Assessment of presence and distribution of Armillaria and Heterobasidion root rot fungi in the forest of Vallombrosa (Apennines Mountains, Italy) after severe windstorm damage
iForest - Biogeosciences and Forestry, Volume 12, Issue 1, Pages 118-124 (2019)
doi: https://doi.org/10.3832/ifor2929-012
Published: Feb 11, 2019 - Copyright © 2019 SISEF
Research Articles
Abstract
One of the main problems for the management and conservation of silver fir stands has long been pathogens causing root rot, in particular Armillaria spp. and Heterobasidion annosum s.l. These opportunistic pathogens are especially threatening now that climate change related stress is increasing tree susceptibility to disease and vulnerability to windstorms. The northern Apennines Mountains (central Italy) are forecast to be one of the areas with the highest temperature increase in the next future. However, no systematic assessment exists of the risk posed by the disturbance due to secondary pathogens in the Apennine forests. In the Nature Reserve of Vallombrosa (northern Apennines), where silver fir forests have been managed and conserved for centuries since the Middle Ages, making it an ideal site for studying these parasites, the high presence of H. annosum was reported already in 1990, while only sporadic observations are available on Armillaria species. The aim of this work was to examine the occurrence of both pathogens, since detailed knowledge about their distribution may assist forest management planning and decision-making. Systematic sampling was undertaken at the intersection of 52 grid points covering the whole forest. Different fungal species from soil and fungal samples (fruiting bodies or rhizomorphs) were identified by combining morphological descriptions with molecular methods. The analyses confirmed the presence of H. abietinum in about 70% of the investigated points. The fungus was detected at two new localities above 1000 metres suggesting a possible expansion of the parasite at upward elevation, which might be associated with climate change. Armillaria was widespread: almost 90% of the samples resulted positive, and four different Armillaria species were successfully identified. The most frequent species were A. cepistipes, whose rhizomorphs were especially abundant, and A. ostoyae, which was often detected just in soil samples. At sites where A. cepistipes was found to coexist with A. gallica, these two species might specialize themselves to necrotrophic and saprotrophic lifestyle, respectively. Besides, there were unexpected findings of A. mellea, supposed to be a residual from the previous rotation of broadleaves.
Keywords
Butt Rot, Climate Change Disturbances, Heterobasidion annosum, Root Rot, Silver Fir, Windstorm Damage
Introduction
Heterobasidion annosum s.l. (Fr.) Bref. and Armillaria species are among the most destructive forest pathogens in many parts of the world. As facultative necrotrophs, these parasitic fungi are able to survive saprotrophically on dead wood, and the same individual can switch from one mode of lifestyle to the other ([18]). Both species complexes cause root rot and decay of the stem, which typically leads to uprooting under intense mechanical stress ([22]), as a consequence of the decreased stability of the tree. Such damages, which are typical in conifer plantations, took place after the windstorm of March 5th, 2015, which destroyed about 50 ha of forest at the Nature Reserve of Vallombrosa (central Italy). This event gave actuality to a complex monitoring in order to shed light on the key factors determining the susceptibility of the forest to extensive windthrows ([7]).
H. annosum s.l. had long been regarded as a single species until mating tests revealed the occurrence of intersterility groups (ISGs - [27]), all of which have later obtained formal description as species. The Eurasian groups were named H. annosum s.s., H. abietinum Niemelä & Korhonen and H. parviporum Niemelä & Korhonen ([38]). H. annosum s.s. is mostly associated with pines, especially Scots pine (Pinus sylvestris L.), but attacks several other conifers and even some broadleaved tree species. H. parviporum shows a relatively strict specialization for Norway spruce (Picea abies Karst.), while H. abietinum is commonly associated with European silver fir (Abies alba Mill.) and other species of the genus Abies ([15]). Root and butt rot caused by Heterobasidion is widespread in coniferous forests of Italy. Along the Apennines, the disease incidence is particularly high in silver fir plantations that are older than 50-60 years ([5]). High economic losses associated with the pathogen have been reported from this area for decades, along with the dieback of fir stands ([4], [35]). Typically, the epidemic begins right after the first thinning, because the remaining live stumps are a favourable substrate for the vegetative spread of the parasite ([6]). Farina et al. ([10]) reported the massive presence of H. abietinum in the forest of Vallombrosa, performing a systematic sampling of the whole area. Their findings imply that root rot is a chronic disease in fir stands, and likely among the most important factors of the species’ decline and high vulnerability to windstorms.
The genus Armillaria is distributed in all continents. In Europe, seven biological species have been distinguished based on sexual incompatibility ([26], [19]). Their geographical distribution has been detailed by Guillaumin et al. ([19]). A. mellea (Vahl:Fr.) Kummer is distributed in the Atlantic and Mediterranean parts of Europe, in various deciduous forests ([45]). A. ostoyae (Romagn.) Herink is less thermophilic and fundamentally linked to conifers ([45], [18]). A. borealis Marxm. & Korhonen mainly populates coniferous forests of Northern Europe ([18]), but it is also common in highlands of Central Europe ([25]). A. gallica Marxm. & Romagn. is regarded as a low-elevation species typical of floodplain forests, while A. cepistipes Velen. occurs most frequently in the zone of European beech (Fagus sylvatica L. - [45], [25], [2]). A. socialis (DC.: Fr.) Fayod, frequently mentioned as A. tabescens in the literature, is a thermophilic species of Southern Europe ([1]). A. ectypa (Fr.) Lamoure is confined to wetlands and extremely rare ([48]). Identification of the various Armillaria spp. present on a site can be of great practical importance because the virulence and host range markedly differ among species. The most pathogenic European species seem to be A. ostoyae and A. mellea ([18]). According to the scheme of Manion ([34]), Armillaria spp. appear sometimes as a predisposing factor, through numerous early infections of the root systems, and sometimes as a contributing factor giving the deathblow to weakened trees, as in fir stands of Vallombrosa ([23]).
Under current and projected changes in climatic conditions, Armillaria species are expected to increase their activity and rhizomorphogenic capacity, and more easily defeat tree defence ([20], [29], [21]). Tree vulnerability to Heterobasidion species is also expected to rise in a warmer climate because of increased fungal growth and sporulation rate ([30], [16], [37]). More generally, climate change might exacerbate drought stress, increasing tree susceptibility to secondary pathogens ([12]), including Heterobasidion and Armillaria species. Considering that increased windstorm damages in the forest of Vallombrosa ([7]), as in European forests in general ([17]), may be exacerbated by higher occurrence and/ or virulence of root rot pathogens, the goal of present work was to provide distribution maps of Armillaria and Heterobasidion species in the area to support forest managers in planning and decision-making. The several DNA-based diagnostic techniques, which were developed since the past studies on root rots in Vallombrosa, allowed for a more straightforward identification of wood rotting fungi in this work.
Material and methods
Data acquisition
The study area is the forest of Vallombrosa (43° 44′ N, 11° 34′ E), a biogenetic reserve located about 50 km east-southeast of Florence, Tuscany, Italy. The forest covers 1273 ha in the northern Apennines Mountains. The climate is characterized by a mean annual air temperature of 9.8 °C and a mean annual precipitation of 1275 mm (thermopluviometric station of Vallombrosa, 980 m a.s.l.). Western and northwestern slopes dominate, with an average inclination of 18.8%. The altitude ranges between 470 and 1440 m a.s.l. The soil units belong to Inceptisols and Alfisols ([46]), formed on Oligocene sandstone. Fragipan soil layers are present in 30% of the area, close to the surface ([3]). Silver fir has been cultivated since the 11th century and occupies more than half of the area today. The native vegetation is mainly represented by beech at higher elevations, oak-hornbeam stands (Quercus spp. mixed with Carpinus betulus L. and Ostrya carpinifolia Scop.) and chestnut (Castanea sativa Mill.) at lower altitudes. Another important change is the introduction of European black pine (Pinus nigra Arn. ssp. nigra and Pinus nigra Arn. ssp. laricio), and to a lesser extent, coast Douglas-fir (Pseudotsuga menziesii [Mirb.] Franco var. menziesii) and Norway spruce.
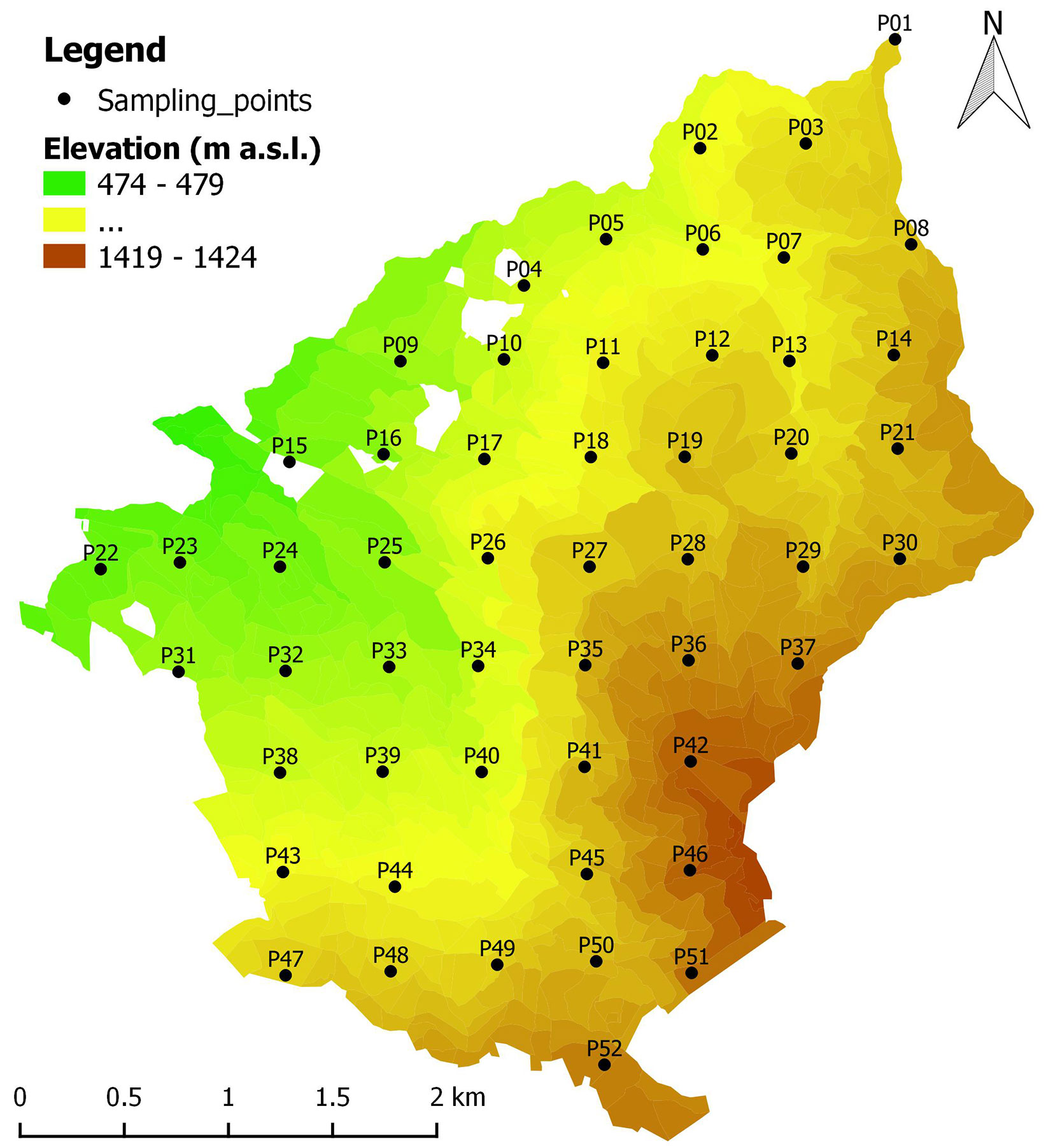
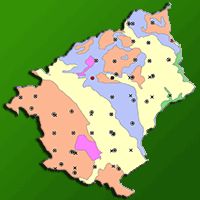
Field surveys were carried out in July 2015. Since summer 2015 had been especially hot and dry, conditions that could restrict the formation of Heterobasidion fruiting bodies ([39]), the survey was repeated in May 2017 for Heterobasidion. The area, where Abies alba dominated more than half of the stands (57.7%), ahead of Pinus nigra (21.2%) and Fagus sylvatica (13.5%) forests, was covered by a 500 × 500 m grid which resulted in 52 sampling points (Fig. 1, Tab. S1 in Supplementary material). The same sampling grid has been used as in the study of Farina et al. ([10]), so it was possible to compare the spatial distribution of H. annosum s.l. over the past 28 years. Points were identified in the field with a handheld GPS navigator (Garmin™ GPSMAP® 62s, containing the forest management map of the Reserve) and a topographic map.
Fig. 1 - Topographic map of Vallombrosa forest with the location of sampling points. Points were unevenly distributed among age classes, with the prevalence of middle-aged stands (Source: database of the forest management plan 2006-2025 of Vallombrosa, central Italy).
Soil sampling was done at all points, through hammering a steel cylinder of approximately 170 cm3 (8.5 cm high × 5 cm inner diameter) to the topmost layer of soil, excluding the organic horizon. Fruiting bodies of Heterobasidion and Armillaria rhizomorphs were collected in an area of about 300 m2 around the points. Samples were put into plastic bags or disposable plastic tubes and conserved at 4 °C until processed.
DNA analysis
All types of samples (Heterobasidion basidiomes, Armillaria rhizomorphs, and soil) were utilized for DNA extraction. Each soil sample was homogenized by mixing; fungal samples were homogenized by grinding the tissue in liquid nitrogen using sterile mortar and pestle. Approximately 0.25 g of samples was used. DNA was extracted using the PowerSoil™ DNA Isolation Kit (Mo-Bio, Carlsbad, USA), according to the manufacturer’s instructions. The extracted DNA solution was conserved at -25 °C.
Morphological traits of basidiomes differ between species within H. annosum s.l. as described by Mugnai & Capretti ([36]). For rapid confirmation of visual identification of H. annosum s.l., the taxon-specific competitive-priming (TSCP-)PCR method was used according to Gonthier et al. ([14]). For amplifying DNA, a mix of four primers (MLS, MLF, Mito 5 and Mito 7) was applied. PCR was performed in a 25-µl reaction mixture containing approximately 50 ng of template DNA, 0.5 µmol of each primer, 5× MyTaq® Reaction Buffer (comprising dNTPs and MgCl2 in a final concentration of 1 mM and 3 mM, respectively) and 1U MyTaq® DNA Polymerase (Bioline, London, UK). The PCR was amplified using a Mastercycler® ep Thermocycler (Eppendorf, Hamburg, Germany). The PCR programme was as follows: 3 min at 95 °C, followed by 35 cycles of 40 s at 95 °C, 20 s at 64 °C and 20 s at 72 °C with a final extension of 7 min at 72 °C. Identification of H. abietinum was confirmed by sequencing of the ITS region (Internal Transcribed Spacer) by the DNA Sequence Service of Macrogen Inc. (Seoul, Korea).
The ITS region was selectively amplified from soil and rhizomorph samples by nested PCR. The first reaction was carried out with external primers ITS1 and ITS4 used for amplification of ITS region of fungi ([49]). In the second reaction, the internal primers AR1 and AR2 for Armillaria ITS region were used ([33]). In both reactions, 1 µl of isolated DNA was used. Reaction conditions were as described for TSCR-PCR. Amplifications were carried out in a Mastercycler® ep Thermocycler (Eppendorf, Hamburg, Germany) with thermal cycling parameters: initial denaturation at 94 °C for 2.5 min, followed by 35 cycles of heat denaturation at 94 °C for 30 s, annealing at 55 °C for 40 s, extension at 72 °C for 30 s and final extension at 72 °C for 5 min for ITS-PCR; initial denaturation at 94 °C for 2.5 min, followed by 35 cycles of heat denaturation at 94 °C for 30 s, annealing at 60 °C for 40 s, extension at 72 °C for 30 s and final extension at 72 °C for 7 min for AR-PCR. In some cases, due to scarce visibility on agarose gel, it was necessary to repeat the PCR with higher volume (2 µl) of extracted DNA. Negative samples were discarded after each reaction; positive ones were further processed.
During restriction fragment length polymorphism (RFLP) analysis, DNA is cut into shorter strands by restriction enzymes that can be visualized after gel electrophoresis. Digestion of unpurified PCR products was carried out using restriction endonuclease HinfI (Fermentas, Lithuania). As shown by Lochman et al. ([32]), this enzyme is able to discriminate the six main European Armillaria species. The restriction mixtures containing 19 µl of PCR product with 1 µl of buffer R and 1 µl of the enzyme HinfI were incubated for 12 h at 37 °C ([33]).
After each reaction, PCR products were electrophoresed in agarose (Serva, Heidelberg, Germany) gel in TBE buffer at 5 V cm-1 for approximately 35 min (ITS, AR) or 80 min (TSCP, RFLP). 1% gel was used for simple identification (ITS, AR) and 2% gel for identification to the species level (TSCP, RFLP). One µl of ethidium bromide or Serva DNA Stain G was added to the gel. Six µl of each sample and control, as well as 3 µl of 100 bp DNA Ladder (New England BioLabs, Ipswich, MA, USA), to determine the fragment size, were mixed with loading dye (New England BioLabs) and loaded into the wells. In the case of RFLP-PCR products, DNA fragment sizes were estimated using ΦX174 DNA/HinfI Marker (Fermentas) as DNA molecular size marker. Amplified DNA of H. annosum s.s. (for TSCP) and A. borealis (for ITS, AR and RFLP) served as positive controls. To visualize DNA fragments, the gel was placed on an ultraviolet transilluminator and documented by digital camera (Olympus™ C8080WZ, Japan).
Data processing
The mapping of sampling points was conducted using the QGIS 3.0.0 software. Spatial data transferred from GPS was linked with the collected attributive data in the program. After georeferencing each sampling location, thematic map layers were created. The effect of environmental factors (altitude, site fertility, soil type, prevalent tree species and age of forest stand) on the presence/absence of H. annosum s.l. was tested using binomial logistic regression. The influence of the above parameters on the detection frequency of different Armillaria spp. was tested by multinomial logistic regression. Species identified from soil and rhizomorphs were pooled for this analysis. Statistical analyses were performed using the software STATISTICA® ver. 12.0 ([47]).
Results
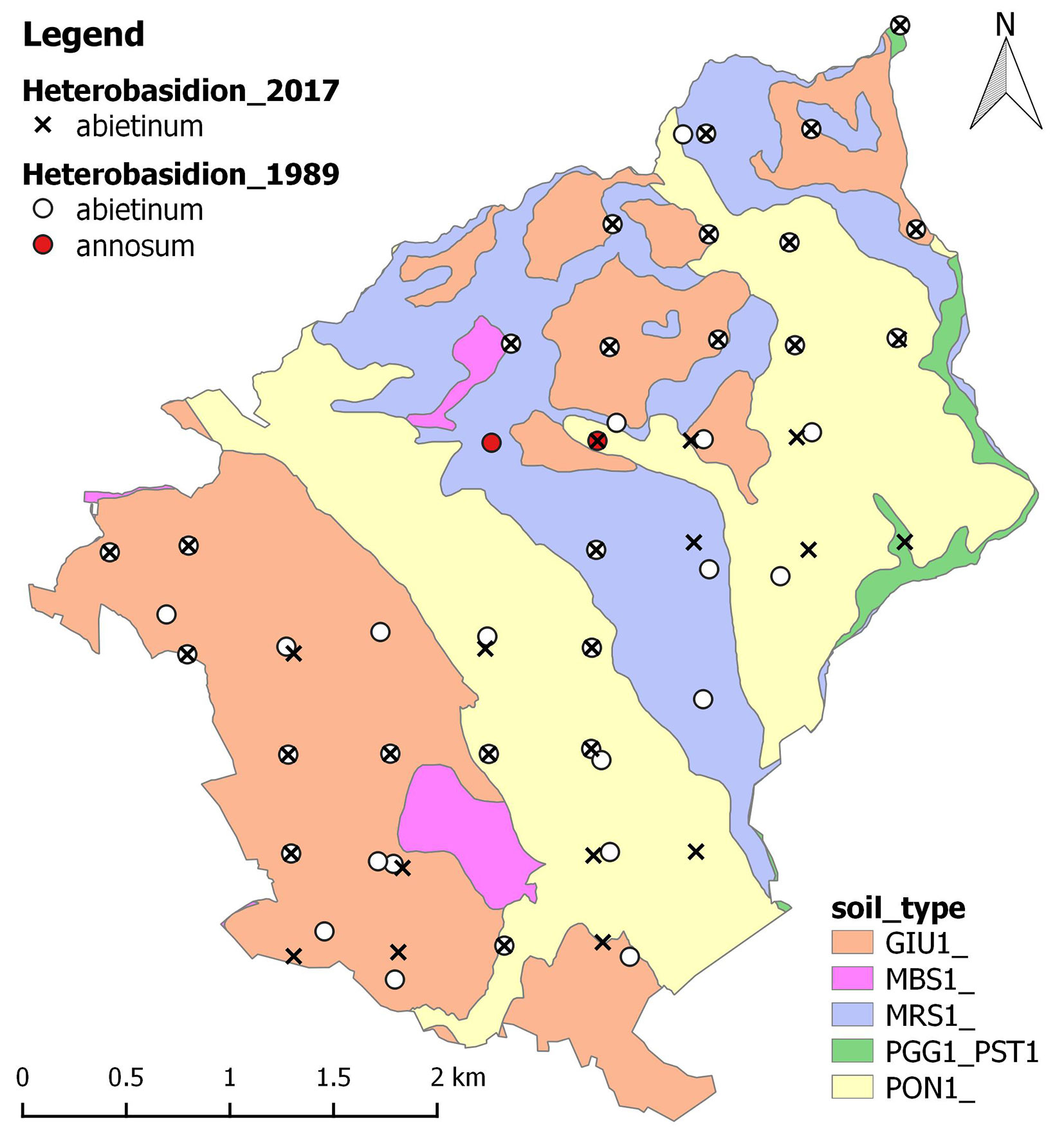
Heterobasidion annosum s.l. fruiting bodies were found on Abies alba stumps (cut or uprooted) at 71% of the sampling points. The pathogen was present in 85% of conifer stands and in only two broadleaved stands (where A. alba was growing sporadically). All samples were assigned to H. abietinum. The parasite was found in all silver fir stands, pure and mixed to other conifers (mainly European black pine and Douglas-fir) or broadleaved species. Fruiting bodies of H. abietinum were also present in about half of the stands where European black pine was the prevalent species. The findings of the present survey are summarized in Tab. 1, and mapped in Fig. 2 together with results by Farina et al. ([10]). No ecological pattern was detected in the distribution of the pathogen.
Tab. 1 - Number of stands with different tree species composition included in the study, and the incidence of Heterobasidion abietinum fruiting bodies in each stand type.
| Prevalent tree species | Number of stands |
H. abietinum incidence (%) |
|---|---|---|
| A. alba | 21 | 100 |
| A. alba with conifers | 5 | 100 |
| A. alba with broadleaves | 4 | 100 |
| P. nigra | 1 | 0 |
| P. nigra with conifers | 3 | 67 |
| P. nigra with broadleaves | 7 | 43 |
| F. sylvatica | 7 | 29 |
| Other broadleaves | 4 | 0 |
| Total | 52 | 71 |
Fig. 2 - Spatial distribution map of Heterobasidion samples identified by Farina et al. ([10]) and by present study (2015-2017). In some cases, where new host species were concerned or the damages from root rot were very evident, Farina et al. ([10]) also collected material outside of the grid points. Soil types according to the Italian soil classification system and the Soil Survey Staff ([46]): (GIU1) Giunchete - Ultic Hapludalfs; (MBS1) Monte Bastione - Typic Hapludalfs; (MRS1) Maresca - Humic Dystrudepts; (PGG1_PST1) Poggio di Petto - Lithic Dystrudepts; (PON1) Pontepetri - Typic Dystrudepts. (Source: ⇒ http://159.213.57.101/pmapper/map.phtml).
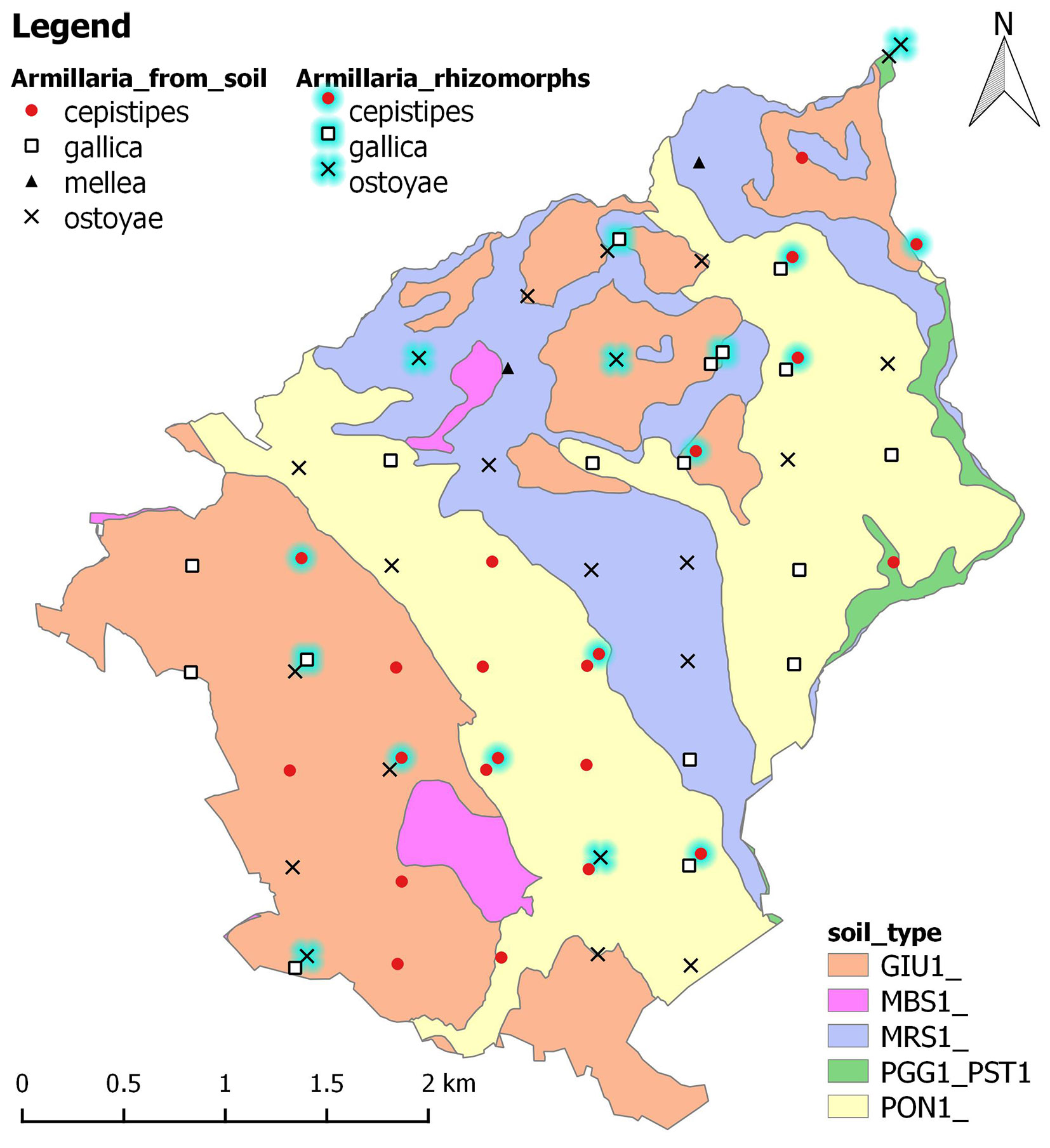
Armillaria rhizomorphs were found at one third of the sampling points. However, Armillaria was widespread throughout the forest in soil (88.5% positive soil samples based on PCR results). The most common species distinguished by restriction were A. ostoyae at 21 points (29% of rhizomorphs/ 37% of positive soil samples) and A. cepistipes at 20 points (53/28). A. gallica was found at 16 points (18/31), while A. mellea was present in only 2 soil samples (0/4). A. cepistipes was twice more frequent in rhizomorph samples than in soil. The spatial distribution of Armillaria species identified from both sample types is shown in Fig. 3. A. mellea was found only in silver fir-Douglas-fir mixed stands in Humic Dystrudepts (MRS1). A. cepistipes was mostly found in silver fir stands (70%), while A. ostoyae and especially A. gallica more abundantly occurred in forests dominated by other tree species, particularly beech.
Fig. 3 - Spatial distribution map of Armillaria samples included in the study. Points with an outer glow indicate rhizomorph samples. Soil types according to the Italian soil classification system and the Soil Survey Staff ([46]): (GIU1) Giunchete - Ultic Hapludalfs; (MBS1) Monte Bastione - Typic Hapludalfs; (MRS1) Maresca - Humic Dystrudepts; (PGG1_PST1) Poggio di Petto - Lithic Dystrudepts; (PON1) Pontepetri - Typic Dystrudepts (Source: ⇒ http://159.213.57.101/pmapper/map.phtml).
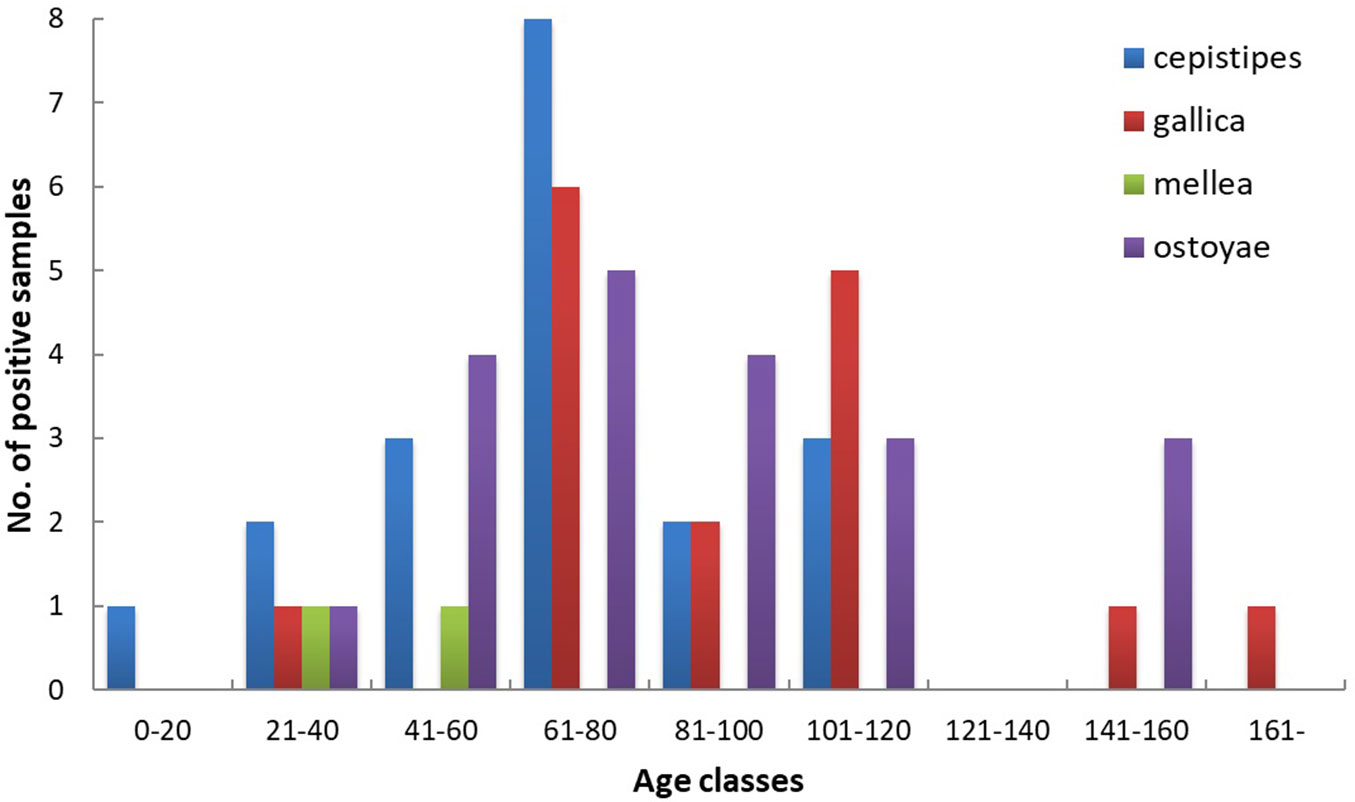
Effects of tree species composition (i.e., prevalent tree species in the stand) and soil type were non-significant. Armillaria spp. were present at a wide range of elevations and various forest sites. There was no statistically significant difference found in the altitudinal distribution of Armillaria species. The difference between A. cepistipes and A. ostoyae occurrence can be explained by the effect of site fertility levels (coef.=0.737, p=0.024), the former species inhabits more productive sites. Another parameter that had a statistically significant effect on the distribution of the pathogen was the age of the stand. A. mellea was only present in pole stands. The majority of A. cepistipes were found in the 61-80 years age class, while the occurrences of A. gallica and A. ostoyae were shifted towards the oldest stands (Fig. 4). The difference between A. cepistipes and A. gallica occurrence can be explained by this pattern (coef.=0.027, p=0.028).
Discussion
The results of the study confirm and extend the knowledge about presence of Heterobasidion in the forest of Vallombrosa since the investigation by Farina et al. ([10]). In that study, H. abietinum (F ISG) was found in the surrounding area of about 73% of the sampling points. The survey carried out in 2015-2017 confirmed the presence of the pathogen at all these points except for three. Nevertheless, in the present survey the fungus was recorded at two new localities at elevation of about 1100 m a.s.l. These detections indicate a possible expansion of the parasite at upward elevation, which might be associated with climate change ([50]).
Contrastingly to the work by Farina et al. ([10]), where H. annosum s.s. (P ISG) was detected in Vallombrosa at a very low frequency (4% of sampling points), this species was not found in the forest in our survey. However, Farina et al. ([10]) isolated the fungus also from wood samples gathered at each point, while the present survey was based solely on the apparent fruiting bodies. Indeed, basidiomes of H. annosum s.s. might be more difficult to detect since they are less thick and less persistent than those produced by H. abietinum ([38]).
Although the majority of H. abietinum occurrences fell to fir stands, basidiomes of the fungus occasionally appeared in stands dominated by Douglas-fir, European black pine and beech, where there was admixture of silver fir. We did not identify the pathogen on other host species than Abies alba. However, in the forest of Vallombrosa H. abietinum had been reported on Norway spruce, Douglas-fir, chestnut, Japanese red-cedar and pine by Farina et al. ([10]). Sedlák & Tomšovsky ([44]) reported H. abietinum on pine and Norway spruce in the Czech Republic, always in stands where the historical presence of A. alba was documented, supporting the evidence that the fungus can survive at sites with a changed tree species composition. Therefore the presence of the fungus might represent a threat for other tree species besides A. alba, especially conifers, but also deciduous trees when growing in mixture with conifers, or at difficult sites ([28]), on high pH (>6) soils ([42]) or under the pressure of climate change, which is expected to be especially strong and sudden in the Mediterranean region ([13], [31]) and which may defeat tree defences against secondary pathogens ([12]). In the case of Douglas-fir, which is only occasionally attacked by the pathogen and seldom suffers early mortality and killing even at an older age ([9], [28]), the highest risk is posed by H. abietinum to young individuals ([43]).
Regarding Armillaria species, the differences in their infection biology must be taken into account. Some species like A. cepistipes propagate directly in soil ([40]), whereas others such as A. ostoyae are restricted to spreading through root contacts ([8]). Therefore, the presence of DNA of distinct Armillaria spp. in soil does not necessarily correlate with their prevalence in the ecosystem.
Armillaria spp. are present in the entire area of the Nature Reserve of Vallombrosa. In many cases, the DNA of the fungus was amplified from both the rhizomorphs and the soil samples collected at the same location, and these isolates often belong to different species. This type of coexistence within the same forest stand is well documented in the literature ([40], [2]), and has been explained by the different ecological strategy of the species, i.e., their specialization to saprophytic or parasitic behaviour.
In many respects, A. cepistipes seems to be the most influential Armillaria species in the Vallombrosa forest. The fact that more than half of the identified rhizomorph samples belong to this species is consistent with observations by many authors who describe it as highly rhizomorphogenic ([45]). Most probably, A. cepistipes finds its ecological optimum in the area and consequently shows pathogenicity in weakened hosts such as pines. Our results foreshadow its ability to overcome host resistance even on the most fertile sites. Given its dominance in relatively young fir stands, this species is considered potentially hazardous in Vallombrosa. A. ostoyae is equally common as A. cepistipes, but its DNA was more often isolated from soil. Despite this species is usually associated with conifers, 38% of its occurrences in the forest of Vallombrosa were broadleaved stands. The third important species is A. gallica. This facultative parasite did not show any preference to forest type, is evenly distributed in the area. In the points where both species are present, A. cepistipes was found as rhizomorphs while A. gallica was determined from soil. This points to the fact that A. cepistipes acts as a parasite whereas A. gallica is a decomposer, which is in line with the abovementioned hypothesis of Prospero et al. ([40]). Surprisingly, A. mellea was detected from Douglas-fir stands. European plantations of this species are considered resistant to Armillaria root rot ([18]). It is reasonable to assume that A. mellea is a residual from the previous rotation of broadleaves in these quite young stands rather than a colonizer of conifers.
Besides root rot disease, two factors are conceived to be crucial in silver fir decline. Soil properties decisively affect fir growth; at Vallombrosa, the high bulk density of the BC horizon hinders deep rooting, thus limiting the stability of trees. Moreover, the almost impermeable layer prevents the soil from accumulating adequate amounts of water. Suffering from water stress, trees are unable to produce inhibitor metabolites for H. abietinum, so their susceptibility increases to the infection ([6]). Mediterranean climate is not very well suited to the needs of silver fir either. In addition to long dry periods in summer, particular weather events, such as heavy snowfalls and cyclones put these conifers under stress ([41]). The situation is getting worse with the advance of the global climate disruption ([24]).
Conclusions
Our results confirmed the widespread presence of root decaying fungi in the forest of Vallombrosa (central Italy). Reviewing the relevant literature has revealed an etiology in which soil properties and climatic circumstances are crucial elements, being able to predispose the trees to fungal infection and determine the disease outcome. When assessing the possibility of intervention to manage the forest so as to limit the damage from root rot caused by H. annosum s.l. and Armillaria spp., it is necessary to know the potential risk posed by the different species regarding their host spectrum, infection biology and ecological needs, considering their possible different behaviour with regard to the applied management strategies and interventions. Abies alba and Pinus spp. are known to be the most vulnerable tree species to these pathogens, but young Douglas-fir individuals may also suffer heavy damage by Heterobasidion attacks. Bearing in mind that the extent of damages at Vallombrosa has crossed a threshold where it does not merely cause large economic loss, but threatens the stability of the ecosystem ([7]), it is advisable to apply practical measures to limit the spread of parasites that have a crucial role in weakening trees and mining their resistance to mechanical stress. Since the area is protected, drastic operations like stump removal are not allowed. Instead, preventive treatment on the stumps utilizing antagonistic fungi is recommended immediately after cutting to inhibit the airborne colonization by H. abietinum and thus reduce the further extension of the rot through root contacts. Cuts should be done in summer when sporulation rate of Heterobasidion is low ([11]). In the case of silver fir, lowering the rotation period to 100-120 years is advisable. Fir stands should be planted only in the most suitable soils of the area, Ultic Hapludalfs (GIU1). Planting of Douglas-fir in areas where the presence of Heterobasidion is documented should be avoided. Creating mixed stands with broadleaves is a preference; as such forests are less susceptible to Heterobasidion ([41]) and Armillaria ([18]) attacks. Our results may help forest managers to decide which tree species are to be planted, based on their resistance to root rot pathogens.
Acknowledgements
Data collection has been done in frame of Erasmus+ internship at the Department of Agrifood Production and Environmental Sciences (DISPAA), University of Florence, Italy. We wish to thank Lorenzo Allighieri and Chiara Aglietti for their help in the field. Samples were analysed in the laboratories of the Department of Forest Protection and Wildlife Management and the Department of Forest Botany, Dendrology and Geobiocoenology, Faculty of Forestry and Wood Technology, Mendel University in Brno, Czech Republic. We are grateful for Ing. Petr Sedlák, Ph.D. and Ing. Tomáš Májek for their guidance during laboratory analyses. Special thanks go to the International Visegrad Fund for supporting the corresponding author’s academic studies by Intra-Visegrad Scholarship.
References
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Supplementary Material
Authors’ Info
Authors’ Affiliation
Libor Jankovský
Department of Forest Protection and Wildlife Management, Mendel University in Brno (ÚOLM), Zemedelská 3, 61300 Brno (Czech Republic)
Luisa Ghelardini
Department of Agrifood Production and Environmental Sciences, University of Florence (DISPAA), p.le delle Cascine 28, I-50144, Firenze (Italy)
Corresponding author
Paper Info
Citation
Dálya LB, Capretti P, Ghelardini L, Jankovský L (2019). Assessment of presence and distribution of Armillaria and Heterobasidion root rot fungi in the forest of Vallombrosa (Apennines Mountains, Italy) after severe windstorm damage. iForest 12: 118-124. - doi: 10.3832/ifor2929-012
Academic Editor
Alberto Santini
Paper history
Received: Jul 22, 2018
Accepted: Dec 28, 2018
First online: Feb 11, 2019
Publication Date: Feb 28, 2019
Publication Time: 1.50 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2019
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 44372
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 36851
Abstract Page Views: 3419
PDF Downloads: 3181
Citation/Reference Downloads: 9
XML Downloads: 912
Web Metrics
Days since publication: 2544
Overall contacts: 44372
Avg. contacts per week: 122.09
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2019): 8
Average cites per year: 1.14
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Temporal development of collar necroses and butt rot in association with ash dieback
vol. 10, pp. 529-536 (online: 05 May 2017)
Research Articles
Regeneration of Abies pinsapo within gaps created by Heterobasidion annosum-induced tree mortality in southern Spain
vol. 7, pp. 209-215 (online: 27 February 2014)
Research Articles
Secondary metabolites of six Siberian and Crimean Armillaria species and their in vitro phytotoxicity to pine, larch and poplar
vol. 15, pp. 38-46 (online: 04 February 2022)
Research Articles
Efficacy of Phlebiopsis gigantea against Heterobasidion conidiospore and basidiospore infection in spruce wood
vol. 13, pp. 369-375 (online: 25 August 2020)
Research Articles
Stand dynamics and natural regeneration in silver fir (Abies alba Mill.) plantations after traditional rotation age
vol. 7, pp. 313-323 (online: 08 April 2014)
Research Articles
Effects of gap size and within-gap position on seedlings establishment in silver fir stands
vol. 1, pp. 55-59 (online: 28 February 2008)
Research Articles
Effects of artificial defoliation and simulated insect damage on the growth of Betula pendula saplings
vol. 9, pp. 95-100 (online: 15 July 2015)
Research Articles
Age trends in genetic parameters for growth and quality traits in Abies alba
vol. 9, pp. 954-959 (online: 07 July 2016)
Research Articles
Wind contribution to yearly silver fir (Abies alba Mill.) compression wood development in the Romanian Carpathians
vol. 9, pp. 927-936 (online: 02 October 2016)
Research Articles
Windstorm disturbance triggers multiple species invasion in an urban Mediterranean forest
vol. 11, pp. 64-71 (online: 25 January 2018)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword