation (September 2006). (*): Significant differences from control (p< 0.05)
In our study the biomass of 100 current year needles was more susceptible indicator of ozone effect than the biomass of other fractions. Right after the fumigation this parameter in all the ozone treatments was significantly lower compared to the control (Tab. 2). This suggests that even though the biomass was not significantly affected, but the saplings were weakened by ozone and were not able to produce needles of usual size.
Tab. 2 - The biomasses of 100 needles of Norway spruce saplings for one month fumigated with 80 µg/m3, 160 µg/m3, and 240 µg/m3 ozone concentrations (July 2005). (*): significant differences from control saplings (p< 0.05).
| Parameter | Treatment (ozone concentration, μg/m3) | |||
|---|---|---|---|---|
| 0 (control) | 80 | 160 | 240 | |
| The biomass of 100 needles, g | 0.081 ± 0.003 | 0.070 ± 0.002* | 0.066 ± 0.003 * | 0.071 ± 0.003* |
The evidences of negative impact of ozone on the plants are often contradictories, there is no accepted uniform species ranking according to their sensitivity ([2], [6]). Norway spruce is included in the “List of European Ozone sensitive Species” ([3]), however, there are many factors that influence the variation in susceptibility of the individuals of the same species and even population ([17], [16]). The timing of bud burst is also a factor that should be taken into account.
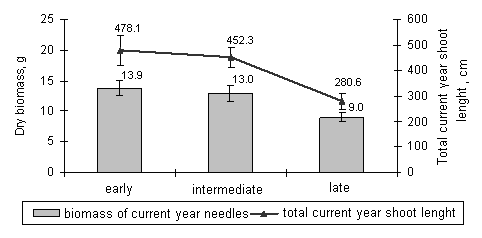
Our results showed that the saplings of early, intermediate, and late timing of bud burst reacted differently to the ozone fumigation. The formation of new needles and shoots of Norway spruce saplings of late bud burst stage was more suppressed during active growth period compared to the saplings of early bud burst stage. The statistically significant difference (p<0.05) between the length of the terminal shoot of saplings of early bud burst stage (7.88 ± 2.85 cm) and the same parameter of the saplings of late stage (4.65 ± 1.81 cm) was detected right after the fumigation. The saplings of the intermediate bud burst stage tended to react more as the saplings of the early stage: the terminal shoot length was 6.76 ± 1.88 cm. Similar patterns were determined in the case of total current year shoot length and in the case of biomass of current year needles (Fig. 3). These results suggest that the generatively younger organs, such as current year needles and shoots during their formation are more susceptible to ozone stress, than the current year needles and shoots that are more progressed in their formation. Therefore Norway spruce trees of late timing of bud burst are potentially more susceptible to the spring episodes of high ozone concentrations.
Fig. 3 - The biomasses of current year needles and the total current shoot length of Norway spruce saplings of early, intermediate, and late timing of bud burst for one month fumigated with 80 µg/m3, 160 µg/m3, and 240 µg/m3 ozone concentrations (July 2005).
Conclusions
The generatively younger organs of Norway spruce saplings, such as current year needles and shoots, are more susceptible to ozone stress, than the older organs.
The timing of bud burst is also a factor that should be taken into account while performing experimental studies or doing monitoring of ozone injuries in Norway spruce. The current year shoots and needles of the saplings of early timing of bud burst were more resistant to ozone fumigation during the active growth period than the same organs of the saplings of late timing of bud burst.
The changes in needle age indicated the start of the recovery process of affected saplings in one year after the end of fumigation keeping the affected saplings in the open field.
In order to get more general view on the plants sensitivity to ozone it is important to choose a set of response parameters for the assessment of ozone effects on vegetation.
The parameters of biomass of current 100 needles and needle age of Norway spruce are useful and relatively cheap indicators for bioindication purposes.
Acknowledgements
This work was supported by the Lithuanian State Science and Studies Foundation under the umbrella of the state project APLIKOM.
References
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Online | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
V Stakenas
Lithuanian Forest Research Institute, Ecology Department, Girionys, LT-53101 Kaunas distr. (Lithuania)
Corresponding author
Paper Info
Citation
Serafinaviciute B, Stakenas V (2009). Ozone fumigation effects on the morphology and biomass of Norway spruce (Picea abies L.) saplings. iForest 2: 15-18. - doi: 10.3832/ifor0483-002
Paper history
Received: Mar 13, 2008
Accepted: Dec 09, 2008
First online: Jan 21, 2009
Publication Date: Jan 21, 2009
Publication Time: 1.43 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2009
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 49460
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 40540
Abstract Page Views: 3593
PDF Downloads: 4255
Citation/Reference Downloads: 44
XML Downloads: 1028
Web Metrics
Days since publication: 6238
Overall contacts: 49460
Avg. contacts per week: 55.50
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2009): 2
Average cites per year: 0.12
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Effects of abiotic stress on gene transcription in European beech: ozone affects ethylene biosynthesis in saplings of Fagus sylvatica L.
vol. 2, pp. 114-118 (online: 10 June 2009)
Research Articles
A new approach to ozone plant fumigation: The Web-O3-Fumigation. Isoprene response to a gradient of ozone stress in leaves of Quercus pubescens
vol. 1, pp. 22-26 (online: 28 February 2008)
Research Articles
Prediction of ozone effects on net ecosystem production of Norway spruce forest
vol. 11, pp. 743-750 (online: 15 November 2018)
Research Articles
Changes in the proteome of juvenile European beech following three years exposure to free-air elevated ozone
vol. 4, pp. 69-76 (online: 05 April 2011)
Research Articles
A comparison between stomatal ozone uptake and AOT40 of deciduous trees in Japan
vol. 4, pp. 128-135 (online: 01 June 2011)
Research Articles
Soil drench of ethylenediurea (EDU) protects sensitive trees from ozone injury
vol. 4, pp. 66-68 (online: 05 April 2011)
Short Communications
Is microbial biomass measurement by the chloroform fumigation extraction method biased by experimental addition of N and P?
vol. 14, pp. 408-412 (online: 04 September 2021)
Research Articles
Ambient ozone phytotoxic potential over the Czech forests as assessed by AOT40
vol. 5, pp. 153-162 (online: 25 June 2012)
Short Communications
Ozone flux modelling for risk assessment: status and research needs
vol. 2, pp. 34-37 (online: 21 January 2009)
Research Articles
Long-term monitoring of air pollution effects on selected forest ecosystems in the Bucegi-Piatra Craiului and Retezat Mountains, southern Carpathians (Romania)
vol. 4, pp. 49-60 (online: 05 April 2011)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword