The effect of silver and copper nanoparticles on the growth and mycorrhizal colonisation of Scots pine (Pinus sylvestris L.) in a container nursery experiment (§)
iForest - Biogeosciences and Forestry, Volume 11, Issue 5, Pages 690-697 (2018)
doi: https://doi.org/10.3832/ifor2855-011
Published: Oct 23, 2018 - Copyright © 2018 SISEF
Research Articles
(§): Authorship and acknowledgements of this article were corrected on 10 April 2019 (see 10.3832/ifor2855-011-bis).
Abstract
Recent research points to the possibility of nanoparticles being used as fertilisers, growth stimulators, and promoters of plant resistance or pesticides. In this study, we sought to determine the influence of nanoparticles of silver and copper (AgNPs and CuNPs) on growth parameters and spontaneous mycorrhizal colonisation of roots in 2-year-old container-grown seedlings of Scots pine. Foliar applications of nanoparticles were made through two growing seasons, four times a season, at concentrations of 0, 5, 25 and 50 ppm. Comparisons of the ultrastructures characterising the needles, stems and roots of the treated or untreated pines were conducted with transmission electron microscopy (TEM). The deployed CuNPs stimulated mycorrhizal colonisation at all concentrations, although the growth of seedlings was only promoted at a concentration of 25 ppm. Higher concentrations of AgNPs (25 and 50 ppm) inhibited the formation of mycorrhizae, though the lowest concentration (5 ppm) produced an increase in both mycorrhizal colonisation and the dry mass of roots. The species of ectomycorrhizal fungi found were Thelephora terrestris, Suillus bovinus and Sphaerosporella brunnea. The TEM results comparing treated and control (untreated) needles revealed changes in the chloroplasts from lens-shaped to spherical. Furthermore, an increase in the number of plastoglobules and the presence of large osmophilic globules in the cytoplasm associated solely with the needles of pines receiving 50 ppm nanoparticles were observed. In contrast, ultrastructural changes in stems and roots associated with the applications of NPs were not found. Overall, the results indicated that CuNPs and AgNPs could be used as stimulators of growth in general, and mycorrhizal colonisation in particular, among container-grown Scots pines. However, further work is needed to determine their optimal doses and concentrations.
Keywords
Nanoparticles, Ectomycorrhizae, Toxicity, Growth Stimulation
Introduction
Nanomaterials are engineered structures with at least one dimension between 1 and 100 nm ([28]). In the past decade, they have gained wide application worldwide owing to electronic, optical, mechanical, magnetic and chemical properties that are unique and can be quite different from those characterising the same materials present in bulk ([36]). Nanotechnology has been applied in plant production (agriculture and forestry) as fertilisers to increase plant growth and disease resistance and as pesticides seeking to improve pest and disease management ([39]).
Thus far, most research on the influence of nanoparticles on plants has been conducted in hydroponic culture ([3]), with only a few trials involving plants grown in soil ([17]) or in the peat-based substrate used in nursery production ([29]). The results of these studies document positive, negative and neutral influences of nanoparticles on plants ([39], [35]). The positive influence is manifested in stimulated germination, increased growth rates, higher plant biomass, a greater number of flowers/fruits/seeds, higher contents of gluten and starch, and higher chlorophyll content with a faster rate of photosynthesis ([24], [16]). Further, an indirectly favourable influence of nanoparticles on plants reflects growth and/or increased metabolic activity within the population of rhizospheric microorganisms ([33], [44]).
The negative influence is manifested in obstructed seed germination, reduced shoot or root length and dry/fresh mass, as well as a reduced chlorophyll content ([40], [27]).
These differences in the results of research carried out so far may reflect that the biological influence of nanoparticles depends on their size, chemical composition, surface structure, solubility, shape, and level of aggregation ([28]). Their influence also seems to depend on the plant species involved, growth conditions, the type of substrate (soil or different nutrient media), temperature, and light intensity and, of course, the concentrations of nanoparticles involved, the manner of the application (foliar or soil) and the dosage ([36], [17], [35]). The large number of factors involved complicates the comparison of results among studies ([43]).
Scots pine (Pinus sylvestris L.) is the most widely distributed conifer species in the northern hemisphere and the most important forest-forming species throughout eastern-central Europe ([21]). In Polish forests, it covers over 58.2% of the forest area, while accounting for 60.8% of the volume ([7]). Pines are thought to be dependent on ectomycorrhizal symbiosis for optimal development under natural conditions. Ectomycorrhizal fungi facilitate both nutrient and water uptake, increase resistance to certain root diseases, and enhance the stress tolerance of trees ([41]).
Currently, little research is being conducted on the influence of nanoparticles on the state and structure of mycorrhizal fungi. The majority of this research concerns arbuscular mycorrhiza, and as in the influence of nanoparticles on plants, the results are ambiguous. Some studies reveal a negative influence on mycorrhizal colonisation of plant roots ([11]), while others suggest no influence on the level of mycorrhization at low nanoparticle concentrations but negative impacts when concentrations are high ([18], [5]). In contrast, Feng et al. ([12]) demonstrated a stimulatory influence of silver nanoparticles (AgNPs) on the formation of an arbuscular mycorrhizae, irrespective of the concentrations applied. Similarly, ambiguous results have been obtained for ectomycorrhizae. Sweet & Singleton ([43]) showed that contamination of soil by AgNPs may result in a drastic reduction in the diversity of ectomycorrhizal fungi in the roots of seedlings of Pinus muricata. Additionally, Olchowik et al. ([29]) documented disparate influences of metal nanoparticles on ectomycorrhizae formation in seedlings of pedunculate oak for both type and concentration. While AgNPs stimulated formation irrespective of concentration, nanoparticles of copper (CuNPs) were found to have a stimulatory effect at low concentration, but an inhibitory impact when the concentration was high.
Notwithstanding the many relevant studies previously conducted, the influence of nanomaterials on plants and the environment remains unclear ([3]). Thus, this paper had the following objectives: (i) to determine the influence of foliar applications of AgNPs and CuNPs at different concentrations on the growth of container-grown 2-year-old Scots pine seedlings; (ii) to compare the ultrastructures of needles, stems and roots of pines treated or untreated with nanoparticles with TEM; and (iii) to determine the extent of root mycorrhization and proportion of different ectomycorrhizal species in relation to the types and concentrations of nanoparticles applied. The basic assumption to be tested was that foliar applications of AgNPs and CuNPs would stimulate both the growth of pines and the formation of mycorrhizae.
Materials and methods
Plant material
The study was conducted in the nursery of the Forest Experimental Station of Warsaw University of Life Sciences, Poland (located at Rogów - 51° 40′ N, 19° 55′ E, 195 m a.s.l). Scots pine seedlings were grown in V-120 plastic container trays (40 pots per tray with a capacity of 120 cm3) in a peat-perlite substrate, purchased from the container nursery in Nedza, Rudy Raciborskie Forest District, Poland. The substrate (pH=4.5) contained 85% sphagnum peat from Estonia and 15% coarse-grained perlite (No. 3) which improves aeration. Seedlings received extra nutrients via a mixture of Osmocote Exact Standard controlled-release fertilisers of different release characteristics: 3-4 M, 5-6 M, and 8-9 M (1:1:1). Fertiliser was added to the substrate at the same time as the pine seeds (of local origin from a managed forest) were sown. On 12 May 2014, two seeds were planted in each pot to provide seedlings. Upon germination, one plant per pot was selected at random and the other was removed. The containers were then placed in a foil-covered greenhouse (height 2.8 m, width 6 m and length 30 m) and watered regularly as necessary. The temperature in the greenhouse during the experiment was 25-30 °C. At the end of the first growing season (30 September 2014), seedlings were replanted in larger pots. We then used V-360 container trays (15 pots per tray with a capacity of 360 cm3) and the same peat-perlite substrate as in the first year. In the second year, seedlings were grown outdoors.
Nanoparticles and treatments
The two types of metal nanoparticle chosen were AgNPs and CuNPs. Samples of commercially available solutions of AgNPs and CuNPs were purchased from Nano-Koloid Sp. z o.o, Warsaw, Poland, as a licensee of Nano Technologies Group, Inc. (NY, USA), manufactured under European patent EP2081672 A2. As the producer notes, these nanoparticles are generated in a physical process, consist of approximately 100 atoms and are suspended in demineralised water. The concentration of nanoparticles in the commercially available product is 50 ppm.
Each year (between May and July), there were four spray treatments of the aerial parts of the seedlings using 1000 l ha-1 (=100 ml m-2) aqueous solutions of NPs (AgNPs, CuNPs) at 4 concentrations: 0, 5, 25 and 50 ppm. The NPs had been suspended in deionised water prior to application by vigorous shaking for at least 10 min. NPs were applied to foliage on 27 May, 11 and 25 June and 9 July in 2014; and on 17 June and 1, 15 and 29 July 2015. Sprayings were performed in the early morning with a manual compressed air sprayer (model KK-PS5000®, Kisan Kraft, Bangalore, India). The experimental design was comprised of two factors: the type of metal nanoparticles (Ag or Cu) and the 4 concentrations (0, 5, 25 and 50 ppm). The experiment was performed using a randomised complete block design with 4 blocks.
Sampling and biometric parameters
At the end of the second growing season (in October), 40 seedlings (10 plants × 4 blocks) were harvested from each treatment at random. Thus, a total of 320 Scots pine seedlings were sampled. At this time, the features of shoot growth measured were shoot length (cm) and root-collar diameter (mm), as well as total root length (cm) determined by analysis of the root systems using an Epson Perfection 4990 Photo scanner integrated with WinRhizo software (Regent Instruments Inc., Quebec, Canada). Then, dry masses of shoots and roots (g) were determined, following drying at 105 °C for 24 h.
Microscopic investigation
For the purposes of transmission electron microscope (TEM) imaging, ten seedlings in each treatment were selected at random following the first growing season. The adsorption and assimilation of NPs in the needles, shoots and roots of the tested pine plants were observed by TEM, 8 weeks after the last treatment. Tissue samples were collected from the middle parts of the needles, shoots and roots of the pine seedlings. The plant material obtained was fixed in 2% (v/v) glutaraldehyde and 2% (v/v) paraformaldehyde in 0.05M cacodylate buffer (pH 7.2), following the methods of Karnovsky ([19]), for 4 h at room temperature. The material was then rinsed four times with cacodylate buffer. The samples were contrasted in 1% OsO4 for 2 h at 4 °C and rinsed four times with the same buffer prior to dehydration in a series of aqueous solutions of ethanol, and subsequently in propylene oxide. Finally, the material underwent gradual saturation with resin (Epon, Fluka) before being polymerized for 24 h at 60 °C. The Epon blocks with plant material were cut for TEM with an ultramicrotome (Leica) into ultrathin sections (~90 nm thick), which were collected on Formvar coated slot-grids and contrasted with 1% lead citrate and 2% uranyl acetate. The material was then examined using a Morgagni 268D (FEI) electron microscope, while photographic documentation was obtained using a Morada (SIS) digital camera and the iTEM (SIS) computer programme.
Assessment of ectomycorrhizae
The roots of harvested seedlings were analysed under a Delta IPOS-808 stereoscopic microscope coupled with a digital camera at 10-40× magnification. The degree of mycorrhization was determined by classifying and counting mycorrhizal and non-mycorrhizal root tips. The proportion of mycorrhizal root tips was calculated as (mycorrhizal root tips) / (mycorrhizal root tips + non-mycorrhizal root tips) × 100 and presented as degree of mycorrhization. Ectomycorrhizal tips were identified under a dissecting microscope by the absence of root hairs, shape (hypertrophy) and colour of fine roots, as well as the presence of mycelial mantles and emanating fungal structures (hyphae and rhizomorphs). The initial identification of morphotypes was based on the literature ([1]). Representative mycorrhizal root tips of each morphotype were photographed and deposited in an internal database, together with fungal descriptions and molecular information. To determine mycorrhizal species/taxa, we collected tip material from three to five mycorrhizal root tips per morphotype and transferred them into Eppendorf tubes filled with 70% EtOH for molecular analysis.
The process of identification of mycorrhizal fungi entailed PCR amplification of selected regions of internal transcribed spacer (ITS) rDNA using the ITS1F / ITS4 primer pair, with sequencing of the resulting PCR product. We used direct PCR amplification of fungal DNA from ectomycorrhizal samples bypassing conventional DNA extraction procedures. PCR products were assessed by loading 2 μL onto a 1% agarose gel (0.5 × TAE buffer) and visualised under UV light using the GeneRuler™ 1 kb Plus DNA Ladder (Fermentas). Bi-directional dye-terminator cycle sequencing was performed using BigDye Terminator v3.1 Chemistry (Applied Biosystems) and one of each PCR primer. The resulting fragments were analysed on a 3730 DNA Analyser at the Department of Botany and Biodiversity Research of the University of Vienna, Austria. Identification of sequenced fungi was based on the results of BLAST searches against the National Centre for Biotechnology Information (NCBI) public database, with subsequent phylogenetic placement and queries to the UNITE database.
Statistical analysis
We tested the relationships among biometric parameters depending on different concentrations of AgNPs and CuNPs using a one-way analysis of variance (ANOVA) with Tukey’s post-hoc tests. Analysis of the effects of the different concentrations of AgNPs and CuNPs on the degree of mycorrhization and abundance of mycorrhizal fungi was performed using generalised linear models (GLM) for a randomised complete block design with a binomial probability distribution. The Tukey’s test was used in pairwise comparisons (as a post-hoc test) between different concentrations of AgNPs and CuNPs. The GLM model for the proportion of the defined root types (mycorrhizal and non-mycorrhizal) is given by (eqn. 1):
where pijk is the numerical proportion of specific root tips (mycorrhizal and non-mycorrhizal) for the j-th concentration of nanoparticles nested in i-th type of metal nanoparticles in the k-th block, θ is the grand mean, ai the effect of the i-th type of metal nanoparticle, bj(i) is the effect of the j-th concentration nested in the i-th type of metal nanoparticle, gk is the random effect of the k-th block and eijk is the random experimental error. The statistical analysis was performed using R version 3.3.3 ([34]) with the accepted level of significance set at p<0.05.
Results
Both types of NPs did not consistently stimulate growth in the 2-year-old pines. Seedlings treated with AgNPs at concentrations of either 5 or 50 ppm had significantly greater dry masses of roots (3.00 and 2.20 g, respectively). The remaining growth parameters in pine seedlings sprayed with AgNPs did not differ from those of untreated seedlings. For the variant involving CuNPs, at the application concentration of 25 ppm, seedlings had significantly greater values for all biometric parameters analysed except girth of the root collar (Tab. 1).
Tab. 1 - Biometrical parameters (mean ± SD) of 2-year-old Scots pine seedlings treated with different concentrations of AgNPs and CuNPs. Ag0, Cu0 - 0 ppm; Ag5, Cu5 - 5 ppm; Ag25, Cu25 - 25 ppm; and Ag50, Cu50 - 50 ppm. Different letters indicate significant differences between treatments with different applied concentrations of AgNPs and CuNPs as assessed using Tukey’s test (α=0.05).
| Treatment (n=40) | Length of shoot (cm) | Root collar diameter (mm) | Total root length (cm) | Dry mass of shoot (g) | Dry mass of root (g) |
|---|---|---|---|---|---|
| Ag0 | 22.2 ± 4.95 a | 4.72 ± 0.91 a | 24.8 ± 5.10 a | 4.67 ± 1.25 ab | 1.42 ± 0.50 a |
| Ag5 | 18.5 ± 4.78 a | 4.82 ± 1.79 a | 25.6 ± 5.46 a | 4.07 ± 1.74 ab | 3.00 ± 0.74 c |
| Ag25 | 19.9 ± 6.46 a | 4.49 ± 0.92 a | 22.9 ± 4.08 a | 3.52 ± 1.88 a | 1.60 ± 0.82 a |
| Ag50 | 19.5 ± 4.48 a | 5.07 ± 0.84 a | 23.5 ± 5.10 a | 4.66 ± 1.85 b | 2.20 ± 0.74 b |
| Cu0 | 21.7 ± 3.57 a | 4.65 ± 0.61 ab | 21.9 ± 3.86 a | 4.01 ± 1.59 a | 1.59 ± 0.57 a |
| Cu5 | 21.6 ± 5.89 a | 5.00 ± 0.58 ab | 25.2 ± 5.23 ab | 4.53 ± 1.45 a | 1.70 ± 0.95 a |
| Cu25 | 26.2 ± 6.73 b | 5.38 ± 0.86 b | 25.8 ± 6.08 b | 5.83 ± 2.21 b | 2.54 ± 1.02 b |
| Cu50 | 19.5 ± 5.05 a | 4.48 ± 0.98 a | 25.0 ± 5.26 ab | 4.05 ± 1.81 a | 2.44 ± 0.84 b |
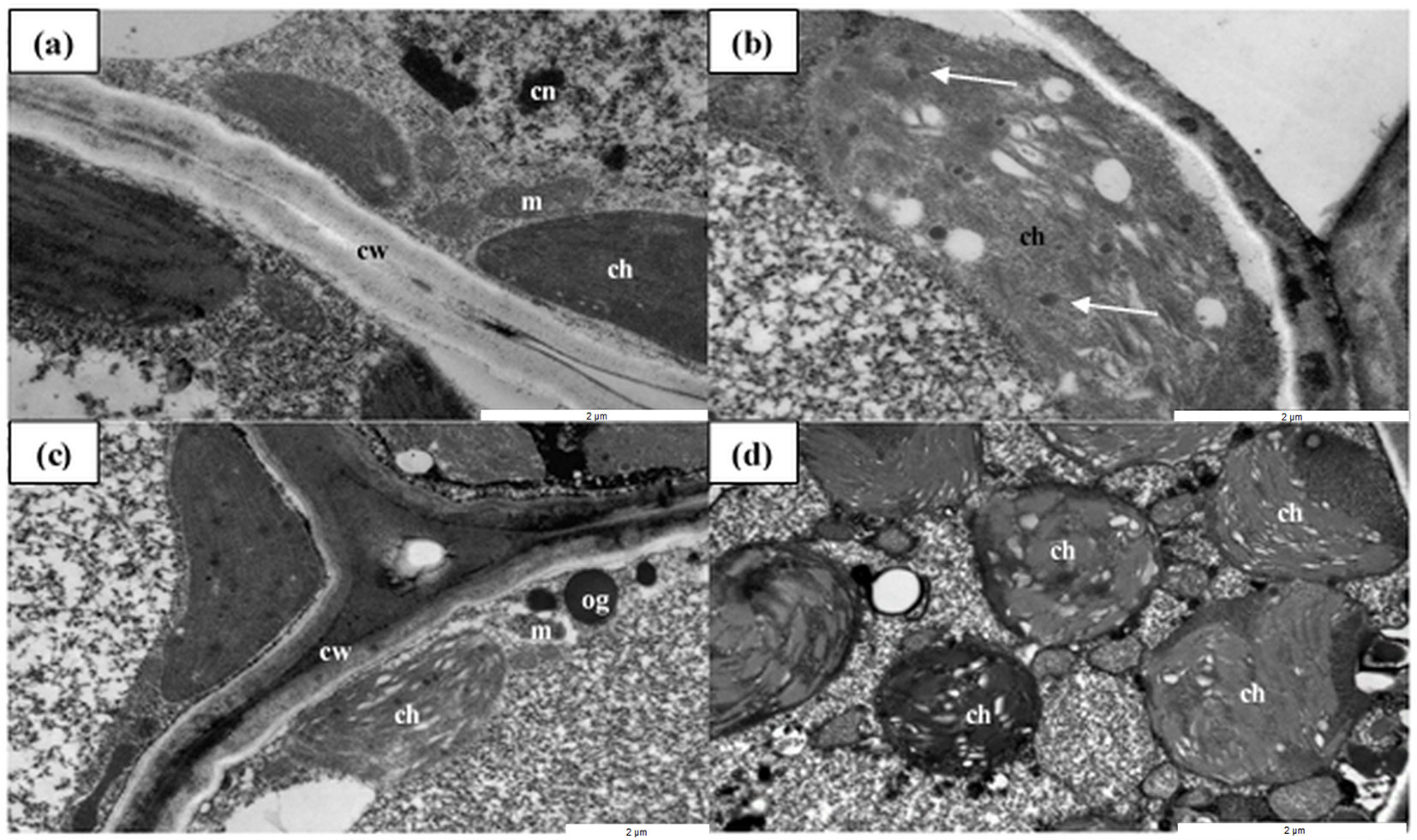
The ultrastructures of the shoots and roots were similar in nanoparticle-treated and control plants (data not shown). In contrast, plants treated with CuNPs and AgNPs (at only the highest concentration of 50 ppm) exhibited disrupted ultrastructure in their needles, especially in the photosynthetic apparatus. In the control plants (no AgNP and CuNP), chloroplasts filled the cells and contained single plastoglobules, while the NP-treated plants exhibited a large number of plastoglobules, and the shape of chloroplasts of NP-treated plants were modified from lenticular to round. In addition, large, osmophilic globules were present in the cytoplasm of NP-treated needle cells. Application of the CuNPs, compared with the AgNPs, resulted in more limited disturbance to the ultrastructure of mesophyll cells in the pine needles. Other organelles (e.g., mitochondria, cell walls and vacuoles) remained unchanged. These results indicate that Cu- and AgNPs at high concentrations (50 ppm) were able to exert an influence on the ultrastructure of chloroplasts in pine needles (Fig. 1).
Fig. 1 - The ultrastructures of Scots pine needles under TEM. (a) Cross-sections of mesophyll cells in control needles. (b, c) Plants treated with 50 ppm CuNPs; chloroplasts with disturbed ultrastructure and a large number of plastoglobules and osmophilic globules are visible. (d) The chloroplasts from the 50 ppm AgNP-treated plants have been modified from lenticular to round. (cw): cell wall; (cn): cell nucleus; (m): mitochondrion; (ch): chloroplast; (og): osmophilic globule; (white arrow): plastoglobule. Scale bars represent 2 µm.
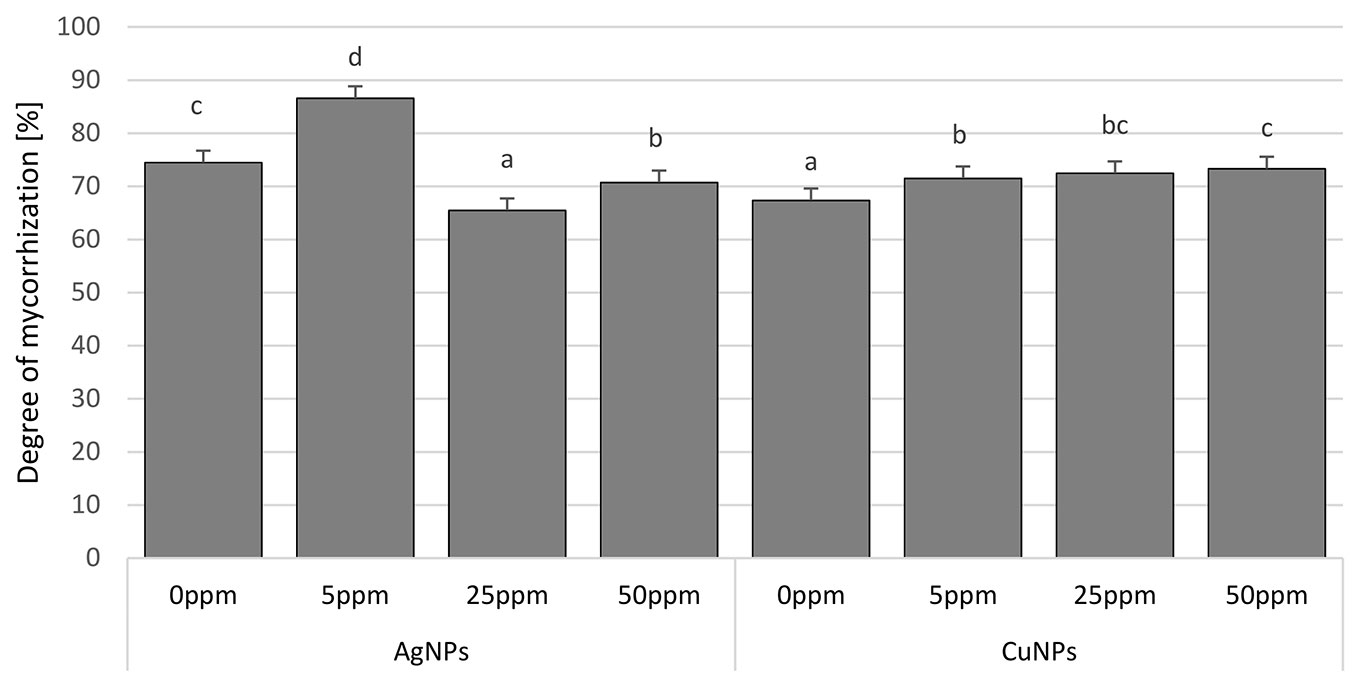
After two years of growth in a peaty substrate in the containers, just over 70% mycorrhization had occurred on average. This process was stimulated by CuNPs at all concentrations and by AgNPs at the lowest applied concentration (5 ppm). Application of AgNPs at the higher concentrations (25 and 50 ppm) was shown to be associated with a more limited role for ectomycorrhizae than in the control (Fig. 2).
Fig. 2 - Degree of mycorrhization (%) of Scots pine seedlings treated with AgNPs and CuNPs. Concentrations of NPs: 0 ppm, 5 ppm, 25 ppm, and 50 ppm. Different letters indicate significant differences between treatments with different applied concentrations of AgNPs and CuNPs as assessed using Tukey’s test (α=0.05).
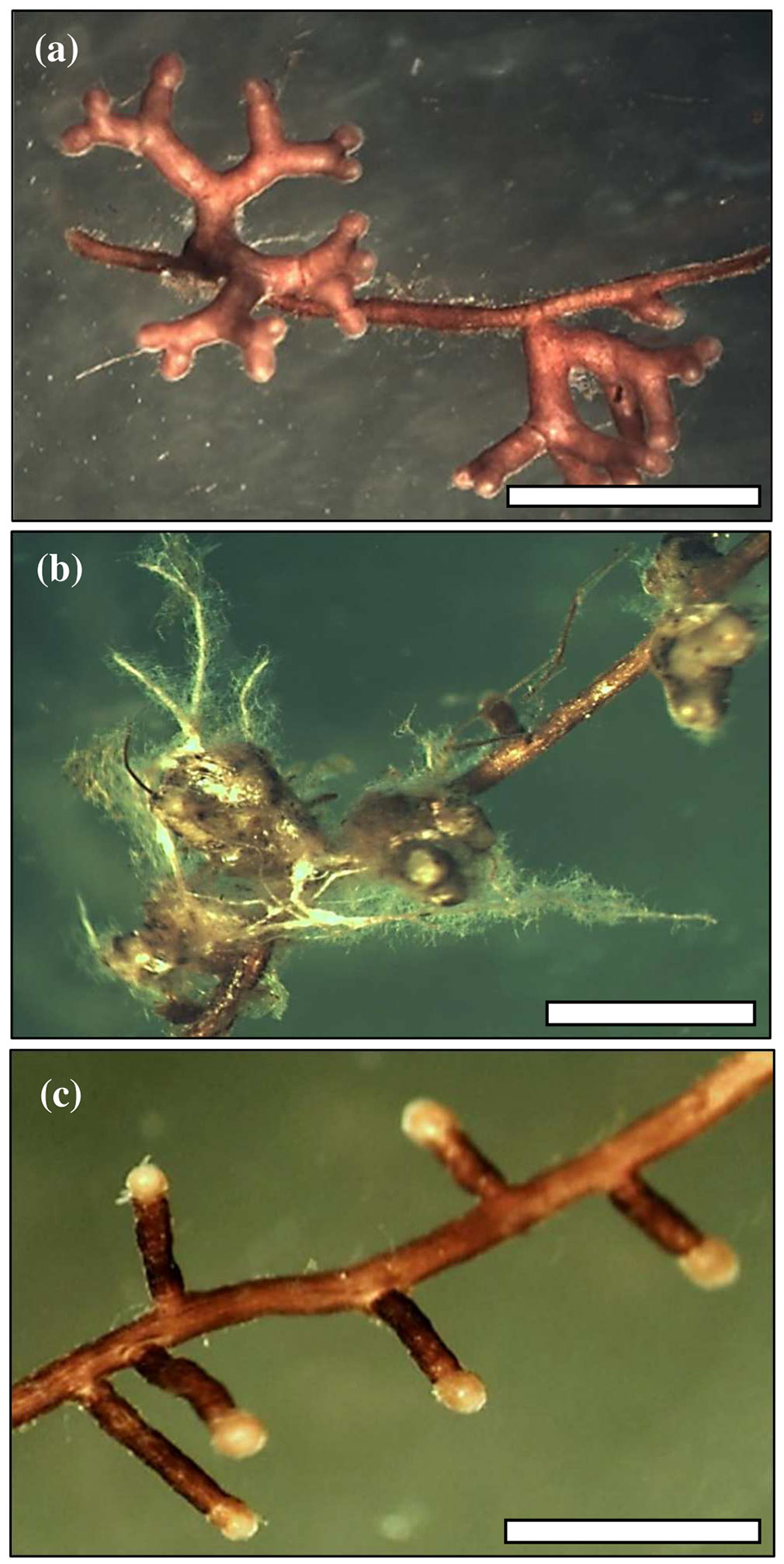
The species of ectomycorrhizal fungi found on seedling roots were Thelephora terrestris, Suillus bovinus and Sphaerosporella brunnea (Fig. 3). In comparison with other species, T. terrestris had higher colonisation and statistically greater growth when concentrations of both NPs were higher. In contrast, S. bovinus was a more abundant coloniser of roots among seedlings not treated with NPs (20.6% and 35.2% with AgNPs and CuNPs, respectively). The participation of this fungus was more limited with successively higher concentrations of the two NPs. The presence of S. brunnea was the most limited of the three and was significantly greater where neither AgNPs nor CuNPs were applied (16.1% and 13.6%, respectively - Fig. 4).
Fig. 3 - Mycorrhizal morphotypes observed on Scots pine seedlings. (a) Thelephora terrestris, (b) Suillus bovinus, and (c) Sphaerosporella brunnea. White bars = 1 mm.
Fig. 4 - Abundance of ectomycorrhizal fungi (%), Thelephora terrestris, Suillus bovinus and Sphaerosporella brunnea, of Scots pine seedlings treated with AgNPs and CuNPs. Concentrations of NPs: 0 ppm, 5 ppm, 25 ppm, and 50 ppm. Different letters indicate significant differences between treatments with different concentrations of AgNPs and CuNPs for each ectomycorrhizal taxon as assessed using Tukey’s test (α=0.05).
Discussion
Two growing seasons of treatment of pine seedlings with AgNPs and CuNPs revealed a significant impact on all of the parameters considered, i.e., growth, the degree of ectomycorrhizal colonisation of roots, species composition of fungi and needle ultrastructure.
Both NPs could stimulate growth, although there was a differential effect, with the use of AgNPs leading, rather unaccountably, to an increase (over the control) in dry masses of roots at doses of both 5 and 50 ppm. In addition, growth stimulation of seedlings treated with CuNP was only observed with the 25 ppm application, although some stimulation was visible for most of the parameters investigated. Copper is a micronutrient essential for proper plant growth. The most abundant copper-containing protein is plastocyanin, which is involved in photosynthetic electron transport in the thylakoid lumen of chloroplasts. Furthermore, copper acts as a prosthetic group of copper/zinc superoxide dismutase, which protects the photosynthetic apparatus from oxidative stress. Despite its physiological importance, excess copper is toxic to plants because of its potential participation in the Fenton reaction ([47]). On the other hand, while organic and peat soils both have copper contents adequate for plant growth, they may be low in forms that can be readily taken up, reflecting the fact that the organic acids (humic, crenic and fulvic) present in peat strongly bind copper in complexes in which the element is rendered plant-unavailable ([37]). Thus, the use of CuNPs with pine seedlings grown in a peaty substrate and in soil in a nursery may yield different results. Our experiment using 25 ppm CuNPs might have represented a source of copper well above that present in the Osmocote fertiliser, with the dose being markedly higher than in the 5 ppm application and hence, capable of influencing the course of photosynthesis with a stimulatory impact on seedling growth. Moreover, the 50 ppm does appear to have induced certain changes in chloroplasts, even if these changes do not actually reflect toxic effects of the CuNPs. Thus, the benefits of any stimulation due to the extra source of Cu may have been suppressed, resulting in the growth of pine seedlings being comparable with that of the control.
The experiment presented here revealed chloroplasts as cell organelles sensitive to the action of NPs. Deliberate exposure of young pines to the highest concentrations (50 ppm) of NPs was sufficient to produce a change in the shape of chloroplasts from lenticular to spherical, with a marked increase in numbers of plastoglobules, and large osmophilic globules in the cytoplasm, which is likely an indication of the final stage of chloroplast disintegration. The application of CuNPs resulted in a more limited disturbance of ultrastructure in needle mesophyll cells than for application of AgNPs. Indeed, similar results were obtained by Lalau et al. ([22]), who found greater numbers of plastoglobules in the aquatic plant Landoltia punctata when it was treated with copper (II) oxide nanoparticles. Plastoglobules are subcompartments of thylakoids, containing enzymes that participate in lipid metabolic pathways. It is well documented that under conditions of biotic and abiotic stress, plastoglobules increase in both size and number ([38], [32]). Clearly, the results obtained demonstrate that the highest concentrations (50 ppm) of both NPs exert limited, but nevertheless phytotoxic, impacts.
To date, only a few studies concerning the influence of NPs on plant growth have been conducted on trees. However, in the cases that are known, involving willows treated with TiO2 nanoparticles, wild pear seedlings receiving nanoparticles of SiO2 and pedunculate oak seedlings treated with AgNPs and CuNPs (as here), negative influences on growth have not been reported ([50], [29]). Only the work by Sweet & Singleton ([43]) revealed a significant inhibitory impact of AgNPs on the growth of bishop pine. However, the fact that the research cited involved different species of trees precludes any comparison with our results. Nevertheless, many factors can affect the impact of NPs on plant growth, with target species of plant being regarded among the most important ([39]).
Scots pine is strongly dependent on ectomycorrhizal symbiosis ([46]). In our studies, ectomycorrhizal colonisation was between 65.5% (with AgNPs at 25 ppm) and 86.6% (with AgNPs at 5 ppm). The level of mycorrhizal colonisation depends on several factors, such as applied cultivation system (bare-root vs. container, outdoor vs. greenhouse), plant species ([25]) and the location and identity of the nursery in which seedlings are grown ([15]). It is typical for bare-root seedlings to achieve almost 100% mycorrhizal colonisation ([23], [31]), while the level is often lower in the case of container-grown seedlings ([25]).
In our case, ectomycorrhizal colonisation of the 2-year-old pines varied in relation to the types and concentrations of NPs applied. Use of CuNPs was observed to stimulate the formation of ectomycorrhizae irrespective of concentration. In contrast, with AgNPs, only the lowest concentration (5 ppm) produced increased colonisation of roots by ectomycorrhizal fungi, with the higher concentrations actively inhibiting the process. Given that the formation and further functioning of ectomycorrhizae is conditioned by a range of factors linked with both host species, fungal partner and the environment ([41]), it is possible to propose several hypotheses that might account for the results we obtained. For example, an inhibitory influence due to higher concentrations (25 and 50 ppm) of AgNPs might reflect the stronger antifungal properties of silver ([20]).
Ectomycorrhizal fungi are very much dependent on products of photosynthesis reaching plant roots ([14]). Many studies reveal how NPs have a favourable influence on chlorophyll content in plants and on the course of photosynthesis ([49], [24], [16]). Thus, the stimulation of the formation of mycorrhizae in our experiment may reflect larger amounts of sugars produced in photosynthesis reaching the seedling roots. This scenario does not have to equate to simultaneous stimulation of growth because seedlings with a higher level of mycorrhization are actually characterised by less-favourable growth parameters ([42]).
The formation and functioning of mycorrhizal symbiosis is influenced by not only the host plant and fungal partner but also interactions with mycorrhizal helper bacteria ([41]). The application of nanoparticles may increase the population of soil microorganisms and their activity in the rhizosphere ([33], [44]). Ge et al. ([13]) have shown that a large group of bacteria, including Bacillus, Pseudomonas, Lactococcus and Serratia, have no response to nanoparticles. As the mycorrhizal helper bacteria may be included in this group, the influence on mycorrhizal associations may be a positive one ([9]).
For the stimulated formation of ectomycorrhizae owing to the use of AgNPs at the lowest concentration, it is also possible to invoke the “ethylene hypothesis”. Ethylene is a plant growth hormone serving in a wide range of functions in plants. Its influence on physiological processes is not merely dose-dependent but also reflects the sensitivity of tissues changes in the course of the development of plant organs ([48]). Ethylene is also involved in plant responses to microbial pathogens and herbivorous insects, as well as in interactions between plants and beneficial microbes and insects ([4]). Ethylene treatment was found to impede fungal colonisation of roots during mutualistic symbiosis between Laccaria bicolour and Populus ([30]). Additionally, it is also known that silver ions block receptors for ethylene in cells, limiting the hormone action on the plant ([26]). Stimulated formation of mycorrhizae may occur in this way.
The roots of our pine seedlings were found to host ectomycorrhizal fungi of the species T. terrestris, S. bovinus and S. brunnea. However, T. terrestris was dominant in all experimental treatments, with a more limited role played by either S. bovinus or S. brunnea. Equally, significant differences from the control (receiving 0 ppm of AgNPs and CuNPs) were noted for treatments with the two types of nanoparticle in relation to the roles played by the different species of fungi. A higher proportion of S. bovinus and S. brunnea was observed where AgNPs and CuNPs applications were absent, while our results clearly demonstrated that the formation of mycorrhizae by these two species was obstructed by applications of both kinds of NP at all tested concentrations. Inhibition of S. bovinus and S. brunnea gave rise to a nearly 90% increase in the role played by what is normally only a weakly competitive species, i.e., T. terrestris ([45]). The major role played by the latter in our experiments may not be surprising as this species is a pioneer mycobiont well adapted to nursery conditions ([8]) and often is the dominant ectomycorrhizal fungus in nurseries ([15]). On the other hand, T. terrestris is regarded as only weakly competitive with other ectomycorrhizal fungi ([45]) and cannot survive, even in the absence of competing species, at sites adversely influenced by the presence of heavy metals ([41]). Silver and copper are typical heavy metals. Similarly, research by Sweet & Singleton ([43]) found that Thelephora sp. was very vulnerable to the action of AgNPs, whose presence was associated with the lack of the mycorrhizae typically formed by the species in infected soil. Furthermore, the high level of sensitivity of S. bovinus to the action of both NPs at all concentrations was surprising, given that Suillus fungi are regarded as very resistant to the impacts of industrial air pollutants, including heavy metals, when compared with other species. Attempts have even been made to use Suillus species in bioremediation ([10]). However, it is possible that the results we obtained reflected the high degree of variability present between one strain of the fungus and another ([2]). Perhaps, the strain of S. bovinus colonising the roots of pines in our experiment are among those more vulnerable to both of the nanoparticles used ([6]).
Conclusion
Both types of nanoparticles were found to have a stimulatory effect on both the growth of seedlings in Scots pine and their generation of ectomycorrhizae. The effect of the application of CuNPs was more favourable than that of AgNPs. Both types of nanoparticles produce minor changes in the ultrastructures of pine needles that relate to the chloroplasts (but only at a concentration of 50 ppm). Our results indicate the possibility of CuNPs and AgNPs being used as stimulators of the growth and mycorrhizal colonisation of container-grown Scots pine trees. However, further research is needed to determine the optimal doses and concentrations of the NPs.
Acknowledgements
MAT conceived of the study and wrote the manuscript, AS carried out the field experiment and lab measurements, MS performed the statistical analysis, MBB performed microscopic investigation, and AU identification of mycorrhizal fungi using PCR amplification.
This work was supported by the Rector of Warsaw University of Life Sciences (SGGW) within the framework of research projects 505-10-030400-L00373-99. Jacek Olchowik gratefully acknowledges the Austrian Agency for International Mobility and Cooperation in Education, Science and Research (OeAD) for supporting his stay at the University of Vienna in 2015.
The editorial help of James R.A. Richards, PhD, is also gratefully acknowledged.
List of abbreviations
The following abbreviations have been used throughout the paper:
- AgNPs: silver nanoparticles;
- CuNPs: copper nanoparticles;
- Nps; nanoparticles;
- TEM: transmission electron microscope.
References
Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Adam Szaniawski
Jacek Olchowik
Department of Forest Protection and Ecology, Faculty of Forestry, Warsaw University of Life Sciences, Nowoursynowska 159, 02-776 Warsaw (Poland)
Department of Experimental Design and Bioinformatics, Faculty of Agriculture and Biology, Warsaw University of Life Sciences, Nowoursynowska 159, Warsaw 02-776 (Poland)
Department of Botany, Faculty of Agriculture and Biology, Warsaw University of Life Sciences, Nowoursynowska 159, Warsaw 02-776 (Poland)
Faculty of Horticulture, Biotechnology and Landscape Architectur, Department of Plant Pathology, Nowoursynowska 159, Warszaw 02-776 (Poland) (since October 2018)
Department of Botany and Biodiversity Research, Faculty of Life Sciences, University of Vienna, Rennweg 14, Wien A-1030 (Austria)
Corresponding author
Paper Info
Citation
Aleksandrowicz-Trzcinska M, Szaniawski A, Studnicki M, Bederska-Blaszczyk M, Olchowik J, Urban A (2018). The effect of silver and copper nanoparticles on the growth and mycorrhizal colonisation of Scots pine (Pinus sylvestris L.) in a container nursery experiment. iForest 11: 690-697. - doi: 10.3832/ifor2855-011
Academic Editor
Claudia Cocozza
Paper history
Received: May 18, 2018
Accepted: Jul 22, 2018
First online: Oct 23, 2018
Publication Date: Oct 31, 2018
Publication Time: 3.10 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 46858
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 38966
Abstract Page Views: 3620
PDF Downloads: 3321
Citation/Reference Downloads: 6
XML Downloads: 945
Web Metrics
Days since publication: 2655
Overall contacts: 46858
Avg. contacts per week: 123.54
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2018): 11
Average cites per year: 1.38
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Conservation of Betula oycoviensis, an endangered rare taxon, using vegetative propagation methods
vol. 13, pp. 107-113 (online: 23 March 2020)
Short Communications
The effects of salicylic acid, oxalic acid and chitosan on damping-off control and growth in Scots pine in a forest nursery
vol. 13, pp. 441-446 (online: 24 September 2020)
Research Articles
Effect of silver nanoparticles on hardness in medium-density fiberboard (MDF)
vol. 8, pp. 677-680 (online: 17 December 2014)
Short Communications
Effect of four levels of shade on survival, morphology and chlorophyll fluorescence of Nothofagus alessandrii container-grown seedlings
vol. 8, pp. 638-641 (online: 08 January 2015)
Research Articles
Use of overburden waste for London plane (Platanus × acerifolia) growth: the role of plant growth promoting microbial consortia
vol. 10, pp. 692-699 (online: 17 July 2017)
Research Articles
Controlled-release fertilizers combined with Pseudomonas fluorescens rhizobacteria inoculum improve growth in Pinus halepensis seedlings
vol. 8, pp. 12-18 (online: 12 May 2014)
Technical Reports
Effects of different mechanical treatments on Quercus variabilis, Q. wutaishanica and Q. robur acorn germination
vol. 8, pp. 728-734 (online: 05 May 2015)
Research Articles
Effects of different nut pretreatments and substrates on germination and seedlings growth of Neocarya macrophylla Sabine in Basse Casamance, Senegal
vol. 17, pp. 346-352 (online: 03 November 2024)
Technical Reports
De novo adventitious root formations in mini-cuttings of Azadirachta indica in response to different rooting media and auxin treatments
vol. 8, pp. 558-564 (online: 09 December 2014)
Research Articles
Growth, morphology, and biomass allocation of recently planted seedlings of seven European tree species along a light gradient
vol. 13, pp. 261-269 (online: 03 July 2020)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword