The importance of tree species and size for the epiphytic bromeliad Fascicularia bicolor in a South-American temperate rainforest (Chile)
iForest - Biogeosciences and Forestry, Volume 13, Issue 2, Pages 92-97 (2020)
doi: https://doi.org/10.3832/ifor2710-013
Published: Mar 10, 2020 - Copyright © 2020 SISEF
Short Communications
Abstract
Bromeliads are a numerous family of vascular epiphytes, though only one epiphytic species inhabits South-American temperate rainforests: the endemic Fascicularia bicolor. This bromeliad is an important driver of canopy biodiversity, but attributes of its hosts are mostly unknown. Here we report (i) the tree species colonized by F. bicolor, (ii) the relationship between tree size and presence of F. bicolor and (iii) the relation between tree size and the number of mats of F. bicolor inhabiting each colonized tree. We sampled 231 trees in seven forest plots recording their species, diameter, heights, and the number of F. bicolor mats growing on them. The dataset was analyzed with a zero-inflated model to relate host tree attributes with F. bicolor occurrence and abundance in a single statistical approach. The occurrence and abundance of F. bicolor depend on host-species identity and diameter. F. bicolor colonization in slow-growing trees started at smaller DBH than that required for other tree species. Nonetheless, the overall occurrence of F. bicolor relies on large trees above 50 cm DBH for most host species. The number of mats occurring on each colonized tree depends on the interaction between tree height and species suggesting the importance of space available for colonization along the tree-trunk, and differential effects due to species’ traits. Currently, large trees and old-growth forests are scarce within the distribution range of F. bicolor, which could seriously affect the long-term conservation of this endemic epiphyte, along with the canopy properties and species associated with it.
Keywords
Forest Canopy, Epiphytes, Bromeliads, South American Temperate Forests
Introduction
Bromeliaceae is the second largest family among Neotropical vascular epiphytes, with 1770 epiphytic species, representing 60% of the family ([32]). Epiphytic bromeliads can provide important habitat for other canopy-dwelling organisms, fostering biodiversity in the upper layer of the forest. For example, tank bromeliads are known to retain water and debris in their rosettes, which support fully-fledged communities in the treetops. Another bromeliad, Tillandsia usneoides, creates intricate shelters which reduce predation risk to invertebrates ([1]). Epiphytic bromeliads can modify canopy environments by creating habitat patches with distinct characteristics, increasing beta-diversity ([1], [22]). Hence, threats to the conservation of bromeliad species could be detrimental for other canopy organisms.
The underlying ecological processes explaining the occurrence and the number of epiphytic bromeliads on individual trees - as well as other canopy dwelling plants - include the increase in surface available for colonization by epiphytic propagules during tree growth ([10]), the time that each tree has been available for epiphyte colonization ([18]), chemical or physical attributes of the bark ([12]), or the distance from propagule sources (i.e., neighbouring trees or stands - [23]). However, most of these processes are tied to tree ontogeny ([27]). As time passes, trees increase their diameter and height until they reach their maximum height, when only diameter and branches continue growing. Over time, branches create a complex crown, the bark of several tree species increases its roughness, and epiphyte colonization occurs whenever diaspores are able to reach their host. Once established, the epiphyte assembly creates its own dynamics, similar to those described in the crowns of long-lived trees in the Northern Hemisphere ([26], [14]). Beyond the value of the specific ecological processes that explain the colonization of epiphytes, most of these processes are intrinsically correlated to variables commonly recorded in forest inventories, such as tree species, diameter at breast height (DBH) and height. Thus, the knowledge about basic attributes of the host-trees is simple but critical information required to include epiphytes conservation in sustainable forest management.
Here we evaluate the relationship between the colonization of trees by Fascicularia bicolor (Ruiz & Pav.) Mez, and DBH, height and species of each host tree. Fascicularia is a single species genus with two subspecies, according to Zizka et al. ([30]): F. bicolor subsp. bicolor (mostly associated with coastal rocky areas) and the epiphytic F. bicolor subsp. canaliculata (hereafter referred to as F. bicolor). The latter subspecies is a trash-basket epiphyte whose mats capture a large amount of organic debris in the forest canopy ([7], [22]). Like other trash-basket epiphytes, F. bicolor influences the presence and abundance of other epiphytic plants and invertebrates in the vertical profile of the forest by creating habitat patches on host trees ([22]). Despite its potential importance for canopy biodiversity, no specific studies relating F. bicolor with the attributes of its hosts have yet been conducted.
While not included in the IUCN or Chilean red lists, Zizka et al. ([31]) recommend monitoring population trends of F. bicolor canaliculata. Besides, a recent industrial project involving forest intervention has proposed to relocate F. bicolor individuals as part of their environmental compensation measures ([5]). However, the selection of host-trees and other environmental management practices concerning F. bicolor rely on anecdotal information and field observations, without a quantitative background. In this context, the goals of our research were to determine: (i) which tree species were colonized by F. bicolor; (ii) what tree size indicated the potential break-point at which tree individuals become suitable hosts for F. bicolor; and (iii) the relation between the number of mats of F. bicolor and host tree species and size. We provide basic knowledge about the host-trees of F. bicolor as a first step to include the species in conservation and management plans of the threatened South American temperate rainforest (SATR - [21]).
Material and methods
Study area
This study was conducted in Parque Oncol (39° 41′ S, 73° 20′ W), a private protected area in the Coastal Range of Valdivia, southern Chile. Parque Oncol is made up of 754 hectares of old-growth and secondary forests between 500-710 m a.s.l. The study site is surrounded by a matrix of exotic pine tree plantations, agricultural grasslands and native forests, the latter with varying degrees of human disturbance. The forest is dominated by broad-leaved evergreen species such as Laureliopsis philippiana (Looser) Schodde (Atherospermataceae), Saxegothaea conspicua Lindl. (Podocarpaceae), Eucryphia cordifolia Cav. (Cunoniaceae) and Drimys winteri J.R.Forst. & G.Forst. (Winteraceae - [22]). The Oncol area was subject to selective logging by the locals up to 1985 (P. Alba, pers. comm.). Then, a Chilean timber company acquired Oncol, and transformed it in a natural reserve in 1989 as a measure of environmental compensation ([13]).
Study design
We established seven 20 × 20 m plots in the old-growth forest in Parque Oncol, with elevation ranging from 500 to 600 m a.s.l. Plots were located at least 100 m from each other. In each plot, we recorded species, DBH, and the height of all trees with DBH greater than 5 cm. Tree height was measured with a hypsometer when possible or estimated by measuring neighbouring trees when necessary. Standing dead trees were grouped as “snags”, since we could not identify their original species.
We performed a ground-based census to record the number of F. bicolor mats growing on each tree, using binoculars (Celestron® outland 10 × 40, CA, USA) when required. Fascicularia bicolor occurs in large mats between 0.5 and 23.2 metres above the forest floor ([22]), and no similar epiphytic species inhabit SATR; therefore, the presence of mats was easily determined from a ground-based perspective. Mats include from one to multiple rosettes growing together, but individual rosettes cannot be counted or measured from the ground. Therefore, we counted each full mat as a proxy of the number of successful colonization events (at least one individual established and passed the seedling stage). We did not count F. bicolor seedlings (plants whose leaves were about 15 cm in length or smaller) both because of the low probability of detecting the ones growing at high height on trees and their uncertain long-term survival rate.
Data analysis
Since the response variable contained many zeros, we used a Zero-Inflated Poisson (ZIP) model to analyse the relationship between the presence of F. bicolor in the sampled trees with DBH, height, and tree species. Zero-Inflated Poisson models are two parts models that fit Poisson and binomial distributions to datasets with a large amount of zeros. Binomial distribution is applied under the assumption that the excess of zeros in the data is produced by the existence of true and false zeros. Then, counts and false zeros (or structural zeros) are modelled with a Poisson distribution. In our case, false zeros could be trees where only seedlings were growing or those with mats that could not be seen from the ground. An additional variable named tree size index (TSI) was added to the dataset as a proxy for the joint effect of height and DBH. We calculated the TSI using the formula to estimate the lateral surface of cones (eqn. 1):
We emphasise that TSI is not a measure of tree surface, since branches and trunk deviations are not considered. However, TSI allowed to evaluate the joint effect of DBH and height on the abundance of F. bicolor mats without testing the interaction between both, thereby decreasing model complexity.
We build a set of nine full models alternating DBH, height, and TSI as fixed effects in the two parts of the model (counts and zeros). In addition, species and the two-way interactions between species and DBH, height, and TSI were included as fixed effects in the count portion of the models. Plot was considered as random effect in both the parts of the models, whereas species was added as random factor in the zero-excess part. Then, we fitted all the possible reduced models by removing interactions and fixed effects from the original set (see Tab. S1 in Supplementary material). The most parsimonious model was selected for interpretation with the corrected version of Akaike’s information criterion ([3]). Snags, host species with less than five trees found, those with only one tree colonized by F. bicolor, and species where F. bicolor was completely absent were excluded from regression analyses, because their inclusion produced unreliable results or complete separation issues. We applied a complimentary Chi-squared test to examine if the number of host-trees per species was associated with tree species abundance in the forest plots. The latter analysis was performed in a data subset with individuals larger than 34 cm DBH of all species (the minimum DBH of a colonized tree in our dataset). Statistical tests were performed in R ver. 3.6.1 ([24]) using the package “glmmTMB” ([2]).
Results
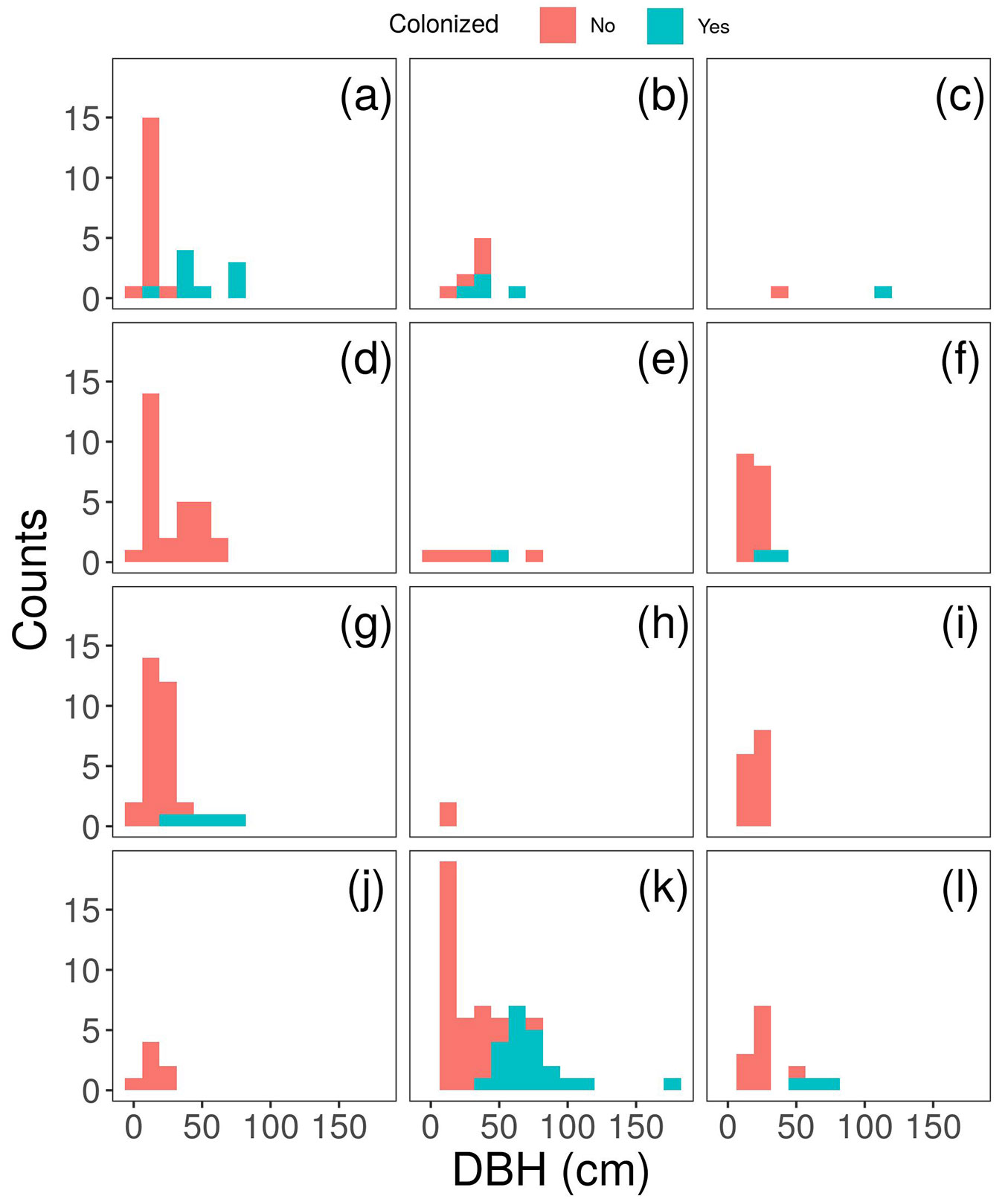
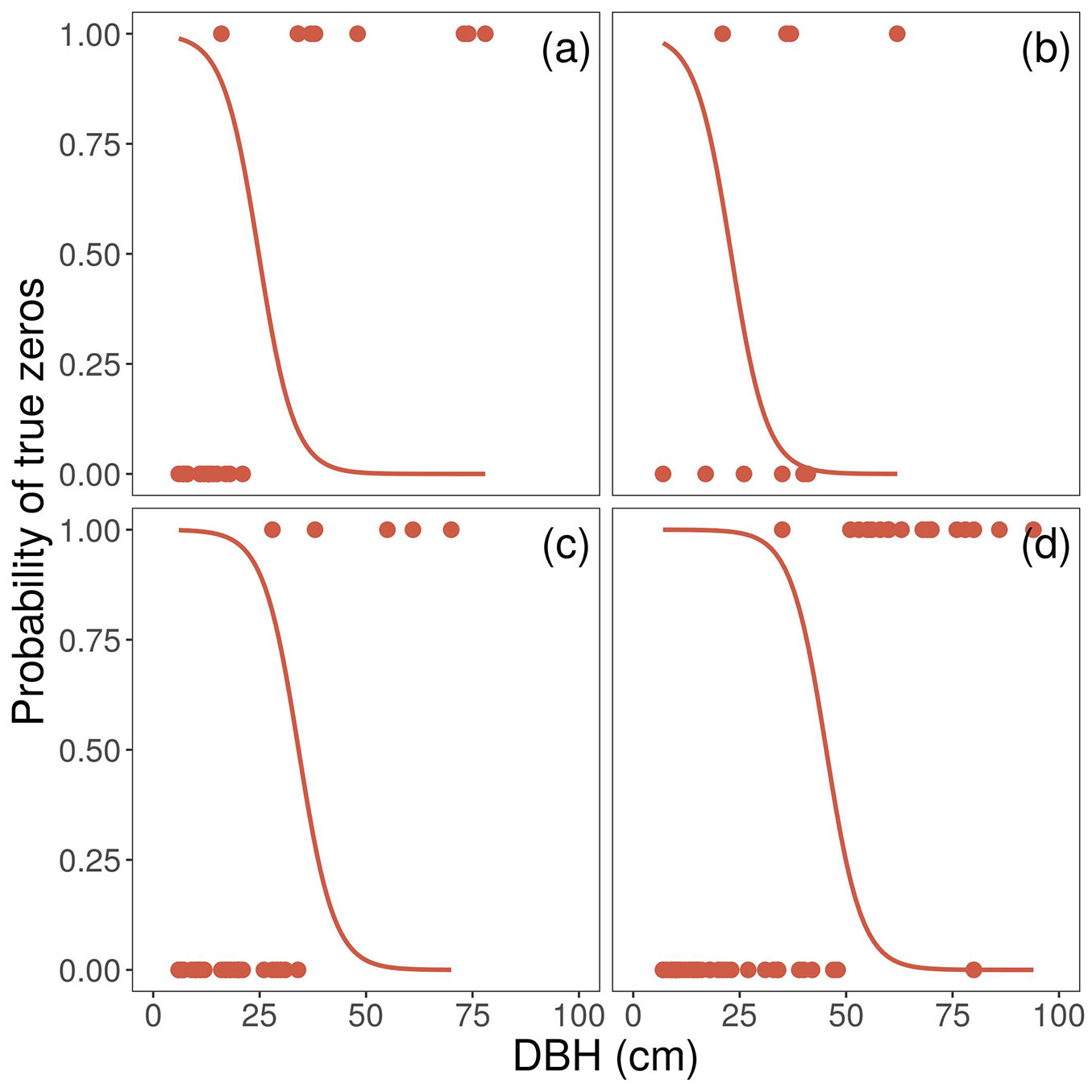
We found 15 tree species totalling 231 individual trees and snags (Tab. 1) with DBH ranging from 5 to 181 cm (Fig. 1), and heights between 3 and 26 m. The most common tree species was Saxegothaea conspicua, followed by Laureliopsis philippiana, Drimys winteri and Amomyrtus luma (Molina) D.Legrand & Kausel (Myrtaceae) (Tab. 1). The DBH distribution was typically skewed with a high abundance of small trees and fewer large ones (Fig. 1). Mats of F. bicolor were found on 20% of the sampled trees, with most of them on S. conspicua (Tab. 1). Colonized trees tend to have higher DBH than non-colonized individuals in each species. No mats of F. bicolor were found on D. winteri or Podocarpus nubigenus Lindl. (Podocarpaceae), despite their high abundance compared to other tree species at the study site (Tab. 1). The number of colonized trees was not related to the total abundance of species (χ2 = 28.8, p<0.001). Our final ZIP model included an interaction between height and species in the count portion and DBH in the zero inflated part (Tab. 2). The probability of having true zeros decay up to 50% between 25 and 50 cm DBH for the species included in the model (Fig. 2). The minimum height of a colonized tree was 10 m (Fig. 3).
Tab. 1 - Total sampled trees per species, number of colonized trees and total mats of F. bicolor found on each colonized tree species in Parque Oncol. Physical features of the bark were classified on a scale from low (+) to high (+++) importance of each variable based on personal observations. Minus sign (-) show the absence of the corresponding feature. Shade tolerances are shown as intolerant (+), semi-tolerant (++), and tolerant (+++) according to Lusk ([17]), Gutiérrez & Huth ([11]), and Donoso Zegers ([9]).
| Species | Bark features | Shade tolerance |
Total trees |
Colonized trees |
Total mats |
||
|---|---|---|---|---|---|---|---|
| Roughness | Peeling | Fissured | |||||
| Amomyrtus luma | - | ++ | - | +++ | 26 | 9 | 50 |
| Amomyrtus meli | - | +++ | - | +++ | 10 | 4 | 7 |
| Dasyphyllum diacanthoides | + | - | + | ++ | 3 | 1 | 22 |
| Drimys winteri | + | - | - | ++ | 30 | 0 | 0 |
| Eucryphia cordifolia | +++ | - | + | ++ | 7 | 1 | 1 |
| Gevuina avellana | + | - | - | ++ | 19 | 2 | 2 |
| Laureliopsis philippiana | ++ | - | - | +++ | 34 | 5 | 30 |
| Lomatia ferruginea | ++ | - | - | + | 1 | 0 | 0 |
| Myrceugenia parvifolia | ++ | + | - | ++ | 1 | 0 | 0 |
| Myrceugenia planipes | ++ | + | - | +++ | 3 | 0 | 0 |
| Ovidia pillopillo | + | - | - | + | 1 | 0 | 0 |
| Podocarpus nubigenus | ++ | + | ++ | ++ | 15 | 0 | 0 |
| Raukaua laetevirens | + | - | - | + | 8 | 0 | 0 |
| Saxegothaea conspicua | + | + | +++ | +++ | 57 | 22 | 161 |
| Weinmannia trichosperma | + | - | + | + | 1 | 0 | 0 |
| Snags | - | - | - | - | 15 | 3 | 22 |
| Total Result | - | - | - | - | 231 | 47 | 295 |
Fig. 1 - Diameter at breast height distribution per tree species in Parque Oncol, Chile. Colours show non-colonized (red) and colonized (green) trees. Panels: (a) Amomyrtus luma, (b) Amomyrtus meli, (c) Dasyphyllum diacanthoides, (d) Drimys winteri, (e) Eucryphia cordifolia, (f) Gevuina avellana, (g) Laureliopsis philippiana, (h) Myrceugenia planipes, (i) Podocarpus nubigenus, (j) Raukaua laetevirens, (k) Saxegothaea conspicua, and (l) snags. Lomatia ferruginea, Myrceugenia parvifolia, Ovidia pillopillo, and Weinmannia trichosperma were excluded because only one individual of each species was found (with 5, 8, 25, and 31 cm DBH, respectively).
Tab. 2 - Estimated parameters of the selected Zero-Inflated Poisson model for the number of F. bicolor mats. Counts of mats were fitted to a conditional model with Poisson distribution (cond), while the zero inflation was evaluated with a logistic model (zi). Intercept (cond) corresponds to Amomyrtus luma. (A:B): interaction terms. Random effects are not shown. A full model selection table is reported in Tab. S1 (Supplementary material).
| Parameter | Estimate | Standard error |
z-value | p-value |
|---|---|---|---|---|
| Intercept (cond) | 2.91 | 0.92 | 3.16 | <0.01 |
| Height (cond) | -0.13 | 0.05 | -2.42 | 0.02 |
| Amomyrtus meli (cond) | -5.07 | 2.20 | -2.31 | 0.02 |
| Laureliopsis philippiana (cond) | -2.76 | 1.81 | -1.52 | 0.13 |
| Saxegothaea conspicua (cond) | -1.94 | 0.90 | -2.17 | 0.03 |
| Height:Amomyrtus meli (cond) | 0.29 | 0.13 | 2.28 | 0.02 |
| Height:Laureliopsis philippiana (cond) | 0.16 | 0.09 | 1.68 | 0.09 |
| Height:Saxegothaea conspicua (cond) | 0.22 | 0.06 | 3.62 | <0.01 |
| Intercept (zi) | 7.68 | 2.25 | 3.41 | <0.01 |
| DBH (zi) | -0.24 | 0.07 | -3.61 | <0.01 |
Fig. 2 - Probability of true zeros. Points show trees colonized and not colonized by F. bicolor. Lines represent the predicted probability of true zeros. Tree species are shown in panels in the following order: (a) Amomyrtus luma, (b) Amomyrtus meli, (c) Laureliopsis philippiana, and (d) Saxegothaea conspicua.
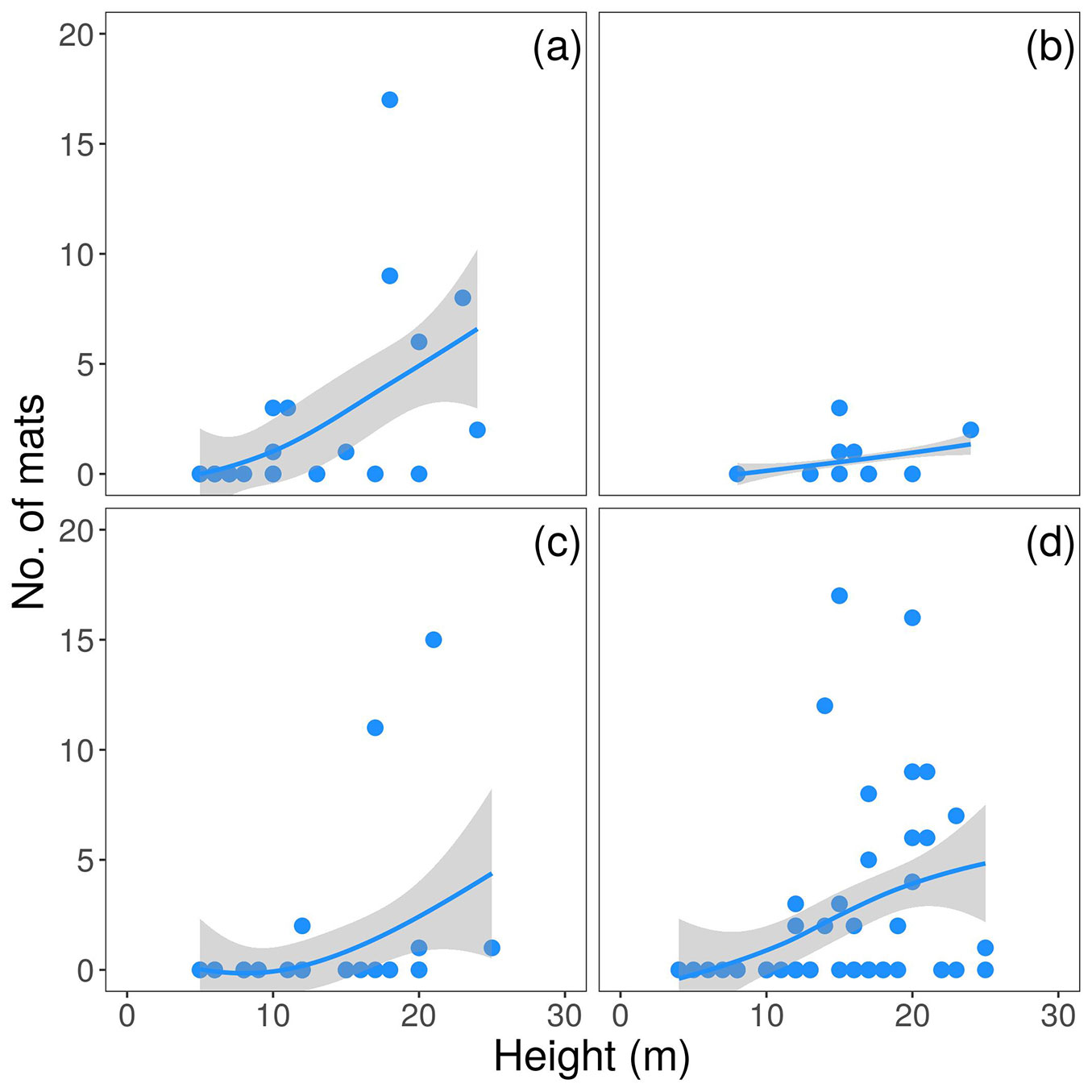
Fig. 3 - Observed (points) and predicted (lines) number of mats per host tree species. Lines show probably LOESS curves fitted to the data. (a) Amomyrtus luma, (b) Amomyrtus meli, (c) Laureliopsis philippiana, and (d) Saxegothaea conspicua.
Discussion
The epiphyte Fascicularia bicolor colonizes many, but not all the tree species in our study site. This could be related to multiple variables, such as bark properties and the processes related to the ontogeny of each tree species (e.g., increase in bark roughness, longevity, size and structural changes in the trunk and branches). For instance, the large and long-living conifer S. conspicua (>750 yrs - [17]) develops a hollow trunk and generates adventitious roots along its internal walls which provide continuous structural strength to assure tree survival ([8]). Such features make large individuals of S. conspicua to have sinuous shapes, cavities, and uneven wrinkled surfaces along the main trunk and branches, which could facilitate the accumulation of detritus, small vascular and non-vascular epiphytes, and arboreal soil, followed by the establishment of large epiphytes like F. bicolor.
The absence of F. bicolor on D. winteri is consistent with Muñoz et al. ([20]), who related low epiphyte richness and abundance on D. winteri to its smooth bark. However, in our study F. bicolor was found on A. luma and A. meli, which have a smoother and decorticating bark ([8]). Other epiphytic bromeliads have also been reported inhabiting tree species with peeling bark ([16]). This evidence suggests that factors other than smooth bark could also explain the absence of F. bicolor on D. winteri. For instance, the bark of D. winteri contains tannins, alkaloids and other substances ([29]) which could negatively affect the establishment of epiphytes. In addition, Drimys winteri reaches large sizes in a short time, while A. luma and A. meli have long lifespans with slow growth rates ([8]), suggesting that the age of the trees could be an important factor. As an example, in an ongoing study, two 30 cm DBH cross sections of A. luma showed an age of around 185 years, while the core of a living tree of D. winteri 1 m in DBH showed an age of 200 years (Díaz & Christie, unpublished data). The slow growth rates of A. luma could also explain why the probability of finding a colonized tree reaches 50% at a lesser DBH than required for other hosts (Fig. 2).
Muñoz et al. ([20]) indicated that large trees of P. nubigena are a common host for many epiphytic species in the SATR; however, we found no P. nubigena individuals colonized by F. bicolor. According to local people, P. nubigena was intensively logged in the area for timber (P. Alba, pers. comm.), and nowadays it is difficult to find large individuals of this species (Fig. 1). We only found P. nubigena individuals with a DBH less than 50 cm, which could explain the absence of F. bicolor mats growing on them. Logging before 1985 could also be related to the scarcity of large E. cordifolia or W. trichosperma individuals. Eucryphia cordifolia is a highly valued source of firewood in southern Chile and W. trichosperma was commercialized to extract tannins for the leather industry ([25]). However, no stumps or other evidence was found to support that logging took place within our study plots. Other sampled species, such as the understory tree L. ferruginea and the hemiepiphytic R. laetevirens do not reach large sizes.
Regarding the number of mats per tree, the interacting effect of height and species suggests height to increase the availability of microsites along the tree-trunk (Fig. 3), but the intensity of such an effect depends on the tree species. Bromeliads suffer dispersal limitation, which could involve that once a tree has been colonized many propagules from the first colonizer individual would establish on the same host or neighbouring trees ([4]). Therefore, it is likely that the higher the first F. bicolor’s individual become established in a host, the larger amount of microsites will be available for the next generation along the tree trunk. As indicated before, the influence of DBH on the probability of finding a colonized tree could result not only from an increased size but from the time that each tree has been available for colonization (Fig. 2).
Implications for sustainable forest management
The functional roles and biomass input of F. bicolor are noteworthy, considering that it can be found associated with forests between 33° and 42° S, one of the most threatened ecosystems on the planet ([21]). Much of these forests are secondary and highly degraded ([6]). The lack of large trees in second-growth or degraded forest stands can limit the long-term viability of local populations of F. bicolor and its ecological role in the forest. This epiphyte is associated with 50% of arboreal soils and epiphytic green tissues ([7]), enhancing the cover of vascular epiphytes ([22]), and providing habitat to invertebrates living along the vertical profile of trees ([22], [28]). Although the conservation status of F. bicolor has not been assessed in the current IUCN Red List, monitoring of population trends is recommended ([31]).
Despite protection efforts, forests in southern Chile are still subject to illegal logging to produce firewood and charcoal ([19]). Therefore, producing basic ecological knowledge to support the development of sustainable forestry in the SATR is necessary. Here we show that conserving tree species large-sized, and with a wrinkled surface (such as Saxegothaea conspicua) could be beneficial for epiphytes like F. bicolor. However, there are open questions regarding dispersal strategies of F. bicolor, or the effects of physical and chemical attributes of the tree-bark on propagule establishment. We also emphasize that further research is needed to elucidate host-tree requirements of other epiphytic species, and to integrate such information in forest management strategies.
Conclusions
Our findings provide an initial approach to evaluate the characteristics necessary to support a substantial population of F. bicolor within a forest stand. F. bicolor does not colonize all tree species, but large trees of its host species could be crucial for the establishment and population’s viability of this bromeliad. Large trees are complex organisms that can support many other species due to their attributes: rough bark, trunk cavities, horizontal and well-developed branches among others ([15]). These features offer a wide microhabitat range, absent in young small trees. Here, we focused on the host’ requirement for F. bicolor, but additional research is needed to elucidate the potential changes in canopy biodiversity related to local extinctions of this large epiphyte.
Acknowledgements
We would like to express our gratitude to the administration and rangers of the Parque Oncol (Valdivia, Chile) for their help throughout the development of this research. We also want to thank Christine Harrower for her valuable service as our English language editor. GO was supported by a doctoral scholarship by the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT). The manuscript was greatly improved by the comments provided by two anonymous reviewers.
References
Gscholar
Gscholar
CrossRef | Gscholar
Supplementary Material
Authors’ Info
Authors’ Affiliation
Unidad de Gestión Ambiental, Dirección de Servicios, Vicerrectoría de Gestión Económica y Administrativa, Universidad Austral de Chile, Las Encinas 220, Valdivia (Chile)
Daniela Mellado-Mansilla
Javier Godoy
Laboratorio de Biodiversidad y Ecología del Dosel, Instituto de Conservación, Biodiversidad y Territorio, Facultad de Ciencias Forestales y Recursos Naturales, Universidad Austral de Chile, Independencia 641, Valdivia (Chile)
Department of Palynology and Climate Dynamics, Albrecht-von-Haller-Institute for Plant Sciences, University of Göttingen, Untere Karspüle 2, 37073 Göttingen (Germany)
Laboratorio de Ecoinformática, Instituto de Conservación, Biodiversidad y Territorio, Facultad de Ciencias Forestales y Recursos Naturales, Universidad Austral de Chile, Independencia 641, Valdivia (Chile)
Escuela de Graduados, Facultad de Ciencias Forestales y Recursos Naturales, Universidad Austral de Chile, Independencia 641, Valdivia (Chile)
Corresponding author
Paper Info
Citation
Ortega-Solís G, Díaz I, Mellado-Mansilla D, Moreno-González R, Godoy J, Samaniego H (2020). The importance of tree species and size for the epiphytic bromeliad Fascicularia bicolor in a South-American temperate rainforest (Chile). iForest 13: 92-97. - doi: 10.3832/ifor2710-013
Academic Editor
Michele Carbognani
Paper history
Received: Dec 08, 2017
Accepted: Jan 04, 2020
First online: Mar 10, 2020
Publication Date: Apr 30, 2020
Publication Time: 2.20 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2020
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 43859
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 36913
Abstract Page Views: 3636
PDF Downloads: 2469
Citation/Reference Downloads: 1
XML Downloads: 840
Web Metrics
Days since publication: 2169
Overall contacts: 43859
Avg. contacts per week: 141.55
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2020): 3
Average cites per year: 0.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Taxonomic distinctness of climbing plants and epiphytes in central-Chilean forests: an alternative diversity measure from unequal species lists
vol. 18, pp. 283-292 (online: 20 October 2025)
Technical Reports
Remote sensing of american maple in alluvial forests: a case study in an island complex of the Loire valley (France)
vol. 13, pp. 409-416 (online: 16 September 2020)
Research Articles
The estimation of canopy attributes from digital cover photography by two different image analysis methods
vol. 7, pp. 255-259 (online: 26 March 2014)
Research Articles
Is methane released from the forest canopy?
vol. 4, pp. 200-204 (online: 03 November 2011)
Research Articles
Influences of forest gaps on soil physico-chemical and biological properties in an oriental beech (Fagus orientalis L.) stand of Hyrcanian forest, north of Iran
vol. 13, pp. 124-129 (online: 07 April 2020)
Short Communications
Estimation of canopy attributes of wild cacao trees using digital cover photography and machine learning algorithms
vol. 14, pp. 517-521 (online: 17 November 2021)
Research Articles
Vertical position of dry mass and elemental concentrations in Pinus sylvestris L. canopy under the different ash-nitrogen treatments
vol. 8, pp. 838-845 (online: 25 March 2015)
Research Articles
Quantifying the vertical microclimate profile within a tropical seasonal rainforest, based on both ground- and canopy-referenced approaches
vol. 15, pp. 24-32 (online: 27 January 2022)
Technical Advances
Thermal canopy photography in forestry - an alternative to optical cover photography
vol. 8, pp. 1-5 (online: 07 May 2014)
Research Articles
A new zoning index for detecting areas of biological importance applied to a temperate forest in Central Mexico
vol. 16, pp. 253-261 (online: 31 August 2023)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword