Evaluating the impact of Hymenoscyphus fraxineus in Trentino (Alps, Northern Italy): first investigations
iForest - Biogeosciences and Forestry, Volume 10, Issue 6, Pages 871-878 (2017)
doi: https://doi.org/10.3832/ifor2486-010
Published: Nov 06, 2017 - Copyright © 2017 SISEF
Research Articles
Abstract
The spread of Hymenoscyphus fraxineus has been causing great concern regarding the survival of European ash (Fraxinus excelsior) throughout Europe since the 1990s. The disease was first recorded in Trentino (southern Alps, Italy) in 2012 and has spread throughout the mountain landscape, where ash trees are scattered in small and isolated stands in different valleys. The status of the disease was checked by monitoring the damage to natural regeneration and adult trees in 90 sites spread over the whole region. The survey confirmed the complete colonization by the pathogen of the whole investigated area, with high levels of damage to both young and adult ash trees. Regeneration (both seedlings and saplings) was observed to be affected by the fungus in 88 plots out of 90. Out of 4486 examined young European ashes, 2261 (50.4%) were affected and 789 (17.6%) were already dead. Ten of the 384 assayed flowering ashes (Fraxinus ornus) showed symptoms on branches and apical stems, similar to those observed for European ash. Isolation and molecular analysis proved the presence of the fungus on both symptomatic European and flowering ashes. The examined 386 adult trees showed different levels of damage, sometimes reaching more than 75% of the crown. Some individual trees (42) growing close to severely damaged trees appeared fully healthy, which suggests the possible existence of some resistant/tolerant individuals in the examined populations.
Keywords
Ash Dieback, Fraxinus excelsior, Fraxinus ornus, Natural Regeneration, Forest Management
Introduction
The appearance and spread of ash dieback is causing great concern in Europe due to the evident impact on stands and trees everywhere the disease has occurred. Hymenoscyphus fraxineus (T. Kowalski) Baral, Queloz and Hosoya, renamed from the previous Hymenoscyphus pseudoalbidus Queloz, Grünig, Berndt, Kowalski, Sieber & Holdenr., is the invasive pathogen mainly involved in these phenomenon ([21], [33], [14], [2]). The fungus, which was introduced most likely from eastern Asia, showed pathogenic behaviour on European ash, overrunning the saprophytic Hymenoscyphus albidus (Roberge ex Gillet) W. Phillips, the common and autochthonous inhabitant of dead ash leaves ( [29]). The disease was first recorded in central Europe during the ’90s, and it is currently spread over almost all the natural range of European ash (Fraxinus excelsior L.), also causing damage to narrow-leaved ash (F. angustifolia L.). Moreover, the fungus has showed its damaging potential on flowering ash (F. ornus L.), which can be infected artificially if seedlings are exposed to high inoculum levels ([18]). The colonization of the whole of Europe is almost complete, and the recent appearance of the disease on the Apennines ridge ([26]) also enables the possibility of severe damage to the Mediterranean area ([13]).
The pathogen can attack different tissues and tree organs; infection initially causes necroses in leaves, leading to the formation of necroses in rachises and petioles, from where the fungus colonizes shoots, twigs or the main stem, causing dieback of the crown, bark lesions (cankers) and sapwood discolouration ([22]). Ash trees of any age can be affected in all growth conditions, natural forests, plantations and nurseries ([39], [22], [14]).
The spread of infection is due to wind-borne ascospores released by apothecia growing on leaf rachises of the previous years. Because of the continuous production of ascospores in all vegetative seasons, the symptoms are mainly due to multiple and repeated infections ([16]).
The concern about the ecological impact of this invasion is confirmed by several investigations carried out throughout Europe ([7], [15], [16], [36]). Recently, the recovering of surviving plants in affected areas open up new possibilities for characterization and breeding of hyposensitive trees ([20], [15], [8]).
In Italy, the disease was reported in 2009 on the boundary with Slovenia in the eastern part of the Alps ([32]), and in the following years, it spread quickly towards the west, on the southern slope of the Alps. The colonization rate in these mountain areas was estimated to be 50-60 km per year ([25]). In Trentino, the first record of the symptoms occurred in 2012 in the eastern part of the Province ([11]). Here, F. excelsior exists in, or dominates, ravine ash-sycamore forests (CORINE biotopes 41.41), although the Trentino forests are mainly dominated by conifers (Picea abies Karst. and Larix decidua Mill). These ash stands cover small surfaces in the humid and fresh sites at the bottom of the valleys, playing an important role in biodiversity despite their size.
European ash is also one of the main species involved in the natural recolonization of abandoned pastures and meadows in endalpic valleys ([40]). Its ability to colonize is also evident in several consolidated coniferous stands ([44]), where it can spread alone or with other hardwood species under cover. These new formation stands could play a potential economic role for the commercial value of the wood ([44]). Here, the presence of trees occurs mainly in small stands of new formation or along edges, or single or groups of trees are isolated in the traditional landscape and regenerate under other species cover. Few mature stands dominated by ash are also present, thus resulting in a highly fragmented distribution.
In 2014, damage due to the disease was reported in all the valleys, confirming the fast spread probably favored by particular weather conditions (high rates of precipitation during the vegetative seasons of both 2013 and 2014 - [28]). This rapid colonization, even in this context of very fragmented stands, enhances the risk of a possible heavy ecological impact of the disease.
Although European ash plays a minor role in the silvicultural management of Trentino forests, it seems important to evaluate and quantify this possible impact. To this purpose, a survey was carried out to estimate the damage to the natural regeneration of ash. Moreover, adult trees were assessed and mapped, aiming to establish permanent points of monitoring the disease progression in the future.
Materials and methods
Field investigations
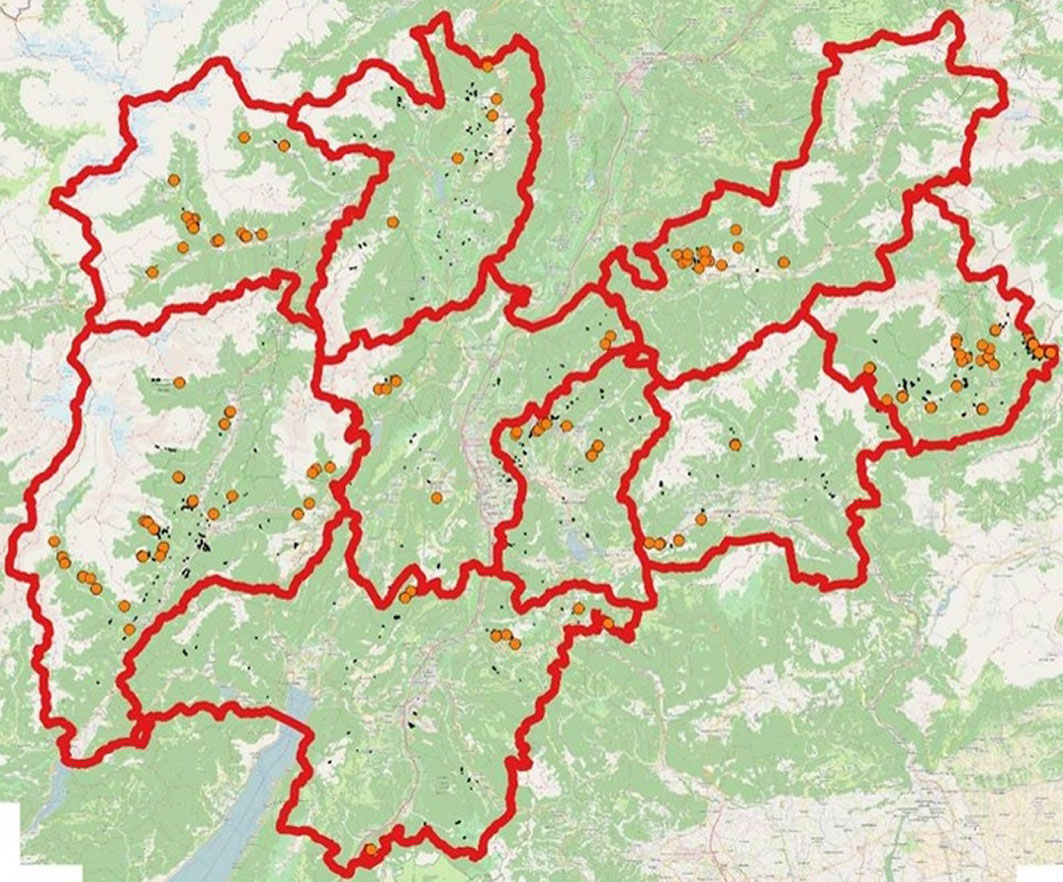
Field investigations were carried out over the whole province of Trento (northern Italy). A distribution map of F. excelsior stands was kindly provided by the Forest and Fauna Service of Autonomous Province of Trento. In each forest administrative district, surveys were carried out aiming to identify the most representative areas of natural regeneration and groups of adult trees, and to cover the whole area reported to be colonized by the tree species. Considering the high irregular distribution of ash stands, each plot was chosen at a distance of approximately 3 km from each other, resulting in total of 90 surveyed sites (Fig. 1). The evaluation of the presence and damage of the disease was carried out during the 2016 vegetative season, starting at the end of July and finishing at the beginning of October.
Fig. 1 - The study area (Trentino, northern Italy). Orange points indicate the investigated plots for H. fraxineus damage to natural regeneration (seedlings and saplings) and adult trees. Red lines delineate the different forest districts in the area. The distribution of European ash stands is displayed in green.
Regeneration evaluation and the presence of H. fraxineus fruiting bodies
The selected plots were geo-located with a high-precision GPS (Garmin Montana 650T). At each identified point, a survey was carried out using a transect 10 m long and 4 m wide. For each transect, the following general characteristics were recorded: (i) altitude; (ii) aspect; (iii) stand type (wood edge, open field regeneration, hedgerow, under cover regeneration).
The whole area of the transect was accurately surveyed, mapping each seedling, sapling or young ash tree (F. excelsior and F. ornus). For each individual ash, height (<20 cm, 21-100 cm, 101-200 cm, >200 cm), diameter at the collar (<2 cm, 2-4 cm, >4 cm) and type of tissue (lignified or herbaceous) were recorded. For the evaluation of the level of damage, the following classes were adopted:
- asymptomatic;
- initial attacks on leaves (dead leaves) and affected minor branches, up to a maximum of 5;
- more than 5 symptomatic or dead branches and/or the presence of stem cankers;
- apical shoot dead;
- dead plant.
Additional data such as the number of cankers on stems and branches, the length of the dead apical shoot and the presence of basal resprouting was also recorded.
Moreover, the presence of H. fraxineus fruiting bodies in the litter of each transect was assessed.
Adult tree evaluation
Wherever possible, groups of F. excelsior adult trees were randomly selected near (50-100 m) the regeneration plots. A maximum of six trees per site, if present, were mapped with GPS and surveyed. Dendrological characteristics (height and diameter at breast height) were recorded together with social position (dominant, codominant, dominated and isolated). The loss of crown due to H. fraxineus was evaluated according to five classes ([30], modified):
- asymptomatic;
- defoliation or desiccation up to 1/5 of the whole crown;
- defoliation or desiccation from 1/5 to 1/2 of the whole crown;
- defoliation or desiccation from 1/2 to 3/4 of the whole crown;
- defoliation or desiccation over 3/4 of the whole crown.
Moreover, the presence of other fungal attacks on stems or necroses on the collars were assessed.
Fungal isolation and molecular analysis
The samples of symptomatic stems or branches were randomly collected in several selected plots to confirm the presence of the fungus: cankers, affected main stems or branches were picked up and transferred to the lab in plastic bags. Eight wood samples of F. excelsior and seven of F. ornus were processed. Following the methods of Stanosz et al. ([42]), the external surface of each sample was disinfested for 10 s in 95% ethanol followed by 4 min in 2% NaOCl solution with 2 drops of Tween 80 per litre. After cutting in aseptic conditions, fragments were excised from discoloured wood. Ten fragments per sample were randomly selected and placed on potato dextrose agar (PDA, Oxoid) in Petri dishes and incubated at 25 °C for ten days in the dark. Other pieces of the same wood samples were placed into sterile Eppendorf tubes for DNA tests. The same procedure was adopted for seemingly symptomatic samples collected from F. ornus seedling or saplings. After 15 days, colonies showing the characteristics reported for those of H. fraxineus were transferred to fresh PDA plates. These colonies were checked after a week to confirm the morphological identification.
The same samples collected for isolation were also assayed for DNA extraction. Plant material was frozen in liquid nitrogen for 5 min, crushed in a Retsch Mixer Mill® MM200 (Qiagen, the Netherlands) for 2 min at a frequency of 25 Hz and then stored at -20 °C until use. DNA was extracted using a DNeasy Plant Mini kit® (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Species-specific primers designed by Johansson et al. ([17]) were used to detect infections directly from plant tissue. The PCR mixture comprised 12.5 µl of GoTaq Green Master Mix® (Promega), 10.5 µl of water supplied with the GoTaq®, each primer at 0.2 mM (Sigma-Aldrich) and 1 µl of DNA. A Biometra T1 Thermocycler (Whatman Biometra, Gottingen, Germany) was used for PCR with the following protocol: 5 min at 95 °C; 35 cycles of denaturation at 95 °C for 30 s, annealing at 62 °C for 30 s and extension at 72 °C for 1 min; and a final extension step at 72 °C for 10 min. PCR fragments were analysed using 1% agarose gel electrophoresis in 1× Tris-boric acid-EDTA buffer (TBE), stained with SYBR Safe® (Life Technologies, Milan, Italy) and visualized under UV light. As a size standard, the GeneRuler DNA ladder mix® (Thermo Scientific, USA) was used. Each DNA sample was assayed in duplicate. Negative controls (sterile water) and a positive control (DNA extracted from ascomata of H. fraxineus, accession number KY593990 - Fig. S1 in Supplementary material) were included in all PCR runs.
Statistical analysis
To analyse the damage level in the regeneration, the level of damage index (LDI) was calculated for each plot as (eqn. 1):
where n is the number of F. excelsior individuals in each damage level i and N is the total number of individuals in the plot. In order to evaluate the degree of spatial autocorrelation between neighbouring plots, Moran’s I test was used in ArcGIS Desktop® 10.2 software ([10]) using the coordinates of each plot and its respective level of damage index ([1], [31]). The collinearity among characteristics of a single seedling (type of tissue, height, diameter) and of the site (altitude, aspect, stand treatment) was first checked based on the Pearson correlation (α = 0.01) using R cran (R Core Team - ⇒ http://www.r-project.org). Subsequently, the association between the level of damage index (transformed to continuous response variable by angular transformation) and predictors was tested using ANOVA. When the result from a dependent variable indicated significant differences (p < 0.05), these were tested with multiple comparison tests (Tukey’s HSD test, α = 0.05, R library agricolae).
The presence of fruiting bodies on the soil was analysed as a function of the main characteristics of the transect (altitude, aspect, stand treatment) and the date of discovery using a conditional inference tree in R cran (library party). Values of site altitude were grouped into 3 classes: (1) ≤ 800 m a.s.l.; (2) > 800 and ≤ 1000 m; and (3) > 1000 m. Aspect was reduced to 8 classes: north, northeast, east, southeast, south, southwest, west, northwest. The performance of the model was evaluated by means of the contingency table (also called confusion matrix).
As for regeneration, the level of damage index for adults was calculated, then spatial autocorrelation between neighbouring plots was tested using Moran’s I test; additionally, the level of damage index was tested against tree (height, diameter, social position) and site characteristics (altitude, aspect, stand treatment) after checking the collinearity of predictors with ANOVA (percentage data were modified by angular transformation), followed by multiple comparison tests (Tukey’s HSD, α = 0.05, library agricolae).
Results
Regeneration
The regeneration of F. excelsior was affected by H. fraxineus in all the forest districts and in all the investigated valleys. No damage was observed only in two plots out of 90, both located in Val di Sole (eastern part of Trentino) and both having only seedlings still in the herbaceous phase. In these areas, however, the disease symptoms (Fig. S2, Supplementary material) were detected on other trees close to the transect. In 5 plots, all the surveyed trees were affected by the disease; in 54 plots, the total number of affected individuals was more than 50%. Regarding mortality, only 10 plots showed no dead individuals, while in 53 plots mortality was > 10%. Among them, 20 plots had more than 20% dead trees. Only in 4 plots the reported mortality was more than 50% of the assayed individuals. The highest level of mortality reached 74%.
A total number of 4870 ash young trees were assessed during the survey (Tab. 1). Among them, 92.1% were European ash, while 7.9% were flowering ash. Regeneration ranged from a minimum of 12 to a maximum of 202, with an average of 54 individuals per plot. The disease affected 50.4% of the young F. excelsior trees in the plots, while only 2.6% of the F. ornus plants showed some symptoms. For European ash trees, most of the symptomatic trees (21.4%) showed heavy damage with the loss of the terminal shoot (class 3) and 17.6% were dead due to the attack of H. fraxineus with no sign of other pathogens. Only 1.5% of F. ornus were badly damaged (class 3) but no individual was dead. Necrotic spots on leaves were observed on another 518 subjects, 383 of F. excelsior and 135 of F. ornus, suggesting a possible (though not confirmed) infection of almost 20% of saplings in damage class 0.
Tab. 1 - Distribution and percentages of the sampled ash regeneration (seedlings and saplings) in the different damage classes.
| Species | Damage classes | Total Individuals |
||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| F. excelsior | 2225 (49.6%) |
364 (8.1%) |
150 (3.3%) |
958 (21.4%) |
789 (17.6%) |
4486 |
| F. ornus | 374 (97.4%) |
3 (0.8%) |
1 (0.3%) |
6 (1.5%) |
0 (0%) |
384 |
| Total | 2599 | 367 | 151 | 964 | 789 | 4870 |
Spatial autocorrelation analysis using Moran’s I test on plot LDI values did not reveal any significant departure from random distribution (p = 0.538, z-score = 0.617).
Height, diameter and type of tissue variables were highly correlated (p < 0.01). The analysis of variance showed no significant differences in LDI among different stand typology (p = 0.6) or different aspect (p = 0.218), whereas significant differences in the level of damage index were observed for seedling height and diameter, type of tissue and altitude (Tukey’s HSD test, p < 0.05).
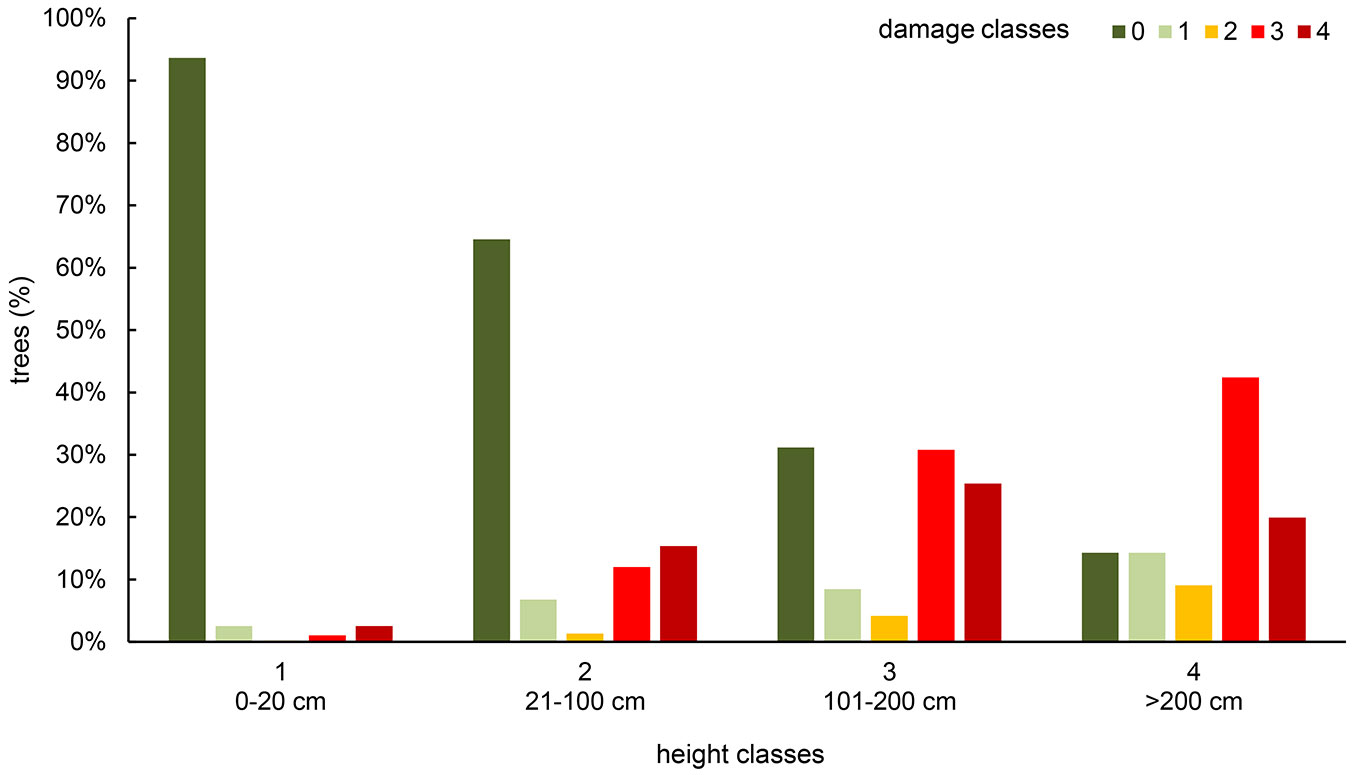
The distribution of the surveyed European ashes in the height classes was as follows: 10.6% in class 1 (<20 cm), 44.2% in class 2 (21-100 cm), 27.7% in class 3 (101-200 cm) and 17.5% in class 4 (>200 cm). Most of the trees in class 1 (94%) were healthy, while single individuals showed heavy damage (1%) or were dead (3%). A significant increase (Tukey’s HSD test, p<0.05) in damaged trees was observed for class 2, where mortality reached 15% and heavy damage reached 12%. In class 3, mortality increased to 25% and heavy damage to 31%, and these values were significantly different (p<0.05) from those in the previous height classes. Similarly, for class 4, the recorded mortality was 20%, and the heavy damage was 42% (Fig. 2).
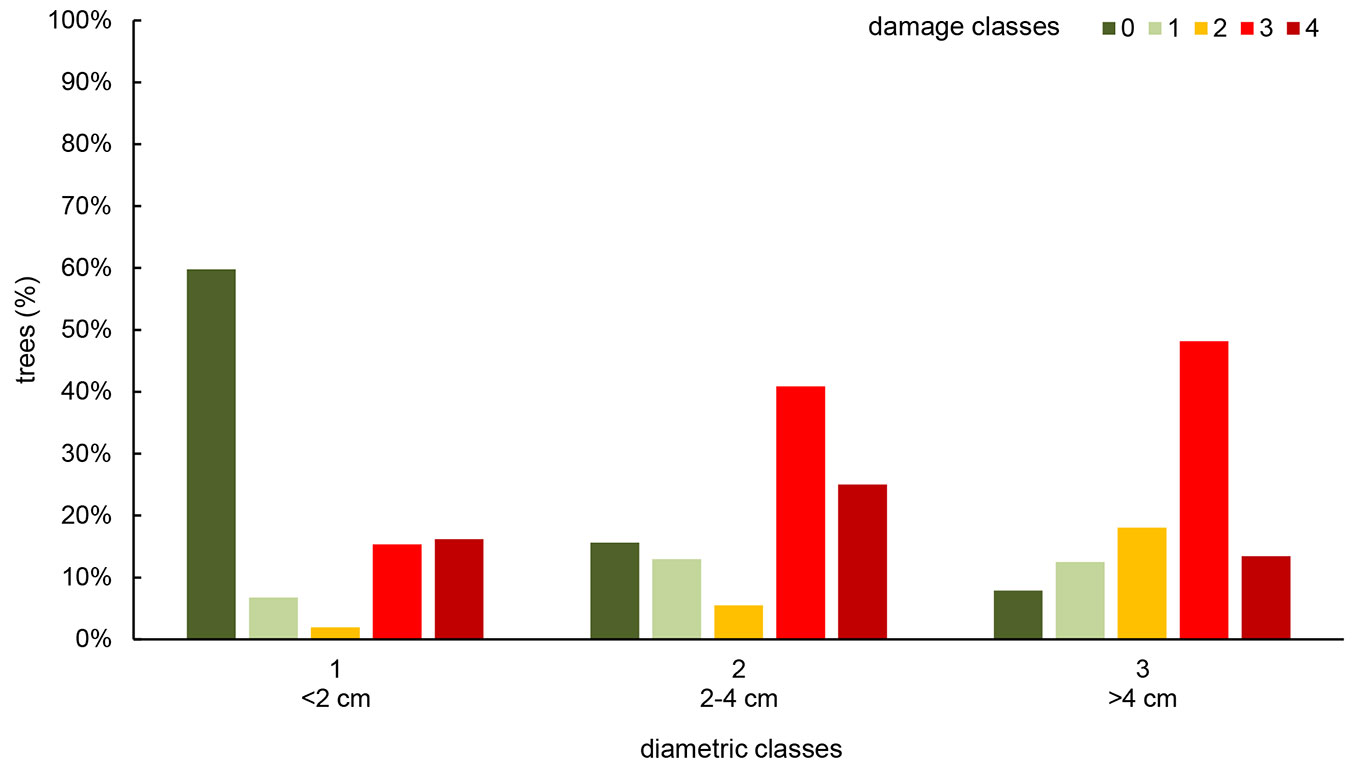
Considering the distribution of diametric classes of F. excelsior regeneration, 77.8% young trees were recorded in the lower class (<2 cm), while 17.4% and 4.8% were recorded in the other two classes (2-4 cm and >4 cm, respectively). Most individuals of the lower class appeared healthy (60%). In the intermediate class, the number of healthy plants significantly decreased to 16%, with a correspondent increase of the most affected ones to 41% (Tukey’s HSD test, p<0.05); 25% of these trees were dead. The upper class showed only 8% of healthy trees, while 48% of subjects occurred in a damage class, and 13% were already dead (Fig. 3); these values were significantly differed from those observed in the first class (Tukey’s HSD test, p<0.05).
Fig. 3 - Distribution (in percentage) of young F. excelsior trees in class of damage, according to diametric classes.
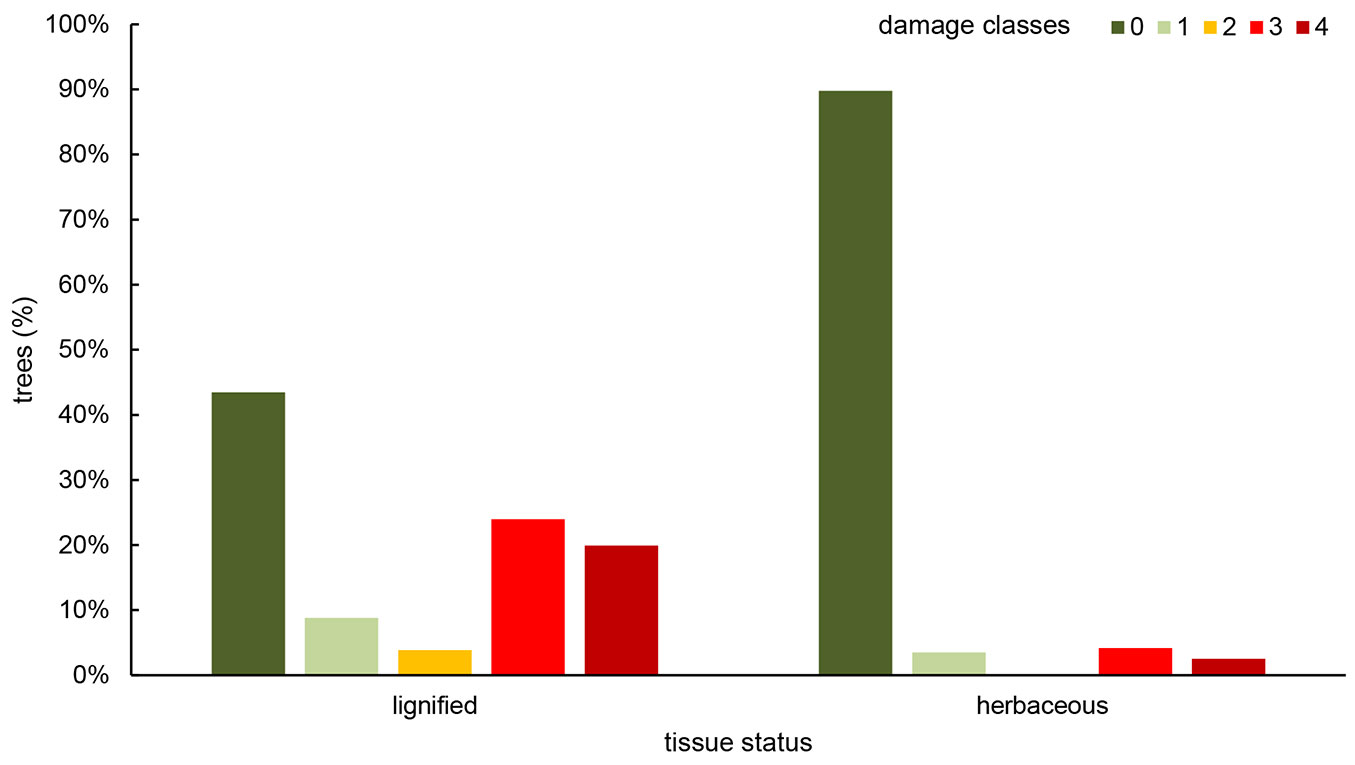
Regarding the tissue status of F. excelsior seedlings and saplings, most of them were already lignified (86.7%), while 13.4% were assessed as herbaceous. As shown in Fig. 4, most herbaceous seedlings were healthy, even if single plants appeared symptomatic or dead (Tukey’s HSD test, p < 0.05).
Regarding the additional data on symptom expression, the highest number of young trees with basal resprouting fell within the third level of damage. Similarly, the highest numbers of necroses on stems and branches and the longest ones were detected in the same class of damage, with a maximum average number of necroses of 0.55 for seedlings taller than 200 cm. With respect to the dead apical shoot, an average length of 134.71 cm was representative for damages in seedlings of the same height category.
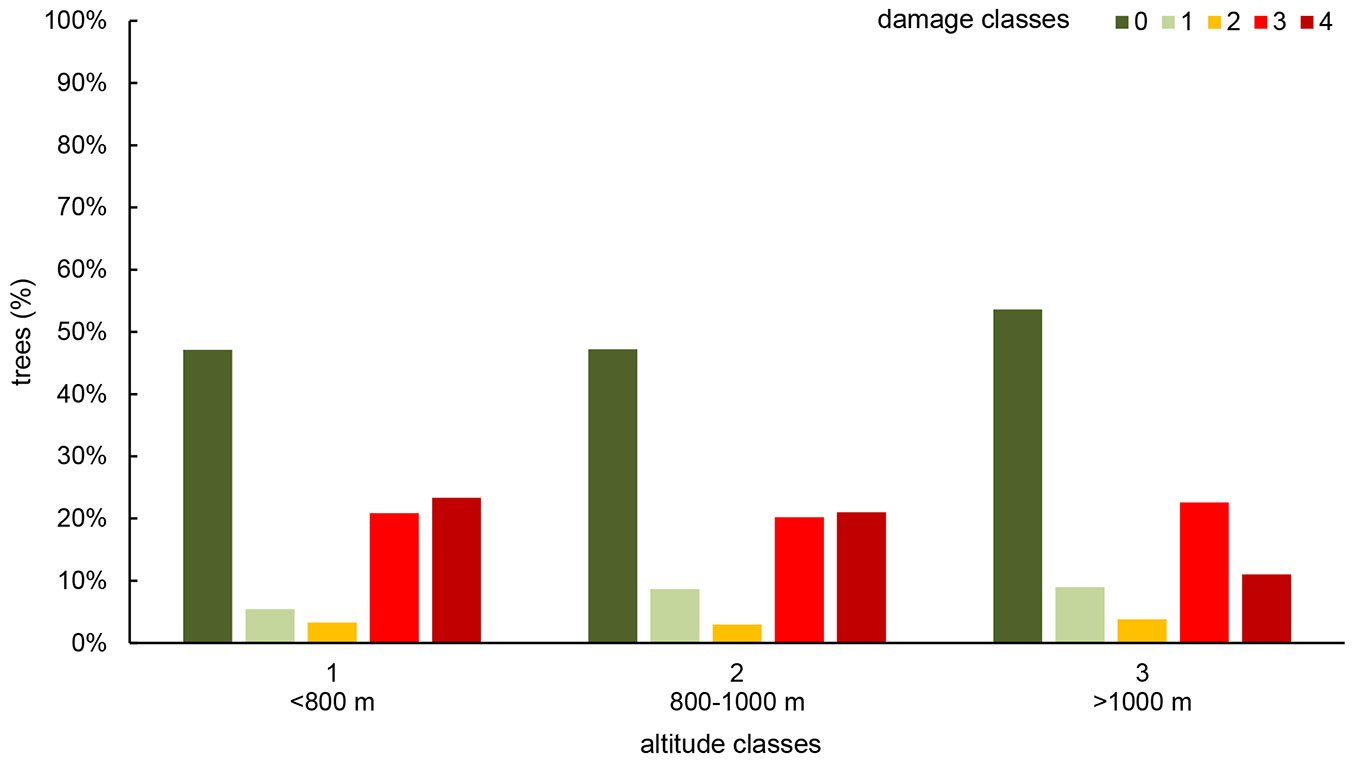
As a further result of the ANOVA, altitude significantly affected the levels of damage of young seedlings (Fig. 5). In particular, the percentage of asymptomatic F. excelsior increased from 47% to 54% at altitudes ranging from 800-1000 m and higher than 1000 m. Similarly, the percentage of ash trees in class 4 decreased from 21-23% to 11%. The number of trees in the intermediate class (1 to 3) did not change by more of 3% among altitude classes.
Fig. 5 - Distribution of young F. excelsior trees in class of damage, according to stand altitude (m a.s.l.).
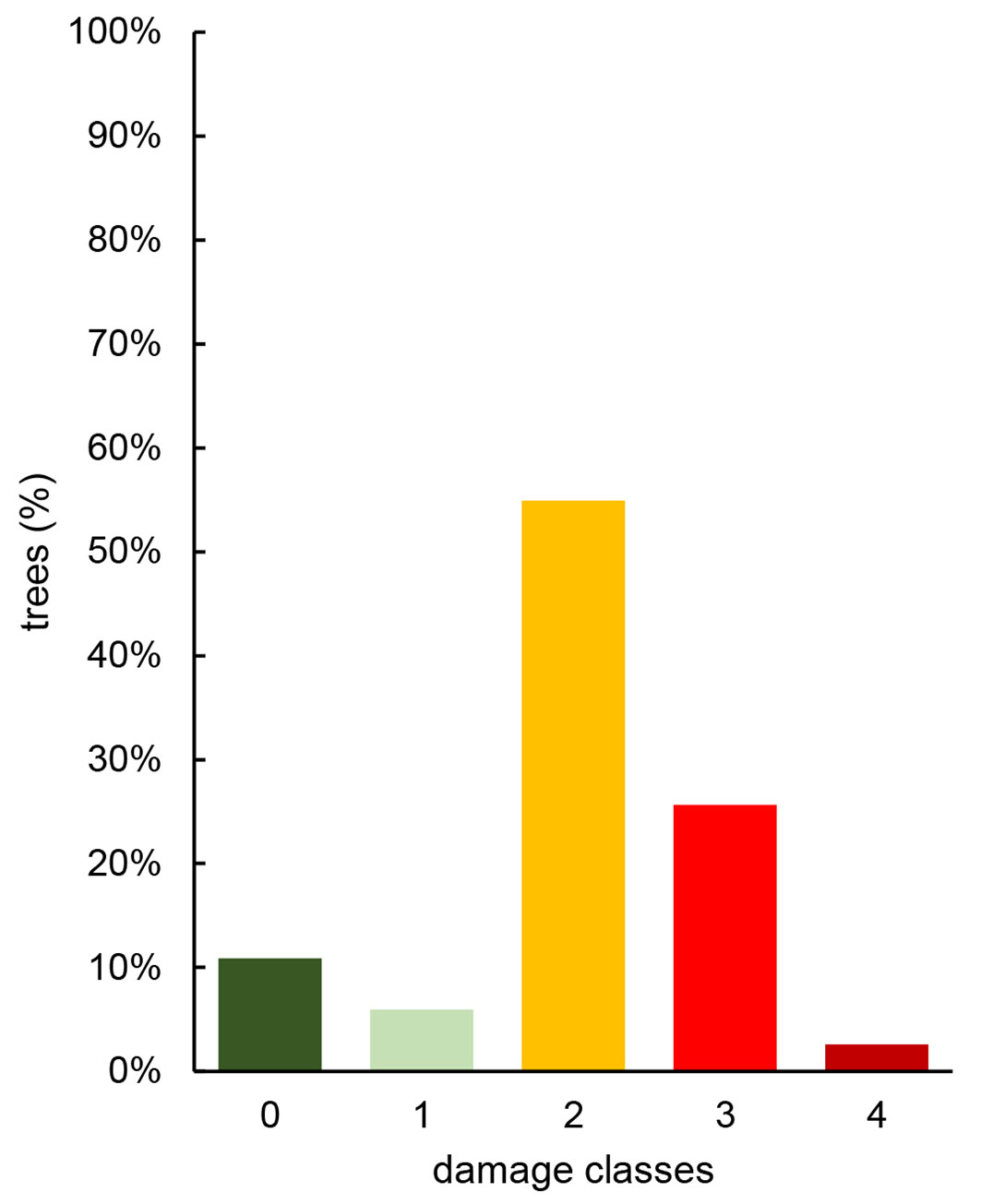
Flowering ash proved to be less susceptible to dieback as compared with European ash. Only 10 individuals out of 384 showed symptomatic branches or stems (Fig. 6). In three plants, small branches were dead and discoloured. In one young tree, more than 5 branches were dead, and in the remaining 6 cases the apical shoot was dead from the terminal bud to the first internode. Discolouration was observed in internal tissues, but generally, it was limited to the wood in correspondence to the external necroses (Tab. 2). No dead foliage attached to dead branches was present, but necrotic spots were observed on the foliage of several subjects. Plants with dead branches or stems were found in 4 of the 10 plots where F. ornus was recorded. In these areas, infection on F. excelsior ranged from 54% to 100% of the trees, while the observed mortality ranged from 0% to 18%.
Fig. 6 - Colonization of F. ornus stem by H. fraxineus: presence of the fungus in wood tissues was confirmed by isolation and molecular analysis.
Tab. 2 - Results of fungal isolations in culture media and molecular analysis carried out on affected samples of F. excelsior and F. ornus. (*): Sample collected near the plot.
| Site | Species | Fungal isolation | Molecular analysis |
|---|---|---|---|
| Val dei Mocheni (Plot 75) | Fraxinus excelsior | + | + |
| Val Genova (Plot 83) | Fraxinus excelsior | + | + |
| Val Maso (Plot 73) | Fraxinus excelsior | - | + |
| Val Ambiez (Plot 63) | Fraxinus excelsior | + | + |
| Vanoi (Plot 65) | Fraxinus excelsior | - | + |
| Val di Sella (Plot 70) | Fraxinus excelsior | + | + |
| Val di Sella (Plot 72) | Fraxinus excelsior | + | + |
| Tione (Plot 72) | Fraxinus excelsior | + | - |
| Val Ambiez (Plot 72)* | Fraxinus ornus | - | + |
| Lavarone (Plot 86) | Fraxinus ornus | + | - |
| Val Maso (Plot 73) | Fraxinus ornus | + | + |
| Val di Sella (Plot 72) | Fraxinus ornus | + | + |
| Val di Fiemme (Plot 6) | Fraxinus ornus | - | + |
| Primiero (Plot 67) | Fraxinus ornus | - | + |
| Primiero (Plot 47) | Fraxinus ornus | - | + |
The fruiting bodies of H. fraxineus were frequently recorded in the investigated area. In particular, their presence was detected in 52 plots out of 90.
The developed conditional inference tree model showed a fairly good fitting of data, with an overall accuracy of 84.4%, a specificity of 84.1% and a sensitivity of 84.6%. Irrespective of the other included variables (altitude, aspect, stand treatment), the final model comprised only the date of the survey as predictor. In particular, the highest number of sites with the presence of fruiting bodies was recorded before the end of September (86.3%), while in October, the detection of fruiting bodies was rarer (20.5%).
Adult tree evaluation
Three hundred eighty-six trees were monitored to assess the disease impact on adult ash trees. Some 344 (89%) were reported as infected even if they displayed different patterns of damage. Most of them (212, i.e., 55%) belonged to class 2, with 20 to 50% of the crown damaged; for 99 (25.5%), the damage reached class 3. As for the other 10 individuals (2.5%), more than 75% of the crown was already damaged, but the trees were still alive. Initial attacks were present on 23 trees (6%). No dead trees were observed. Forty-two trees (11%) were recorded as completely free of symptoms and with complete foliage (Fig. 7).
Spatial autocorrelation analysis indicated the absence of any spatial correlation of affected stands in the region (p = 0.928, z-score = 0.09). Most of the trees were in class 2 of damage and both healthy trees and the most damaged ones were observed in each social, diametric and height classes (Tab. 3). The analysis of variance showed no significant differences in terms of height, diameter and social position of single trees or in terms of altitude, aspect and stand treatment used as predictors (Tab. 3).
Tab. 3 - Distribution of adult trees in the different damages classes for social (D: Dominant; CD: Codominant; d: dominated; F: free), diametric and height classes.
| Group | Class | Damage classes | Total | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |||
| Social position | D | 21 | 12 | 85 | 39 | 4 | 161 |
| CD | 16 | 2 | 70 | 26 | 3 | 117 | |
| d | 1 | 1 | 18 | 15 | 1 | 36 | |
| F | 4 | 8 | 39 | 19 | 2 | 72 | |
| DBH classes | <25 cm | 17 | 14 | 119 | 63 | 7 | 220 |
| 25-50 cm | 22 | 8 | 77 | 32 | 1 | 140 | |
| 50-75 cm | 2 | 1 | 13 | 4 | 1 | 21 | |
| 75-100 cm | 1 | 0 | 3 | 0 | 1 | 5 | |
| Height classes | <8 m | 1 | 4 | 17 | 8 | 1 | 31 |
| 8-16 m | 24 | 11 | 127 | 50 | 4 | 216 | |
| 16-24 m | 15 | 7 | 59 | 39 | 5 | 125 | |
| 24-32 m | 2 | 1 | 9 | 2 | 0 | 14 | |
Healthy trees were observed across all the valleys, even in those already infested in 2012 (Vanoi, Primiero and Valsugana). In some cases, healthy individuals were observed very close to severely affected trees (Fig. S3 in Supplementary material).
No fruiting bodies of other fungi were detected on the surveyed trees, including those severely affected by the disease. On these trees, no basal necroses or other symptoms of decline were observed.
Fungal isolation and molecular analysis
Fungal colonies with morphological characters resembling those described for H. fraxineus were obtained from 6 samples out of 8 branches or apical shoots of F. excelsior. Positive isolation occurred in 3 of 7 infected samples of F. ornus (Tab. 2).
Molecular analysis using species-specific primers revealed the presence of H. fraxineus in seven wood samples of F. excelsior and in six samples of F. ornus assayed. DNA amplification allowed to identify the presence of the pathogen based on characteristic bands which were compared to those of the positive control of H. fraxineus.
The two above methods (culture media and molecular analysis) showed different results in F. excelsior and in F. ornus samples. The molecular analysis was more effective for diagnosing the presence of the pathogen in the wood samples, as compared the culture media method.
Discussion and conclusion
This survey confirmed that ash dieback has already colonized all the valleys of Trentino. The complete colonization occurred in approximately 4 years, considering the first record of the symptoms ([11]). The colonization apparently was not hindered by ecological barriers due to the mountainous topography. It is noteworthy that no movement of plant material nor plantation of ash seedling occurred between the valleys. Moreover, ash trees and wood are not commercialized in this region. The rate of colonization corresponds to 50 km per year, as already suggested by Luchi et al. ([25]) for Northeast Italy. Such efficient spread in Trentino was probably favored by the particular weather conditions that occurred in 2013 and 2014, with high temperatures and precipitation during the entire growing season that were favourable to the fungus ([28]). Moreover, local microclimatic conditions in the valley, where humidity values can be elevated during the growing season, can also enhance the production of fruiting bodies, thus contributing to the spread of the pathogen.
Our results showed a clear impact of the disease on European ash regeneration, with damage and mortality observed almost everywhere. Only the seedling and the current year’s growth seemed less affected by the disease, due to the modalities and the time of infections. Moreover, considering the individuals that displayed some foliage damage, the infection rates could increase by 10%. Remarkably, a high levels of damage was recorded to the established regeneration plots, in which almost all trees were infected. The loss of apical shoots was regularly observed, which could lead to alteration of polar transport of auxin and to marked modifications of wood biometric traits, progressively leading to tree death ([43]). Moreover, the apex loss was always associated with other attacks on branches or with cankers on the stems, thus further reducing the chance of survival. Therefore, though natural regeneration still occurred in all the surveyed plots, its survival up to the adult stage seems severely compromised. As observed in other countries (Germany and Latvia) where the disease has been occurring for a longer period, natural regeneration of European ash trees occurs also under a severe disease pressure, with damages modulated by different environmental conditions, as reported by Enderle et al. ([9]) and Pušpure et al. ([35]). Both the cited works reported that the health status of ash trees worsen with increasing height and age, suggesting that in our context tree survival needs to be monitored for a longer period to assess the real impact of ash dieback. Consistently, our results strengthened the hypothesis of less symptom expression in the youngest seedlings.
Regarding the relationship between the level of infection and stand characteristics, we observed that the average level of damage was lower at higher altitudes (>1000 m a.s.l.). Elevation is highly correlated with many environmental variables that also influence vegetation type ([34]), and the presence of H. fraxineus is conditioned by summer precipitation and air temperature ([5]). Investigations are needed to evaluate a possible role of these environmental factors in affecting the spread of the pathogen in higher mountain areas.
The recovery of affected F. ornus trees in natural conditions confirmed the pathogenicity of H. fraxineus on this species, as previously reported by Kirisits & Schwanda ([18]) which proved the fungus’ ability in colonizing flowering ash leaves. The colonization of leaves (but not stems) was confirmed on seedlings of F. ornus grown in a severely affected F. excelsior area ([19]). In our study, the pathogen was detected in dead and discoloured wood tissues, showing a symptomatology similar to that observed on F. excelsior. In the few observed cases, the fungus seemed confined to the first internode and was unable to spread throughout the wood. Similar observations have been reported in Friuli Venezia Giulia (north-eastern Italy), where affected F. ornus trees were observed in mixed stands with F. excelsior ([3], [4]). Therefore, flowering ash seems to be susceptible to the fungal infections with evident damage, at least when growing together with severely affected F. excelsior or in presence of high loads of ascospores ([19]). The ability of H. fraxineus to spread and colonize pure and mixed stands of F. ornus needs deeper investigations, as this forest type cover more than 40.841 ha in Trentino and approximately 852.202 ha across Italy ([12]), mainly along the Apennines. The possibility of a dangerous spread of the disease also in these kinds of woodlands could also potentially lead to new troubling scenarios in the Mediterranean mountain region ([13]).
Several adult trees with severe damage were observed in this study, though no dead trees were observed during the survey or were reported by the Forest Trees Damage Monitoring (FTDM) survey carried out for the Trentino forests ([38]). However, the observed trees having the crown already desiccated could further decline in the next few years, as already reported in northern and central Europe ([33]). Furthermore, the observed damage has already a strong visual impact, which could be reduced by appropriate pruning or endotherapy ([6], [27]) wherever the affected trees assume any landscape, touristic or ornamental values.
The extent of ash decline observed in Trentino is similar to that observed throughout Europe, as reported in several recent papers ([36], [16], [23]): damage is severe and increasing in both juvenile and adult trees. However, the presence of asymptomatic trees in the surveyed area seems to suggest the existence of resistance or tolerance to the disease ([36], [24]). These plants could constitute the genetic reservoir for implementing the conservation of the species. Nevertheless, much research is needed to confirm this observation and delineate proper management techniques to exploit this potential tolerance ([37], [9], [41]). Apparently, seedlings in the investigated plots seem susceptible to dieback even where asymptomatic adult trees were observed. Moreover, the exclusive sexual reproduction of the pathogen could easily overcome restrictive genetic resistance if evolutionary processes favour fungal genotypes of higher virulence ([24]).
The level of damage has drastically hindered the silvicultural perspectives of European ash in Trentino forests. Also, its potential economical role as the major colonizer of forest gaps or neo-formation stands, as suggested by Wolynski ([44]), might be completely lost.
The impact of H. fraxineus in the Alpine region is substantial. In any case, constant monitoring of the disease is needed in order to evaluate any possible evolution of future scenarios.
Acknowledgements
We would like to thank Emanuel Endrizzi, Elisa Zadra, Maria Claudia Ferretti, Matteo Poda, Alex Dallago, Giorgio Cordin, Chiara Facchinelli for their technical assistance in the field. We also thank Felice Dorna and Massimo Miori (Forest and Fauna Service, PAT, Trento, Italy) and Maurizio Salvadori (Parco Paneveggio and Pale S. Martino, Trento, Italy) for their help in the localization of ash stands.
References
Gscholar
Gscholar
Online | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Online | Gscholar
Online | Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Giorgio Maresi
Fondazione Edmund Mach, Centre for Technology Transfer, v. E. Mach 1, 38010 San Michele all’Adige, TN (Italy)
Fondazione Edmund Mach, Research and Innovation Centre, Department of Sustainable Agroecosystems and Bioresources, San Michele all’Adige, TN (Italy)
Lucio Montecchio
University of Padova, Department of Land, Environment, Agriculture and Forestry, v.le dell’Università 16, 35020 Legnaro, PD (Italy)
Corresponding author
Paper Info
Citation
Giongo S, Oliveira Longa CM, Dal Maso E, Montecchio L, Maresi G (2017). Evaluating the impact of Hymenoscyphus fraxineus in Trentino (Alps, Northern Italy): first investigations. iForest 10: 871-878. - doi: 10.3832/ifor2486-010
Academic Editor
Alberto Santini
Paper history
Received: May 08, 2017
Accepted: Sep 20, 2017
First online: Nov 06, 2017
Publication Date: Dec 31, 2017
Publication Time: 1.57 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2017
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 49039
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 40449
Abstract Page Views: 3280
PDF Downloads: 3919
Citation/Reference Downloads: 20
XML Downloads: 1371
Web Metrics
Days since publication: 3027
Overall contacts: 49039
Avg. contacts per week: 113.40
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2017): 11
Average cites per year: 1.22
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Effect of cadmium (Cd) and lead (Pb) soil contamination on the development of Hymenoscyphus fraxineus on Fraxinus excelsior and F. angustifolia seedlings
vol. 16, pp. 307-313 (online: 09 November 2023)
Research Articles
Lenticel infection in Fraxinus excelsior shoots in the context of ash dieback
vol. 12, pp. 160-165 (online: 04 March 2019)
Research Articles
Seasonal development of lesions caused by Hymenoscyphus fraxineus on young Fraxinus excelsior trees in Latvia
vol. 11, pp. 17-23 (online: 09 January 2018)
Research Articles
Genetic variation of Fraxinus excelsior half-sib families in response to ash dieback disease following simulated spring frost and summer drought treatments
vol. 9, pp. 12-22 (online: 08 September 2015)
Research Articles
Temporal development of collar necroses and butt rot in association with ash dieback
vol. 10, pp. 529-536 (online: 05 May 2017)
Research Articles
Species-specific responses of wood growth to flooding and climate in floodplain forests in Central Germany
vol. 12, pp. 226-236 (online: 03 May 2019)
Research Articles
A resource capture efficiency index to compare differences in early growth of four tree species in northern England
vol. 10, pp. 397-405 (online: 24 March 2017)
Research Articles
Effect of soil-applied lead on mineral contents and biomass in Acer cappadocicum, Fraxinus excelsior and Platycladus orientalis seedlings
vol. 10, pp. 722-728 (online: 27 July 2017)
Research Articles
Cryptogamic epiphytes and microhabitat diversity on non-native green ash (Fraxinus pennsylvanica Marsh., Oleaceae) in urban habitats
vol. 14, pp. 393-399 (online: 01 September 2021)
Research Articles
Application of fungicides and urea for control of ash dieback
vol. 8, pp. 165-171 (online: 13 August 2014)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword