Carbohydrate metabolism during new root growth in transplanted Larix olgensis seedlings: post-transplant response to nursery-applied inorganic fertilizer and organic amendment
iForest - Biogeosciences and Forestry, Volume 10, Issue 1, Pages 15-22 (2016)
doi: https://doi.org/10.3832/ifor1988-009
Published: Sep 22, 2016 - Copyright © 2016 SISEF
Research Articles
Abstract
Sustainable agriculture often requires the incorporation of organic matter into cultural protocols as an amendment to mitigate problems caused by chemical inputs, but the responses of transplanted seedlings to such additions have not been well quantified. In this study, bare-root Changbai larch (Larix olgensis Henry) seedlings were applied with 100 or 200 kg nitrogen (N) ha-1 of inorganic fertilizer with or without chicken manure added at a rate of 10.000 kg ha-1 during nursery cultivation, obtaining four treatment combinations designated as F100+, F200+, F100-, and F200-, respectively. Over-winter seedlings were transplanted into pots and placed in a growth chamber, where the carbohydrate metabolism, biomass accumulation, root respiration, and new root number were quantified. Both initial soluble sugar and total non-structural carbohydrate (TNC) accumulation were the lowest in the F100+ treatment. However, two months later, root soluble sugar content was the highest in this treatment, while coarse-root (diameter > 2mm) carbohydrate content was the highest in the low rate of inorganic fertilizer treatment. During the two-month post-transplant period, the net carbohydrate accumulation rate (NCAR) for starch was negative for all treatments, but the NCAR value for soluble sugars was the highest in the F100+ treatment at both the root and whole-plant scales. Relative to the F200- treatment, the NCAR value for soluble sugars, final sugar content, and biomass accumulation in coarse roots, respiration rate of fine roots (diameter ≤ 2 mm), and new root number were all greater in the F100+ treatment, while new root number was increased by organic matter additions. In conclusion, the use of chicken manure as an organic amendment had the potential to enhance transplanted larch seedling performance by improving post-transplant new root number, but this application must be considered within the context of the interaction between organic amendment treatments and inorganic fertilizer applications.
Keywords
Changbai Larch, Organic Additive, Mineral Fertilizer, New Root Egress, Starch, Soluble Sugars
Introduction
Larch species (Larix spp.) have both ecological and commercial values in their native range. They are the focus of many restoration, afforestation, and commercial reforestation efforts in both boreal and temperate forests of the northern hemisphere ([25], [1]). However, larch seedlings are facing many challenges to successfully establish due to harsh environments caused by climate change and soil degradation ([35], [15]). Consequently, to maintain low mortality and high productivity of newly planted larch seedlings through improving seedling quality will have both economic and ecological ramifications for larch afforestation.
Root characteristics strongly influence the performance of transplanted tree seedling ([8]). Seedling root quality is considered as a critical parameter relating to post-transplant seedling performance ([1]) in terms of architecture and developmental patterns ([42]). At nursery stage, nitrogen (N) fertilization has been proven to foster seedling root growth, and subsequently to enhance post-transplant seedling performance ([8], [9]). However, over-application of inorganic N fertilizers beyond the optimal rate may induce inherent toxicity in seedlings ([28], [14]) and result in chemical contamination of groundwater through leaching ([20], [25]). Sustainable agriculture often requires organic matter to be incorporated into cultural protocols as an amendment to mitigate problems associated with chemical inputs and to improve soil physical properties ([37]). Organic matter may be added to soils as a substitute for some inorganic fertilizers during tree seedling cultivation so as to alleviate the potential negative impacts of chemical inputs on the environment. For larch seedlings, simultaneous uses of both organic amendments and inorganic fertilizer were found to have a positively interactive effect on nutrient reserves and root quality during nursery culture ([37]). However, to our knowledge, current literature has not documented sufficient information about post-transplant larch seedling performance with regard to the responses of root growth and carbohydrate metabolism to nursery-applied organic amendments and inorganic fertilizer.
New root production in post-transplant tree seedlings has been demonstrated to rely on stored carbohydrates for several tree species ([30], [32]). Stored carbohydrates are required by newly planted seedlings not only as metabolic substrates but also as regulatory signals for new root growth ([41]). A high proportion of total non-structural carbohydrates (TNC) are consumed by root respiration ([11], [12]) for maintaining ion gradients across cell membranes and for the maintenance and replacement of proteins, membranes, and other constituents. Roots within different diameter classes have different respiration rates ([5], [19]), mostly due to changes in diameter-related surface-areas without significant changes in lignification area. However, root carbohydrate metabolism during new root growth has not been thoroughly investigated in newly planted seedlings. It is not clear whether the response of carbohydrate metabolism would change if post-transplant seedlings had different initial carbohydrate statuses.
In China, about 31% of the total forested area is in the northeast region of the country (including Heilongjiang, Jilin, and Liaoning Provinces), corresponding to a forested area of ~47×104 km2 ([46]). Larch has been promoted for planting as a typical fast-growing tree species by government policy in China for years ([47]). The northeast Chinese region accounts for 73% (~4.7 million hectares) of the total larch plantations in China ([44]). Changbai larch (Larix olgensis Henry) is one of the most important larch species in Northern China and many other areas of eastern Asia. Recently, it was reported that Changbai larch plantations are declining in distribution or being replaced by other tree species, which was partly the result of low quality of locally cultured Changbai larch seedlings ([39], [40], [14]). In a previous study, we found that the interaction between a nursery-applied organic amendment and inorganic fertilizer had a positive effect on the quality of Changbai larch seedlings ([37]). This implied that Changbai larch seedlings receiving these nursery treatments would exhibit improved post-transplant performance, but currently no evidence is available to support this hypothesis. In the present study, the same lots of seedlings cultured from the previous study were transplanted to a controlled environment to quantify root performance characteristics. It was hypothesized that: relative to the application of inorganic fertilizer at nursery stage, the combined effects of organic amendment and inorganic fertilizer would result in (1) more carbohydrate reserves and biomass accumulation, (2) higher rates of root respiration, and (3) more new roots.
Materials and methods
Nursery culture of seedlings and fertilization treatments
Changbai larch seedlings were cultured as bare-root stocks in an open-air nursery (43° 45′ N, 126° 45′ E) near Jilin City, Jilin Province, Northeast China. In local region, the annual precipitation is 650 to 750 mm with less than 200 mm from May to mid-June, and the mean annual temperature is 3 to 5 °C with a mean temperature of 6.5 °C in the early growing season. Soils in the nursery were determined to have 4.79 mg kg-1 NH4+-N, 192.88 mg kg-1 NO3--N, 1.60 mg kg-1 available P, 0.10 dS m-1 EC2.5, and a pH of 6.51. More specific soil properties can be found in Wei et al. ([37]).
Seeds were collected by employees of Xiaobeihu Forest Station (44° 03′ N, 128° 28′ E) from a local wild stand in Heilongjiang Province, Northeast China. After collection, seeds were transported to the nursery and stored at temperature 0-4 °C. Before sowing, locally prescribed ammonium phosphate (18-46-0) was applied into surface soils (0-20 cm) at a rate of 54 kg N ha-1 in late April. The use of ammonium phosphate and its application rate has been employed as a practical fertilization protocol for Larch culture in Jilin for many years ([36]). Seeds were soaked in 5% potassium permanganate (w/w) solution for 24 h, stratified for 5 days at 0-4 °C, and sown at a density of 700 seeds m-2 on 3 May 2009. In mid-June 2009, germinated seedlings were thinned to a density of about 550 seedlings m-2. Thinned seedlings were then fertilized at the rate of 200 kg N ha-1 (F200) using ammonium nitrate (21-0-0) and urea (46-0-0). This rate was performed as the standard practical dose for inorganic fertilizer application of bare-root Changbai larch seedlings locally in Jilin ([37], [14]). The other inorganic fertilizer treatment was applied at a lower rate of 100 kg N ha-1 (F100), which was found to result in a fair seedling quality at the end of the nursery production stage ([38]). Chicken manure was chosen as the organic additive, which had undergone natural decomposition outdoors for nearly 12 months prior to the experiment and consisted of a local mixture of chicken feces and some soil from the southeastern part of the nursery. Chemical analysis revealed that total N, P and K contents within the chicken manure were 11.93, 8.22 and 12.34 g kg-1, respectively, with an EC of 3.51 dS m-1 and a pH of 8.23. High pH values above 8.0 have also been reported in other chicken manures and their mixtures in compost in other regions ([10], [17]). More specific properties of the chicken manure used in the present study can be found in Wei et al. ([37]). Chicken manure was incorporated evenly into amended soils to a depth of 10-15 cm by hand-raking at a rate of 0 (-O) or 10.000 kg (fresh weight) ha-1 (+O). Chicken manure was applied immediately after the seedling beds were shaped but before the application of basal inorganic fertilizer in late April. Therefore, fertilization treatments were performed as two inorganic fertilizer regimes (the F100 and F200 treatments) combined with or without an organic matter addition (the +O and -O treatments, respectively), which was conducted as a 2 × 2 factorial design containing 4 combined treatments (F100+, F100-, F200+, F200-). Therein, the combined treatment of inorganic fertilizer without organic matter added as an amendment (F200-) was considered to be the control treatment. This treatment is also used as the standard practice for fertilization of bare-root Changbai larch seedlings locally in Jilin Province ([37]). The experiment was arranged as a randomized complete block design. Three replicated plots were randomly placed for one combined treatment. Each plot had an area of 1 × 1 m with 0.1 to 0.2 m-width buffers between adjacent plots, where plastic barriers were inserted to a depth of 0.2 m to eliminate the potentially lateral movement of nutritional elements between plots.
Seedling sampling and over-winter storage
On 12 October 2009, when seedlings had formed apical buds and needles were about to fall, they were carefully hand-lifted with a shovel so as to maintain the whole root system intact. Excavated seedlings were separated into three categories according to their sizes. Sixteen seedlings per nursery plot were then randomly collected from the large-sized seedling category (height ≥ 11 cm, root collar diameter - RCD ≥ 3 mm). Rhizosphere conditions were assumed to be uniform for all investigated seedlings. Large seedlings were used because they met the top I grade of the national standard for larch seedling quality in China and are the preferred size class for use in local larch afforestation. Sampled seedlings were grouped and labeled by treatment combination and nursery plot, then carefully washed to clean residual soil from roots using tap water and rinsed with distilled water. Roots were handled carefully throughout this procedure to avoid unnecessary damage. Cleaned seedlings were then transported to the Laboratory of Silviculture and Forest Management, Beihua University (43° 84′ N, 126° 61′ E), Jilin City, Jilin Province, Northeast China, and stored in darkness at 0-4 °C to overwinter.
Growth chamber transplant experiment
On 22 April 2010, one hundred and ninety-two seedlings, i.e., 16 seedlings per plot at nursery stage, were transported on ice to the laboratory of Beijing Forestry University (40° 00′ N, 116° 35′ E). Two seedlings per plot at nursery stage were measured for their initial height, RCD, and biomass, while an additional two seedlings were destructively harvested to determine initial shoot and root biomass and carbohydrate content. Hence, a total of 48 seedlings were used for initial measurements. The other 144 seedlings (12 seedlings per plot at nursery stage) were moved to an artificially controlled climatic chamber in the Experimental Forest Station of Beijing Forestry University (40o 07′ N, 116o 11′ E) at Jiufeng Forest Park, Beijing, China. Seedlings were transplanted into pots (10 × 7 × 20 cm, top diameter × bottom diameter × height, one seedling per pot) filled with 1:1 (V/V) Pindstrup® substrate (SP., Pindstrup Mosebrug A/S, Ryomgaard, Denmark) and vermiculite. In the climatic chamber, day/ night air temperature was set to be 26/20 °C with 70% relative humidity (RH) and an 18 h photoperiod. An RH of 70% approximates the annual ambient RH of the native range of Changbai larch near Changbai Mountain ([45]). This RH also approximates to occur at one m height aboveground where bare-root Changbai larch seedlings are cultured in Jilin ([43]). Illumination was provided by 200-W plant growth lamps (OudiTM Co., Huzhou, Zhejiang, China), which promised a photosynthetic photon flux density (PPFD) to be 150 µmol m-2 s-1 measured at seedling apical tip. The 18-h photoperiod has been considered sufficient for seedling growth and a PPFD of ~150 µmol m-2 s-1 was the light intensity observed in the environment where Changbai larch saplings were regenerated ([49], [39]). All transplanted seedlings were watered using nutritional solutions every two days at a rate of 200 ml seedling-1 each time. The solution included essential mineral elements which have been found to benefit growth of transplanted Changbai larch seedlings ([39]). Briefly, solutions delivered a total sum of nutrients per year: 4 mM NH4NO3, 0.5 mM K2HPO4, 0.5 mM KCl, 1 mM CaCl2, 0.6 mM MgSO4·7H2O, 20 µM FeCl3·6H2O, 6 µM MnCl2·4H2O, 16 µM H3BO3, 0.3 µM ZnCl2, 0.3 µM CuCl2·2H2O, and 0.3 µM NaMoO4·2H2O. All pots were randomly placed and rearranged every two days so as to eliminate possible edge effects.
Sampling, measurement, and chemical analysis of transplanted seedlings
Transplanted seedlings were harvested and transported to the laboratory of Beijing Forestry University on 22 June 2010. Twelve harvested seedlings per plot at nursery stage were separated into three groups with each including four seedlings. New roots were characterized as white, supple, and un-lignified ones, while old roots were brown and lignified. Roots of seedlings were washed free of substrates before being cut and separated into shoots and roots, and roots was further separated into three diameter classes: coarse roots (diameter > 2 mm), medium fine roots (diameter of 1-2 mm), and very fine roots (diameter of 0-1 mm). The number of new roots for each seedling was measured by counting for white juvenile root tips. Thereafter, biomass of each separated seedling sections was measured after being oven-dried at 70 °C for 48 h.
The respiration rates of roots within different diameter classes were measured using an infrared gas analyzer (IRGA) Model 7000 (Li-Cor, Inc., Lincoln, NE, USA), which was connected by a columnar cuvette (9.5 × 3 cm, length × inner diameter). During the measurement, root sections were folded in wet paper towels and placed into the cuvette, which was wrapped with aluminum foil so as to keep the inside completely dark. Both ends of the cuvette were sealed with rubber plugs through which two tubes were inserted to create both airflow entrance and exit. Each measurement was allowed to equilibrate for 15 min until the maximum CO2 concentration reached approximately 1.000 μmol mol-1 and a RH of 80% with a temperature range from 27 to 28 °C. The CO2 efflux rate was logged every 0.5 s from its steady-state, with an air velocity of approximately 1.25 L min-1. During the measurement, a technical control was involved with no sampled roots placed in the cuvette, and the mean respiration rate of controls was subtracted from root respiration values.
Carbohydrate concentrations of seedling organs were analyzed after the fresh mass measurement. Determination methods were adapted from Wei et al. ([40]): a 0.5 g sample was placed in a 10 ml glass test tube with 5 ml distilled water inside, covered by a plug, and incubated in a boiling water bath for 1 h. After removal of the supernatant (which was used for soluble sugar determination as described below), the residue was washed with 25 ml distilled water and oven-dried at 70 °C for 48 h. Starch was extracted from this dried residue with an HCl extraction method: 5 ml of 3% HCl was added to a test tube with the residue, which was then hydrolyzed in a boiling water bath for 3 h. Soluble sugar and starch contents were determined by a colorimetric method: one ml extracted solution was filtered through Whatman No. 42 filter paper into a 50 ml volumetric flask to which 1 ml of 25 % HCl was then added, and the solution was hydrolyzed for 30 minutes; next, 1 ml of a 28 % phenol solution was added, immediately by 5 ml of concentrated sulphuric acid added rapidly, directing the stream on to the liquid surface. The flask was then shaken by hand for 1 min, cooled at room temperature for 5 min, neutralized with NaOH solution, and diluted to the volume of 50 ml with distilled water. Carbohydrate concentration was determined using a spectrophotometer at 490 nm (UV-Visible 8453, Agilent Technologies Inc., Santa Clara, CA, United States).
Parameter calculation and data analysis
Net carbohydrate accumulation rate (NCAR) was calculated as (eqn. 1):
where CC1 and CC2 were the total non-structural carbohydrate contents (µg) in the seedlings sampled at day t1 and t2, respectively; t1 (d) and t2 (d) represent the time when seedlings were transplanted and harvested, respectively.
For post-transplant data analysis, new root numbers, biomass, carbohydrate (sugar, starch, and TNC) concentrations and contents (concentration × biomass), NCAR and root respiration rates were tested as dependent variables. The inorganic fertilizer treatment and organic amendment were included as main effects, and each treatment combination was represented by three replicates (n = 3). Data were analyzed using the General Linear Model (GLM) in SAS 9.0 (SAS Institute Inc., Cary, NC, USA). For pre-transplant seedlings, root biomass and carbohydrate contents were analyzed as dependent variables as described above. For transplanted seedlings, new root number, biomass, carbohydrate contents, NCAR, and root respiration rate were compared separately for each root diameter class. When analysis of variance (ANOVA) indicated significant effects (P < 0.05), Tukey’s Studentized Range Test was used to identify the significant differences among treatments (α = 0.05).
Results
Seedling carbohydrate storage in roots before transplant
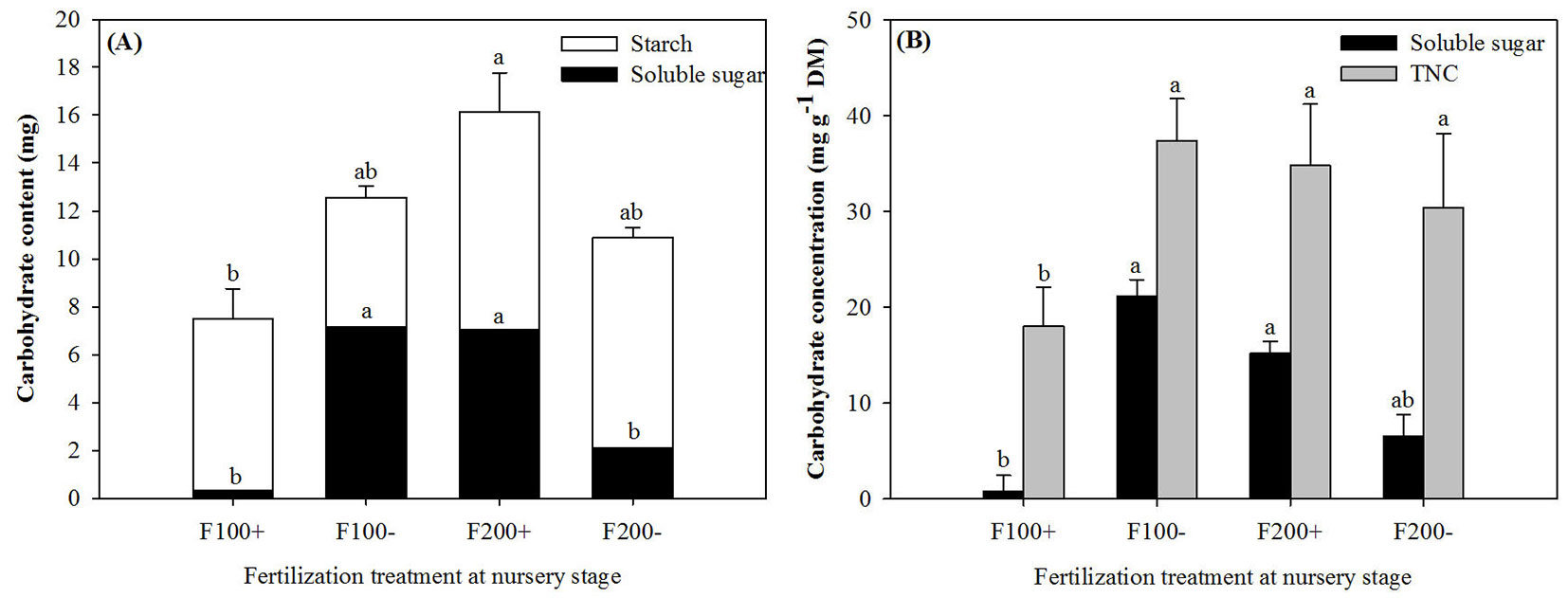
On 22 April 2010, initial root soluble sugars and TNC contents and concentrations in pre-transplant seedlings showed significant responses to nursery-applied treatments (Tab. 1). Initial root soluble sugar content and concentration in the F100- and F200+ treatments were at least 20% higher than those in the F100+ treatment (Fig. 1), and the highest TNC content was detected in the F200+ treatment (Fig. 1A). The main effect of organic amendment on soluble sugar concentration was found to be significant (Tab. 1).
Tab. 1 - F values from ANOVA for the influence of inorganic fertilizer (F), organic amendment (O), and their interaction (F×O) applied at nursery stage in 2009 on root carbohydrate contents, concentrations, and biomass accumulation in Changbai larch (Larix olgensis Henry) seedlings before and after transplant on 22 April and 22 June 2010, respectively. (*): P < 0.05; (*): P < 0.01; (***): P < 0.001.
| Before/After Transplant |
Root Diameter (mm) |
Source of variation |
Carbohydrate content | Carbohydrate concentration | Biomass | ||||
|---|---|---|---|---|---|---|---|---|---|
| Sugars | Starch | TNC | Sugars | Starch | TNC | ||||
| Before (22 April 2010) |
Whole roots | F | 0.75 | 2.65 | 2.56 | 0.01 | 2.78 | 1.93 | 0.66 |
| O | 1.00 | 0.41 | 0.01 | 13.97** | 0.29 | 4.44 | 5.24 | ||
| F×O | 38.09*** | 0.19 | 5.63* | 85.30*** | 0.76 | 11.19* | 0.09 | ||
| After (22 June 2010) |
0-1 | F | 2.84 | 0.50 | 2.71 | 1.18 | 0.18 | 1.18 | 2.74 |
| O | 3.09 | 0.38 | 0.65 | 1.42 | 1.78 | 0.01 | 4.85 | ||
| F×O | 1.84 | 2.55 | 0.01 | 1.11 | 3.95 | 0.44 | 1.35 | ||
| 1-2 | F | 0.80 | 0.71 | 0.95 | 1.29 | 2.12 | 2.02 | 3.59 | |
| O | 6.30* | 0.37 | 4.39 | 6.45* | 0.10 | 4.03 | 0.30 | ||
| F×O | 3.28 | 0.19 | 2.28 | 3.90 | 0.17 | 2.65 | 0.18 | ||
| >2 | F | 11.68** | 6.03* | 9.95* | 1.98 | 0.88 | 1.62 | 94.83*** | |
| O | 1.95 | 0.16 | 0.82 | 0.77 | 1.52 | 1.50 | 101.51*** | ||
| F×O | 5.50* | 0.05 | 7.86* | 3.32 | 0.27 | 2.52 | 5.82* | ||
| Whole roots | F | 7.85* | 1.83 | 5.52* | 0.52 | 0.09 | 0.35 | 32.62*** | |
| O | 0.82 | 0.07 | 0.05 | 2.33 | 2.16 | 3.71 | 34.39*** | ||
| F×O | 5.83* | 0.81 | 0.01 | 0.84 | 1.41 | 0.27 | 3.75 | ||
Fig. 1 - Initial soluble sugar and starch contents (A) and soluble sugar and total non-structure carbohydrate (TNC) concentrations (B) in roots of Changbai larch seedlings before transplant on 22 April 2010 in response to combined treatments of inorganic fertilizer (F200 and F100) and organic amendment (“+” and “-”) applied during nursery stage in 2009. Results for starch content were not statistically different. Different lowercase letters above columns indicate significant differences at P < 0.05 level. Upper letters were labeled for TNC content and lower letters were labeled for soluble sugar content. Error bars indicate standard errors.
Carbohydrate reserves in roots after transplant
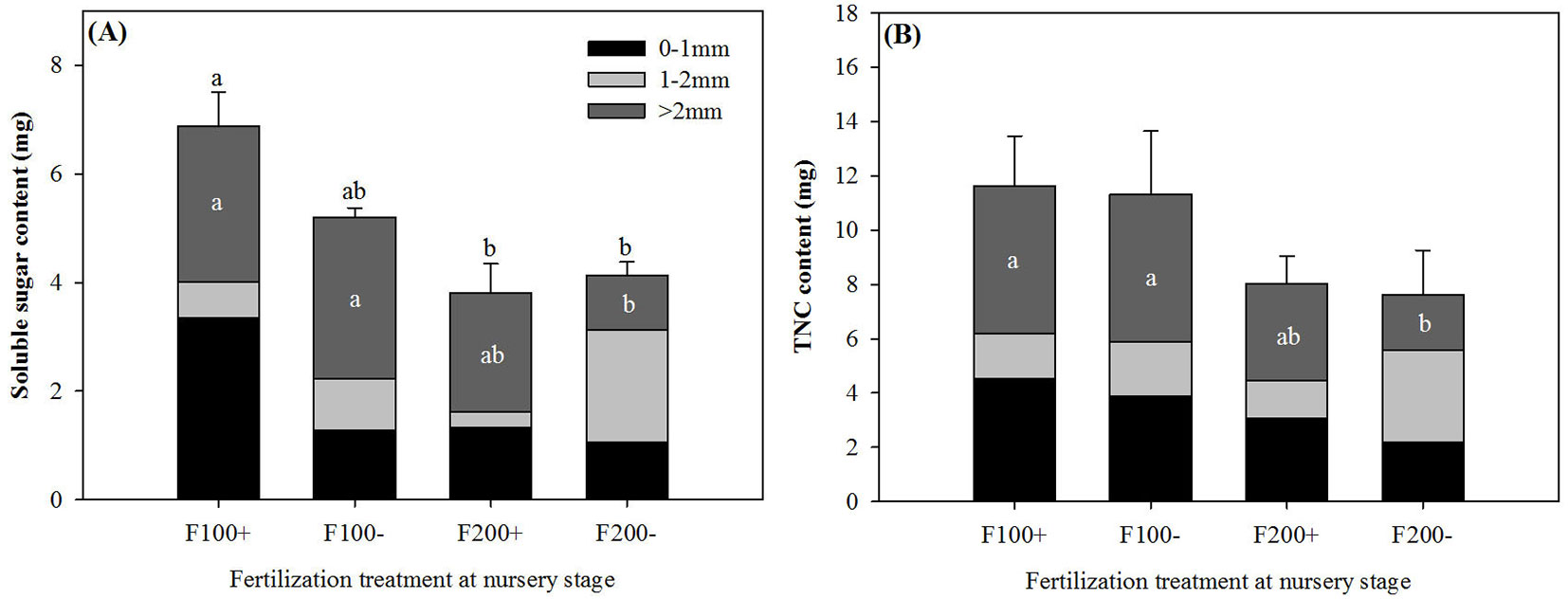
On 22 June 2010, both soluble sugar and TNC contents of coarse roots were greater in the F100+ and F100- than in the F200- treatment (Fig. 2). For whole roots, soluble sugar content in the F100+ treatment was greater by 81% and 66% than that in the F200+ and F200- treatments, respectively (Fig. 2A). Inorganic fertilizer applications during the nursery stage significantly influenced root carbohydrate contents after transplant (Tab. 1). In coarse roots, starch content in the F100 treatment (2.52 ± 1.04 mg) was greater than that in the F200 treatment by 105% (1.23 ± 0.26 mg). Organic amendment treatments during the nursery stage resulted in decreases of 67% and 69% for soluble sugar content (+O: 0.49 ± 0.26 mg; -O: 1.50 ± 0.99 mg) and concentration (+O: 10.10 ± 5.42 mg g-1; -O: 32.62 ± 22.15 mg g-1) in roots with diameter of 1-2 mm, respectively. Soluble sugar concentrations in post-transplant shoots on 22 June were 42.91 ± 15.80 mg g-1, which was 120% higher relative to that in pre-transplant seedlings (n = 6, P = 0.0075).
Fig. 2 - Soluble sugar (A) and total non-structure carbohydrate (TNC) contents (B) of roots by diameter class (0-1 mm, 1-2 mm, and >2 mm) in Changbai larch seedlings two months after transplant on 22 June 2010 in response to combined treatments of inorganic fertilizer (F200 and F100) and organic amendment (“+” and “-”) applied during nursery stage in 2009. Different lowercase letters above columns indicate significant differences at P < 0.05 level. Error bars indicate standard errors.
Post-transplant carbohydrate metabolism
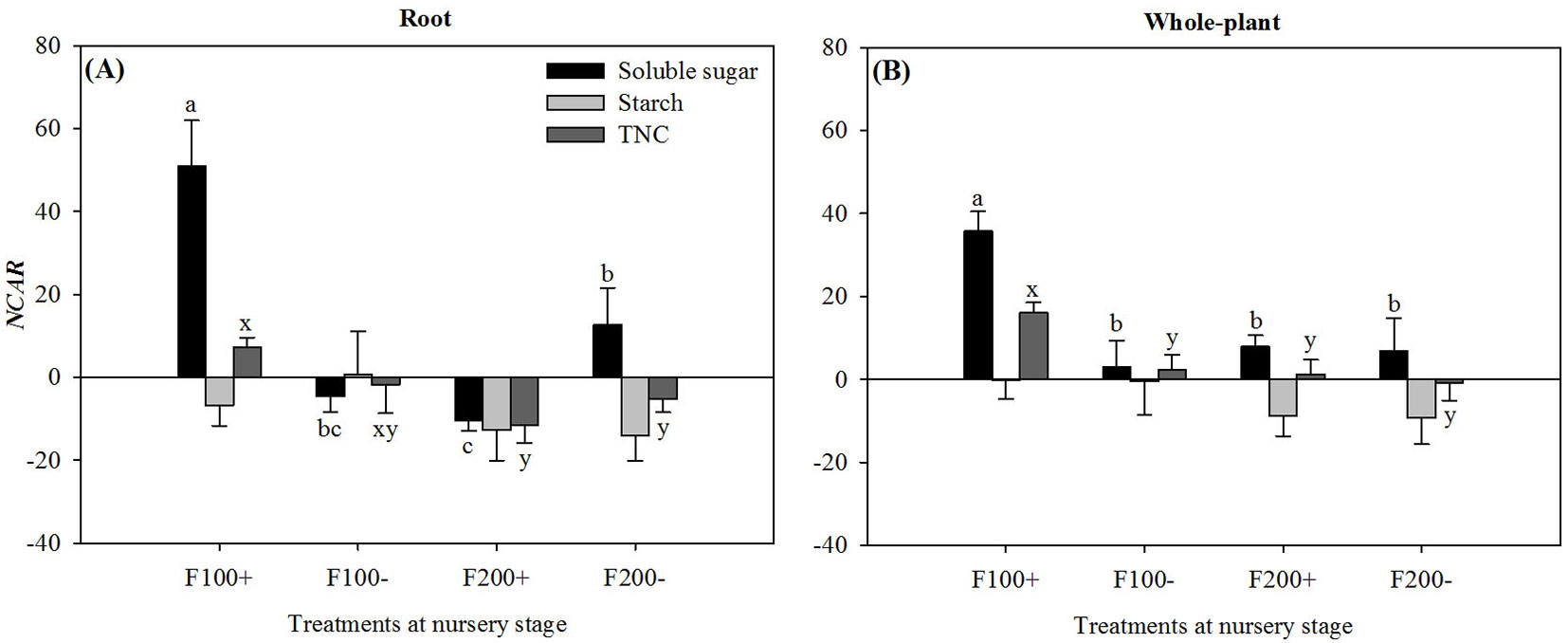
During the first two months after transplant, there were significant interaction effects between nursery-applied inorganic fertilizer and organic amendment treatments on NCARs for soluble sugars (P = 0.0227 and P = 0.0264) and TNC (P < 0.0001 and P = 0.0020) at both root and whole-plant scales. NCARs for both soluble sugars and TNC were the highest in the F100+ treatment. Most values of NCAR for starch were negative and not statistically different among treatments (Fig. 3). Root NCAR for soluble sugars was higher in the F200- treatment than in the F200+ treatment.
Fig. 3 - Net carbohydrate accumulation rates (NCAR) for soluble sugars, starch, and total non-structural carbohydrates in roots (A) and whole plants (B) for Changbai larch seedlings two months after transplant on 22 June 2010 in response to combined treatments of inorganic fertilizer (F200 and F100) and organic amendment (“+” and “-”) applied during nursery stage in 2009. For a given chemical fraction, different lowercase letters above columns indicate significant differences at P < 0.05. Significant means separations for soluble sugars are labeled as “a”, “b”, and/or “c”, while those for TNC are labeled as x or y. Error bars indicate standard errors.
Post-transplant biomass accumulation in roots
On 22 June 2010, coarse root biomass was the greatest in the F100+ treatment (194.36 ± 11.98 mg), followed by the F100- and F200+ treatments (118.98 ± 12.83 mg and 121.01 ± 14.86 mg, respectively), and reached the least value in the F200- treatment (74.76 ± 2.17 mg - P = 0.0423). Seedlings receiving organic amendment during nursery stage had greater whole-root biomass by 38% (+O: 0.33 ± 0.06 mg; -O: 0.24 ± 0.04 mg - P = 0.0004), while seedlings in the F100- treatment had greater whole-root biomass than those in the F100+ treatment (0.33 ± 0.07 mg and 0.24 ± 0.04 mg, respectively - P = 0.0003).
New root growth and root respiration
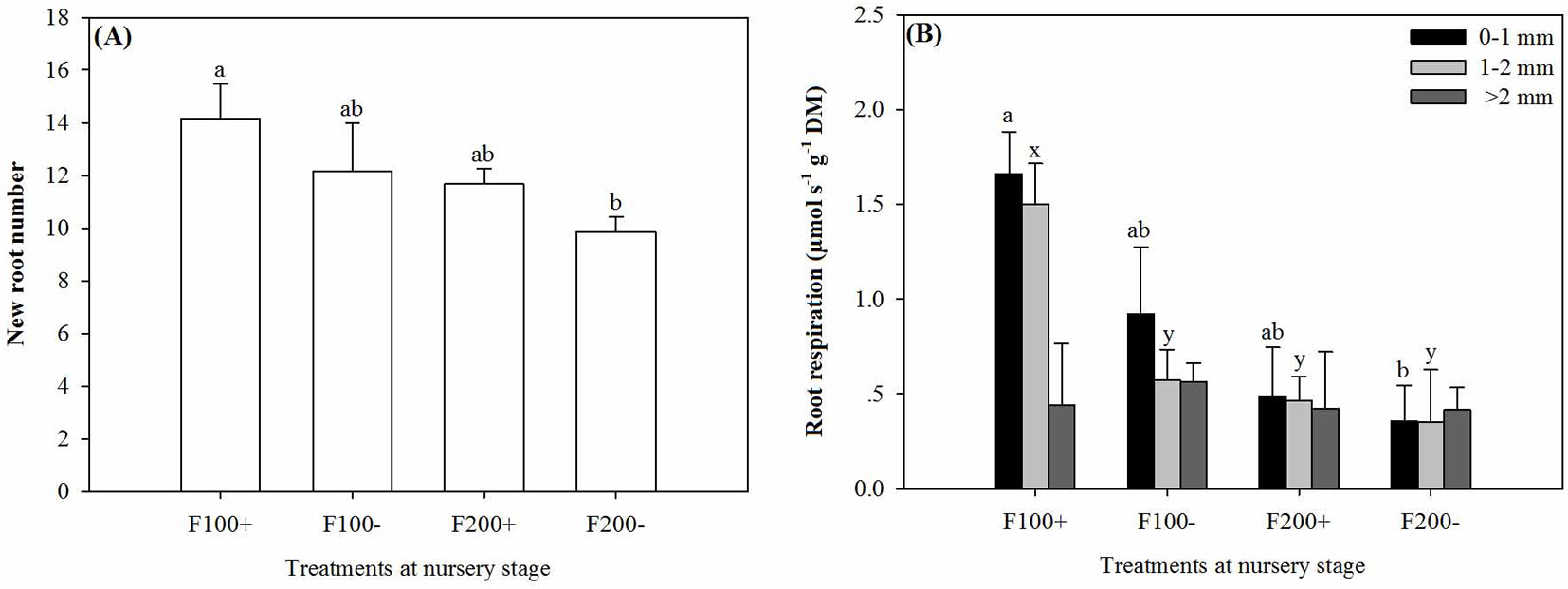
The interaction between organic amendment and inorganic fertilizer treatments significantly affected new root numbers (Tab. 2). From 22 April to 22 June 2010, seedlings in the F100+ treatment developed the most new roots, which was 44% more than those observed in the F200- treatment (Fig. 4A).
Tab. 2 - F values from ANOVA for the influence of inorganic fertilizer (F), organic amendment (O), and their interaction (F×O) applied at nursery stage in 2009 on root respiration rate and new root numbers in Changbai larch (Larix olgensis Henry) seedlings after transplant. (*): P < 0.05.
| Source of variation |
Root respiration | New root number |
||
|---|---|---|---|---|
| 0-1 mm | 1-2mm | >2 mm | ||
| F | 11.21* | 3.60 | 0.35 | 6.96* |
| O | 2.84 | 5.79* | 0.16 | 11.07* |
| F×O | 5.14* | 8.50* | 0.20 | 6.01* |
Fig. 4 - New root number (A) and respiration rate of roots by diameter class (0-1 mm, 1-2 mm, and >2 mm) (B) in Changbai larch seedlings two months after transplant on 22 June 2010 in response to combined treatments of inorganic fertilizer (F200 and F100) and organic amendment (“+” and “-”) applied during nursery stage in 2009. Different lowercase letters above columns indicate significant differences at P < 0.05. Significant means separations for roots 0-1 mm diameter are labeled with “a” and “b”, while those for roots of 1-2 mm diameter are labeled with “x” and “y”. Error bars indicate standard errors.
The interaction of organic amendment and inorganic fertilizer treatments could also influence root respiration rate (Tab. 2). Respiration rates in fine roots of 0-1 mm and 1-2 mm in diameter were higher in the F100+ treatment than in the F200- treatment (P = 0.0286 and 0.0195, respectively), but similar results was not detected for coarse roots (Fig. 4B).
Discussion
During nursery culture, Changbai larch seedlings were applied with approximately 18 or 36 mg N seedling-1, respectively, but received a total dose of about 112 mg N seedling-1 in solution during the first two months after transplant. Thus, the overall fertilizer dose in the second year was higher than that applied in the first year, and the high level of N supplied in solution after transplant likely had some effects on transplanted seedling roots. The dose of 112 mg N seedling-1 in our study was much lower than those used for transplanted stocks in studies from Spain ([23]) and USA ([18]). All seedlings received a uniform dose during the post-transplant growth phase; consequently, post-transplant solution-supplied N was unlikely to result in different root responses observed in this study. In an applied context, the nutrient-enriched solution provided to seedlings in this study could simulate transplant conditions in necessary situations where seedlings are supplied with controlled-released fertilizer at out-planting to improve their potentially future performance ([23], [31]).
Biomass accumulation
The great root biomass observed in the F100+ treatment resulted from TNC accumulation in coarse roots where root biomass and TNC accumulation had a significant positive correlation (n = 12, R = 0.5764, P = 0.0498). Coarse roots provide structural support to transplanted seedling growth through secondary thickening ([7]). However, according to Wilson et al. ([42]) and Wei et al. ([39]), greater root biomass with larger tap and woody roots may lead to two main results of N uptake by fine roots: to limit the proliferation of fine roots but to promote the rate of N influx into fine root cells. In the present study, greater root biomass was observed in the lower rate of inorganic fertilizer treatment and in the organic amendment treatment, both of which coincided with TNC accumulation in coarse roots. Thus, according to these biomass accumulation results, we accept our first hypothesis.
Root respiration and new root growth
The trend in declining respiration rates with increasing root diameter which appeared in our study has already been identified for mature trees ([5], [19]). Fine root respiration rate changes among treatments coincided with general patterns observed for soluble sugars, TNC, and biomass accumulation in coarse roots, potentially suggesting the function of root carbohydrate storage to fuel root respiration ([2]). Intensive soluble sugar depletion by respiration during new root growth has also been shown in cuttings of some Pelargonium species ([13]). Additionally, both carbohydrate decline ([12]) and root respiration ([27]) are related to inherent N status, which was controlled by nursery-applied treatments. Hence, it is reasonable to accept our second hypothesis.
Higher respiration rates of roots of post-transplant seedlings in the lower rate of the inorganic fertilizer treatment may resulted from a greater seedling N reserve ([11]), which usually contributes to more new root egress ([8], [9]) because many critical functions during new root growth depend upon root respiration for an energy supply ([27], [3]). In a previous study, Wei et al. ([37]) reported that a lower rate of the inorganic fertilizer treatment promoted seedling quality by enhancing N reserves. This conclusion was continuously strengthened in our study, likely due to more new root egress in the same treatment ([3], [30], [4]). On the other hand, the nursery-applied organic amendment treatment also resulted in more new roots after transplant, which was attributed to better initial root quality as characterized by tap root length and lateral root number before transplant ([37], [42]). Therefore, the final result of more root number in the F100+ treatment was related to a continuous influence from nursery treatments through the changes of root respiration.
Carbohydrate accumulation before transplant
Before transplant, starch storage in initial roots was not different for either content or concentration among nursery treatments. Temperature during storage can lead to starch depletion throughout the period from seedling lifting at fall to transplant at spring ([24]). During this time, starch is depleted mainly through respiration. Also carbohydrate-metabolism-related enzymatic activity can at least partly result in starch depletion, such as starch granule bound endoamylase and starch phosphorylase ([16]). Therefore, the null effect of nursery treatments on initial starch status might result from the temperature impact during storage. However, the proportion of starch to TNC reserves was as high as 68% in initial roots of Changbai larch seedlings in this study, which was higher than that in roots of Sabina prezewalskii Kom. (SP), S. Chinensis (Lin.) Ant. (SC) (~38% - [6]), and some poplar species (~35% - [26]). In our study, although Changbai larch seedlings suffered some level of transplant shock during processes of seedling lifting, moving out of storage, and removal of soils at root-surface ([11], [33]), the high proportion of starch to TNC suggests that the substantial starch reserves within seedlings may not have been depleted intensively at the beginning of the growth chamber experiment. Transplant shock was unlikely to seriously interfere with initial carbohydrate status. Nevertheless, decline of soluble sugars in the F100+ treatment in initial seedling roots led to differentiation of carbohydrate statuses among treatments. According to former results, greater N reserves were created in annual organs of seedlings in the F100+ treatment at the end of nursery cultivation due to greater biomass accumulation and higher N concentration in stems ([37]). These additional N reserves in seedlings in the F100+ treatment may have resulted in increased carbohydrate consumption and the devotion of more assimilated carbon to growing structures ([22], [11], [12]). For Changbai larch seedlings, it has also been reported that nursery fertilization would result in starch depletion ([40]).
Post-transplant carbohydrate metabolism and accumulation
During the period from 22 April to 22 June 2010, starch was intensively depleted, resulting in negative values of NCAR for starch at both root and whole-plant scales. Starch reduction in early stages of post-transplant seedling growth was caused by shoot growth ([41]) and new root egress ([30], [32], [41]). We employed the low light intensity of 150 µmol m-2 s-1 with the aim of mimicking the natural conditions of Changbai larch regeneration, but this light intensity was also found to result in poor performance of regenerated Changbai larch seedlings ([49]). Some studies have reported that light compensation point (LCP) for Changbai larch seedlings is ~40 µmol m-2 s-1 ([48], [21]), while the LCP for this species ranged from 100 to 170 µmol m-2 s-1 ([34]). Hence, we surmise that the light intensity at 150 µmol m-2 s-1 in the present study may be insufficient to support the growth of Changbai larch seedlings and may have impaired their net C assimilation ([29], [12]). According to our results about starch metabolism, lower starch concentration in the F200+ treatment could be attributed to a greater starch depletion in shoots due to inefficient starch production under the low light intensity. The nursery-applied organic amendment treatment resulted in a significant effect on fine roots in initial seedlings, but had no effect on N reserves ([37]), which led to the lack of response observed for current-season starch metabolism.
In the F100- and F200+ treatments, values of NCAR for soluble sugars were negative in roots but positive at the whole-plant scale. Aside from the impact of insufficient photosynthetic production under the low light intensity, these results also suggest that most soluble sugars were retained in shoots, leading to intensive soluble sugar depletion in roots ([22]). Compared to these two treatments, final root soluble sugar contents were greater in the F100+ treatment, suggesting that seedlings in the F100+ treatment were more efficient to assimilate soluble sugars for new root formation ([13]) compared to those in the F100- treatment. Greater soluble sugar content in whole roots of seedlings in the lower rate of inorganic fertilizer treatment resulted from the greater soluble sugar accumulation in coarse roots, suggesting a low-cost of consumption of soluble sugars for new root growth in this treatment.
Conclusions
This study indicated that, relative to the inorganic fertilizer treatment at nursery stage, a lower rate of inorganic fertilizer treatment with organic additive could benefit roots of post-transplanted Changbai larch seedlings in carbohydrate assimilation, biomass accumulation, and fine root respiration. The effect of the single treatment of inorganic fertilizer supplied at 200 kg N ha-1 can be overcome by the treatment of half-dose-rate of 100 kg N ha-1 with chicken manure added at the rate of 100 kg N ha-1. Obviously, the implementation of organic amendment contributed to the relief of contamination through the replacement of some inorganic fertilizers. Our results also highlight the potential of incorporating organic amendments into the cultural protocol of larch seedling culture so as to improve their quality and transplant performance, but the application should be considered to be employed in the context with interactive inorganic fertilizers at a reasonable rate.
Acknowledgements
This work was supported by the “Dalian Science and Technology Project (2014B11 NC078)”, “Key Deployment Project of Chinese Academy of Sciences (KFZD-SW-302-03)”, “Natural Science Foundation of China (31170168)”, “National Spark Program project (2013GA651006)”, “National Torch Plan Project (2012GH531899)”, “Program for Liaoning Excellent Talents in University (LR2013055)”, and “Liaoning province science and technology plan (2011209001)”.
References
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Online | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Northeast Institute of Geography and Agricultural Ecology, Chinese Academy of Sciences, Changchun City, Jilin Province, 130102 (China)
Environment and Resources College, Dalian Nationalities University, Dalian City, Liaoning Province, 116600 (China)
Corresponding author
Paper Info
Citation
Wei H, Guo P (2016). Carbohydrate metabolism during new root growth in transplanted Larix olgensis seedlings: post-transplant response to nursery-applied inorganic fertilizer and organic amendment. iForest 10: 15-22. - doi: 10.3832/ifor1988-009
Academic Editor
Claudia Cocozza
Paper history
Received: Jan 19, 2016
Accepted: Jun 21, 2016
First online: Sep 22, 2016
Publication Date: Feb 28, 2017
Publication Time: 3.10 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2016
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 49753
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 41275
Abstract Page Views: 3155
PDF Downloads: 3984
Citation/Reference Downloads: 47
XML Downloads: 1292
Web Metrics
Days since publication: 3387
Overall contacts: 49753
Avg. contacts per week: 102.83
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2017): 20
Average cites per year: 2.22
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Evergreen Quercus aquifolioides remobilizes more soluble carbon components but less N and P from leaves to shoots than deciduous Betula ermanii at the end-season
vol. 11, pp. 517-525 (online: 01 August 2018)
Research Articles
Effects of nitrogen loading under low and high phosphorus conditions on above- and below-ground growth of hybrid larch F1 seedlings
vol. 11, pp. 32-40 (online: 09 January 2018)
Research Articles
Impact of thinning on carbon storage of dead organic matter across larch and oak stands in South Korea
vol. 9, pp. 593-598 (online: 01 March 2016)
Research Articles
Relationship between volatile organic compounds released and growth of Cunninghamia lanceolata roots under low-phosphorus conditions
vol. 11, pp. 713-720 (online: 06 November 2018)
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Research Articles
Changes in organic compounds during leaf litter leaching: laboratory experiment on eight plant species of the Sudano-guinea Savannas of Ngaoundere, Cameroon
vol. 1, pp. 27-33 (online: 28 February 2008)
Research Articles
Fine root morphological traits and production in coniferous- and deciduous-tree forests with drained and naturally wet nutrient-rich organic soils in hemiboreal Latvia
vol. 16, pp. 165-173 (online: 08 June 2023)
Research Articles
Tree-ring-based reconstruction of larch budmoth outbreaks in the Central Italian Alps since 1774 CE
vol. 12, pp. 289-296 (online: 27 May 2019)
Short Communications
Variation in growth, photosynthesis and water-soluble polysaccharide of Cyclocarya paliurus under different light regimes
vol. 10, pp. 468-474 (online: 04 April 2017)
Research Articles
Auxin (IAA) and soluble carbohydrate seasonal dynamics monitored during xylogenesis and phloemogenesis in Scots pine
vol. 11, pp. 553-562 (online: 01 September 2018)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword