Seasonal development of lesions caused by Hymenoscyphus fraxineus on young Fraxinus excelsior trees in Latvia
iForest - Biogeosciences and Forestry, Volume 11, Issue 1, Pages 17-23 (2018)
doi: https://doi.org/10.3832/ifor2283-010
Published: Jan 09, 2018 - Copyright © 2018 SISEF
Research Articles
Abstract
The spread of the ascomycete Hymenoscyphus fraxineus, causing dieback of common ash (Fraxinus excelsior) in Europe, is rapid and the damage is pronounced, as young ashes can perish over the course of only a few months following infection. The objective of this study was to investigate the rate and extent of lesion formation on young (5-8-year-old) ashes during a vegetation season in the hemiboreal zone in Latvia. Continuous surveys (with monthly intervals) of the health condition of 30 young ash and measurements of lesion area in three stands were performed during the vegetation season of 2015. From June to September of that year, the number of observed lesions gradually rose from 58 to 87. New lesions emerged on branches (55%, 0.5 per tree), top shoots (28%, 0.3 per tree), and stems (17%, 0.2 per tree), mostly appearing at the beginning of the observation period (45%, 52%, and 3% in June, July, and August, respectively). During the vegetation season, 20% of the existing and 28% of the newly-emerged lesions on branches, as well as 20% and 25% of top shoot lesions, respectively, reached the main stem. Some (< 20% of cases) transitions of lesions from the tops and branches to the stems were observed. The extension of lesions was significant until August, and ceased afterwards in a similar fashion in all stands. The mean extension of area significantly differed between the previously-existing and newly-emerged lesions. During the vegetation season, the new lesions expanded by 25.1 ± 4.8 cm2, whereas the existing ones grew by only 7.3 ± 1.1 cm2. The extension of the new lesions varied according to their location on a tree. The spread of emerging lesions on stems was considerably slower than on branches or top shoots (1.9 ± 0.7, 7.3 ± 1.5, and 14.5 ± 4.1 cm2 per lesion per month, respectively). During the studied vegetation season (summer), the overall health score of trees decreased twice, yet the relationship between heath status and development of lesions lacked significance.
Keywords
Introduction
The ascomycete Hymenoscyphus fraxineus is a disease agent that has caused dieback of common ash (Fraxinus excelsior L.) in Europe in recent decades ([23]). Since the 1990s, ash dieback has been observed in Lithuania ([34]) and Latvia ([17]), although in Latvia, it was only confirmed in 2007 ([10]). The number of countries affected by ash dieback continues to increase and already exceeds 20 ([6]). This dieback affects stands of different ages and compositions ([24], [29]), yet young stands are the most susceptible to infection, and hence are critical for development of the pathogen ([31], [2]).
The spread of H. fraxineus within its host is rapid, irrespective of tissue type, and proceeds in three dimensions ([29]). Some of the affected trees can be destroyed promptly, particularly as the fungus girdles the main stem, whereas others can have chronic symptoms ([32], [23]). Primary symptoms of the disease are macroscopic cankers on leaves and leafstalks ([31]), brown spots on buds ([2]), and wilting of leaves and/or top shoots ([29]). These symptoms are followed by the formation of necrotic lesions spreading along rachises onto shoots, branches, and stems, resulting in dieback of the affected parts of a tree ([1], [31], [2]). Skovsgaard et al. ([31]) noted, however, that cankers may also appear before wilting and dieback of shoots occurs. The appearance of lesions on undamaged stems suggests that the fungus could have entered through the lenticels ([8]). In addition, several strains of the fungus can attack a host simultaneously ([2]). After dieback of the primary shoots, the affected trees can recover growth by formation of epicormic shoots, resulting in a bushy appearance of tree crowns ([6]). Nevertheless, for most ash trees, lifespan is considerably reduced ([31]).
Due to the threatened existence of ash ([23]), natural resistance against the pathogen and methods for improving this resistance have been among the most commonly investigated issues regarding the dieback ([14], [21]). Seasonal dynamics of lesion development, specifically balance between emergence and entering the latent phase, has been shown to be a proxy of the dieback process ([2]). Pliura et al. ([26]) concluded that none of the tested provenances or progenies of ash had complete resistance to the infection or development of the disease, yet their susceptibility notably differed. In contrast, McKinney et al. ([21]) were more sceptical, arguing that much more time is required for ash to form a resistance to H. fraxineus by means of natural selection. Alternatively, varying susceptibility to the dieback might be related to phenological differences in the seasonal cycles of trees and the fungus ([20], [2]). Among abiotic factors, seasonal temperature has been shown to significantly affect the development of lesions ([2]); however, McKinney et al. ([20]) observed development and spread of the fungus during the dormant period, suggesting only a partial role of this factor. Hence, more comprehensive information concerning the factors affecting the formation of lesions is required.
The aim of this study was to assess the pattern of lesion development caused by H. fraxineus in young ash trees during a growing season. We hypothesised that the development of lesions varied during the vegetation season, and that it was more intense at the beginning of summer when the newly-formed tissues of ash had not yet matured. We also hypothesised that the development of emerging lesions occurred more quickly than that of those already existing.
Materials and methods
Studied sites
This study focused on 30 young ash trees (5-8 years of age, according to inventory data) growing in three naturally regenerated post-clear-cut stands in Latvia (Fig. 1) that were dominated by ash in the preceding rotation. These trees were monitored in 2015 from June to September, when the greatest fungal activity is expected ([33], [2]). The stands were fertile and corresponded to the Aegopodiosa site type, according to the national classification by Bušs ([3]). Trees grew on flat terrain with a well-drained fertile loamy soil, although water excess occurred during the moist springs. The climate could be classified as moist continental, with the mean annual temperature (± standard error) during the most recent three decades ranging from 6.2 ± 1.6 to 7.1 ± 1.5 °C and the mean annual precipitation ranging from 665 ± 13.9 to 618 ± 12.4 mm in the eastern and central parts of Latvia, respectively. The highest monthly precipitation occurred in July and August. The mean temperature in June, July, and August 2015 was 14.9 ± 0.3, 16.9 ± 0.4, and 18.7 ± 0.6 °C in the central part, and 14.7 ± 0.3, 16.2 ± 0.4, and 17.6 ± 0.6 °C in the eastern part of Latvia, respectively, which were ca. 0.3 °C cooler than the 30-year mean values of the respective regions. The precipitation in these months was similar to the long-term mean (ca. 76.9 ± 6.5, 70.8 ± 6.2, and 62.6 ± 5.1 mm, respectively).
The densities of the Bauska and Aizpurve stands were the greatest (ca. 8000 and 3500 trees ha-1, respectively), and their compositions were mixed. The Bauska stand was dominated by ash (ca. 5000 trees ha-1), with an admixture of common aspen (Populus tremula L.) and goat willow (Salix caprea L.; ca. 1500 trees ha-1 each). In the Aizpurve stand, ash had a considerably lower density (ca. 1500 trees ha-1), and was mixed with Norway maple (Acer platanoides L.) and common aspen (ca. 1500 and 500 trees ha-1, respectively). In the Limbaži stand, ash was the dominant species (density ca. 1900 trees ha-1), with a small admixture of silver birch (Betula pendula Roth; ca. 200 trees ha-1). The dieback process was apparent in all studied stands, as indicated by ash saplings with obvious damage (lesions on top shoots, branches, and stems), confirming the presence of the pathogen. No obvious signs of other diseases or damages were observed.
Sampling and measurements
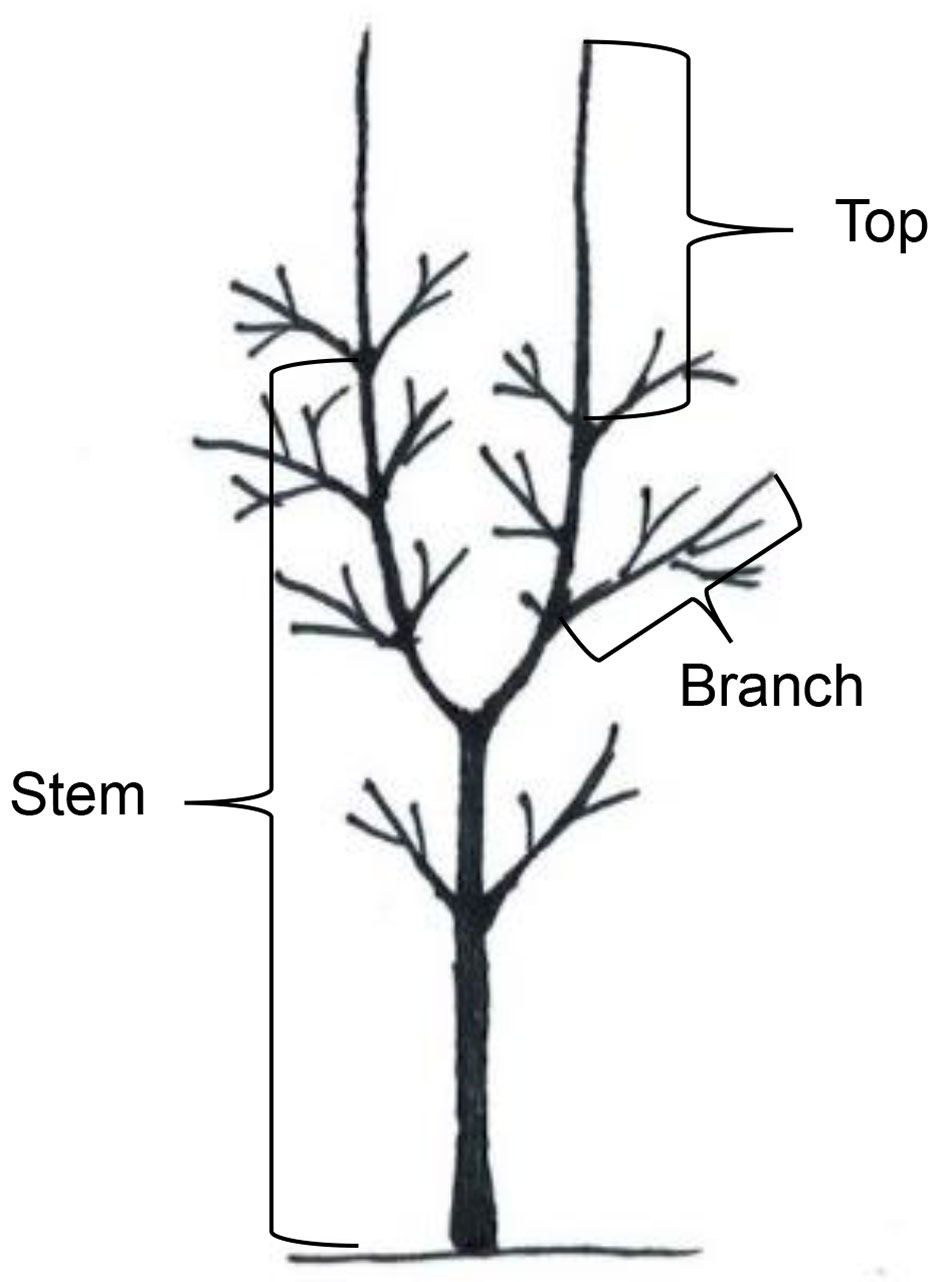
In each stand, 10 dominant unsheltered ash saplings with heights of 2.5-3.0 m were selected. At the beginning of the observation, all chosen saplings had one to three lesions that were necroses (discolorations) on stems (40%), tree tops (32%), or branches (28% - Fig. 2). Only trees with a small initial lesion area (the maximum area of the lesions on stems, branches, and tree tops were 37 cm2, 5 cm2, and 18 cm2, respectively) were selected. At the beginning of the survey in June, height and diameter at breast height of the sample trees were measured with accuracies of 5 cm and 0.5 mm, respectively. For four months (from the 10th to the 15th of each month), the health condition of each sapling (quality of crown, and proportion of damaged leaves and shoots) was graded (Tab. 1) according to the methodology reported in Pušpure et al. ([27]). In June, all visible lesions on branches and stems were marked on a transparent film. Development of lesions - the area of the extension since the last measurement, as well as the emergence of new lesions - was marked on the films at monthly intervals. All measurements were taken by the same person. The location of each lesion, such as necrosis on stems (elongated axis from the apical shoot to the root collar), or necrosis and wilting of top shoots (top shoot down to the uppermost axil) and branches (from stem to top of shoots), was recorded (Fig. 2). For bifurcated trees, all tree tops were considered individually (Fig. 2). After the final survey in September, the damaged parts of the saplings were sampled, bark was removed, and the area of the discoloured wood was marked on the film. To confirm the presence of H. fraxineus in the studied trees, samples from the symptomatic material, i.e., from the inner bark or wood from the largest lesions, where the quantity of H. fraxineus mycelium presumably was the highest ([29]) were collected. In the Bauska, Limbaži, and Aizpurve stands, seven, six, and four samples were collected, respectively.
Tab. 1 - Grades of ash sapling health condition. (AGB): aboveground biomass.
| Grade | AGB Damage (%) |
Damage visual characteristics |
|---|---|---|
| I | 0 - 10 | Tree looks healthy or slightly damaged individual leaves |
| II | 11 - 25 | Damaged several leaves, some necroses of the bark |
| III | 26 - 60 | Fully damaged/dead separate branches; damaged part of the foliage; necroses of the bark on large areas |
| IV | 61 - 99 | Completely broken up dead part of the crown; partially damaged the entire crown; live separate branches in secondary crown |
| V | 100 | Tree is completely dead |
In a laboratory, the areas of the lesions from each tree and month were measured on the films with the accuracy of 0.01 cm2 using a TAMAYA digital planimeter PLANIX 10S “Marble”. To isolate H. fraxineus, samples of the infected material were surface sterilised by submersion in 35% hydrogen peroxide for 30 seconds and washed twice in distilled water for one minute. After draining, the samples were placed on a Petri dish containing 1% malt agar and incubated in darkness at 20°C for four weeks. As the study was focused on H. fraxineus, any other emerging fungi were mechanically removed from the samples every three days to facilitate development of the target species. The systematic affiliation of the isolate was confirmed microscopically according to Kowalski ([16]).
Data analysis
Based on the rate of extension during the observation season, lesions were divided into three groups: active, inactive, and latent. A lesion was considered active if it had expanded since the last measurement, and considered latent if no expansion occurred during the entire observation period. Newly-emerged lesions (NL), which appeared during the observation period, exhibited patterns of development that differed from those of existing lesions (EL), and were therefore analysed separately. The differences in size and rate of extension of the lesions (per tree) according to their location on a tree (Fig. 2), their age (NL or EL), overall health condition of the sapling at the beginning of the observation period, and site were evaluated using analysis of variance (ANOVA). A paired-sample t-test was applied to assess differences in the extension of the lesions between the consecutive months, and a Bonferroni transformation was used to adjust the p-values of the differences. A generalised linear model (GLM) utilising the binomial distribution of residuals was applied to assess differences in the activity of the lesions (active or inactive, as well as latent or non-latent) according to their location, date of observation, health condition of the sapling, and site. Differences in the number of active/inactive/latent lesions per tree, as well as NL and EL, were assessed according to the same factors via GLM, using a Poisson distribution of the residuals. In all cases, Tukey’s honest significant difference post-hoc test was applied to compare the levels of significant factors. Relationships between the lesion area above and below the bark at the end of the survey were quantified through bootstrapped ([9]) Pearson correlation analysis, whereas relationships between health condition class at the end of the study and sapling height and diameter were quantified via bootstrapped ([9]) Kendall correlation analysis. The sapling was considered as the statistical unit. All data analyses were conducted in R ver. 3.3.3 ([28]) using the “multcomp” package ([7]), with a significance level of α = 0.05.
Results
Activity of lesions
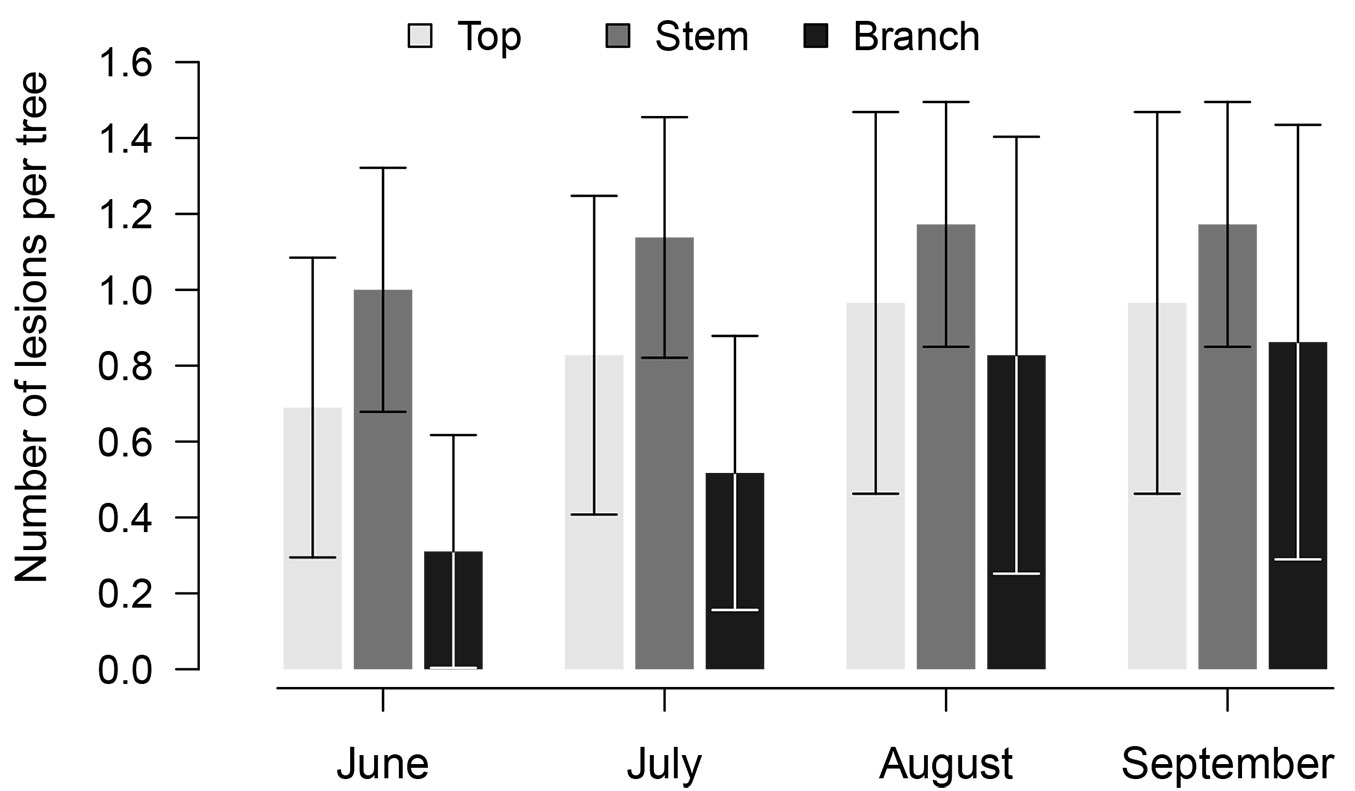
In total, 319 observations of 87 lesions were made. The observed lesions (Fig. 3) were necroses on stems (39.8%, the mean ± standard error number of lesions per tree was 1.2 ± 0.8), tree tops (31.8%, 0.9 ± 0.5 per tree), and branches (28.4%, 0.9 ± 0.3 per tree). Most of the lesions (67%) were EL, of which 26% were latent; NL comprised 33% of all observed. Among the EL, 50%, 34%, and 16% were located on stems, tree tops, and branches, respectively, whereas 17%, 28%, and 55% of the NL appeared on those same parts, respectively. The number of NL significantly (p-value < 0.001) differed among months and sites. Overall, NL appeared at the beginning of the observation period (45%, 52%, and 3% in June, July, and August, respectively). The number of NL was significantly (p-value < 0.001) lower in the Limbaži and Aizpurve stands (12% and 20% of the accounted lesions; 0.3 and 0.1 lesions per tree that emerged in June and July, respectively) than in the Bauska stand (62% of the accounted lesions), where 0.7, 1.3, and 0.1 lesions per tree emerged in June, July, and August, respectively. H. fraxineus was isolated in six of the 17 samples collected (35% - four in Bauska and two in Aizpurve).
Fig. 3 - The number of the accounted lesions per tree during the observation period according to their location on trees. Whiskers indicate standard errors.
The mean proportion of latent lesions was similar (p-value = 0.23) among the locations on trees (25 ± 10 %), yet it was significantly (p-value = 0.007) higher in Limbaži than in Bauska (39 ± 9% and 12 ± 6%, respectively); in the Aizpurve stand, it was intermediate (30 ± 11%). The proportion of active lesions (Tab. 2) differed between EL and NL (p-value < 0.01), and among locations on trees (p-value = 0.02), and varied during the season, with significant differences observed among months (p-value < 0.001). Nonetheless, the activity of lesions was similar among the stands (p-value = 0.13). During the entire observation period, the highest activity was observed for lesions on stems (68 and 75% for EL and NL were active, respectively) and tree tops (58 and 67%, respectively). The activity of lesions on branches was the lowest, as 52% of EL and 50% of NL expanded during the observation period. For both EL and NL, activity was the highest in July (83% and 100%, respectively), and then sharply decreased in August (39% and 50%, respectively).
Tab. 2 - The proportion of active (expanding since the last observation) lesions during the observation period according to their location on tree. (N_sept): number of accounted lesions in September.
| Period | Existing lesions | Newly emerged lesions | ||||
|---|---|---|---|---|---|---|
| Top | Stem | Branch | Top | Stem | Branch | |
| June (%) | 58 | 83 | 43 | 33 | 75 | 17 |
| July (%) | 67 | 83 | 86 | 100 | 100 | 92 |
| August (%) | 50 | 39 | 29 | 67 | 50 | 42 |
| Total (% mean) | 58 | 68 | 52 | 67 | 75 | 50 |
| N_sept | 12 | 23 | 7 | 6 | 4 | 12 |
Extension of lesions
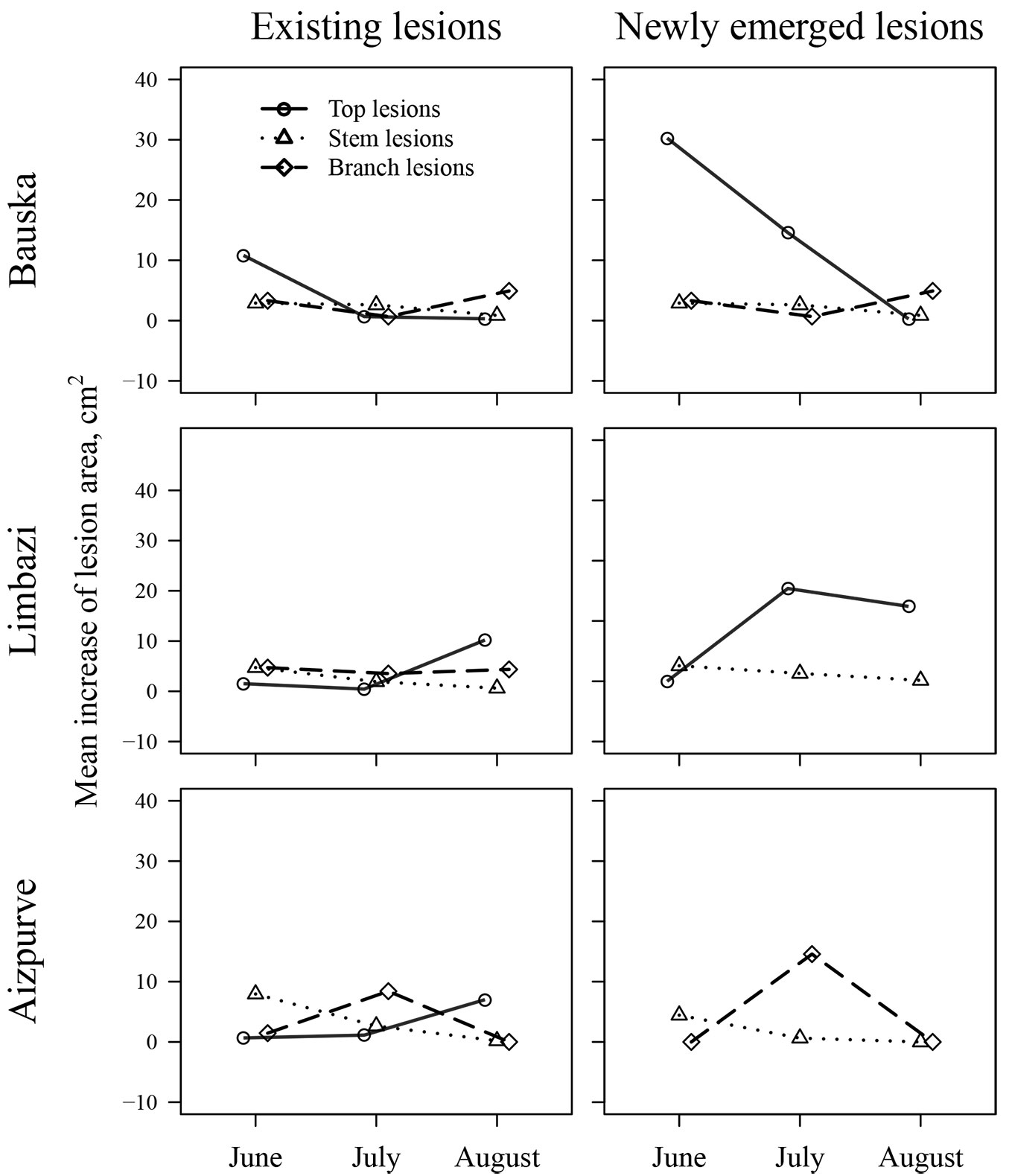
During the season, the area of lesions ± standard error increased from 52.5 ± 11.3 cm2 per tree in June to 92.1 ± 14.7 cm2 per tree (91.9 ± 60.1, 42.3 ± 11.5, and 26.6 ± 15.9 cm2 per tree for top, branch, and stem lesions, respectively) at the end of the observation period (Fig. 4). The extension of the lesions was highly significant (p-value < 0.001) until August, but ceased thereafter, similarly across all stands. The mean extension of lesion area significantly differed between EL and NL (p-value < 0.01). During the season, NL extended by 25.1 ± 4.8 cm2, whereas EL only expanded by 7.3 ± 1.1 cm2. The rate of extension of EL was similar among stands (p-value = 0.84) and locations on trees (p-value = 0.47; 2.48 ± 0.70 cm2 per lesion per month). The extension of NL was also similar among the stands (p-value = 0.50); however, it differed among locations on trees (p-value = 0.02). The extension of lesions on the stems was considerably slower than on the branches and tree tops (1.9 ± 0.7, 7.3 ± 1.5, and 14.5 ± 4.1 cm2 per lesion per month, respectively). The development of lesions on the stems and branches culminated in June and July, respectively, similarly for both EL and NL. The patterns of development of EL and NL on tree tops differed; NL had the fastest enlargement after appearance (mostly in June), whereas EL exhibited a maximum extension later in the season (in August; Fig. 4). A strong correlation (r = 0.99) between the area of the lesions above and below the bark in late September suggested a clear linear dependence of these variables (Fig. 5). Nevertheless, the difference between the lesion areas below and above the bark was significantly (p-value < 0.001, by ca. 6%) higher for EL than it was for NL (ca. 2%), yet it was similar among stands and locations on trees.
Fig. 4 - The mean monthly increase in area of the lesions existing before the observation period and newly emerging lesions in the studied stands according to the location on trees. The symbols are shifted for clarity.
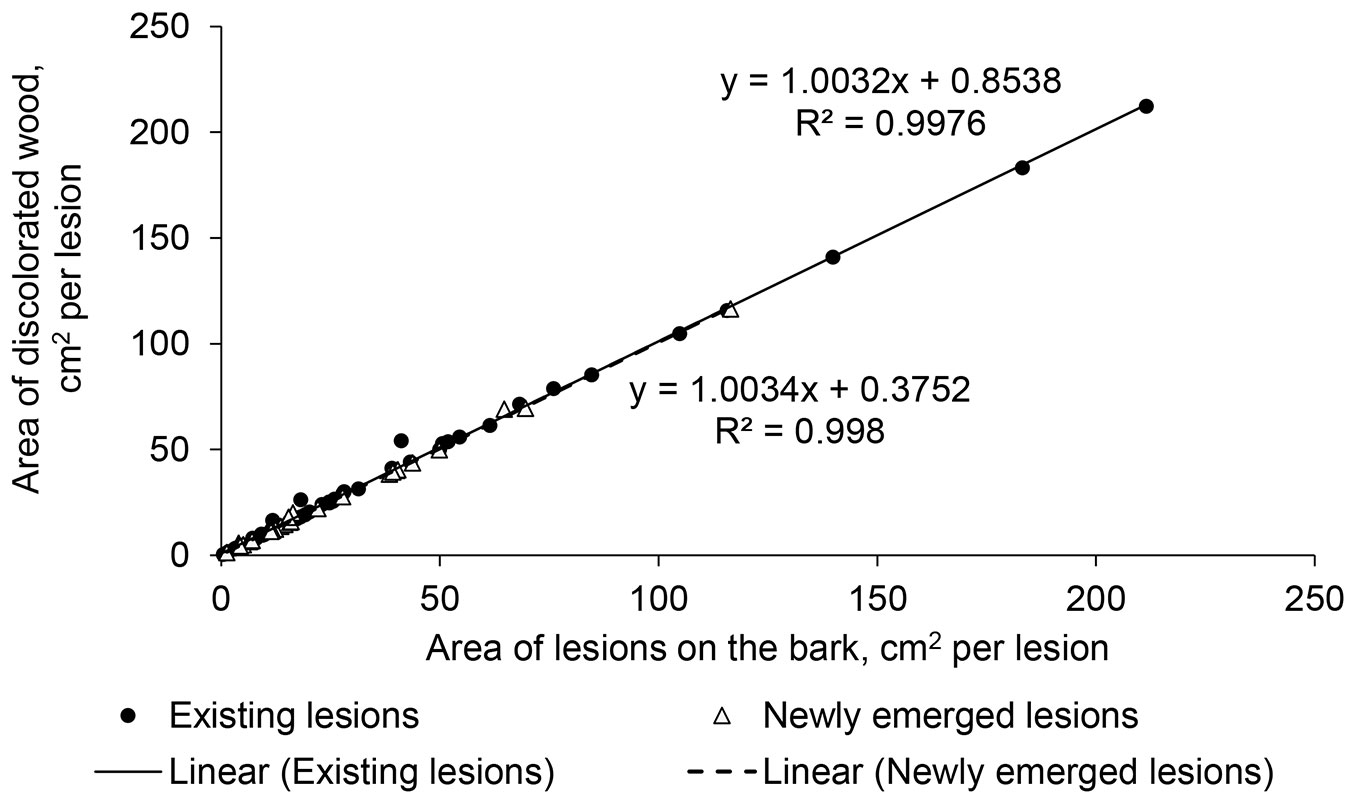
Fig. 5 - The relationships between the visible area and area below the bark of the lesions existing before the observation period and newly emerging lesions.
As the season advanced, 28% of EL and 20% of NL girdled branches, causing their complete dieback. Some transitions of lesions from tree tops and branches to stems were also observed. At the first observation, five necroses on stems coincided with dead branches, whereas in the following months, three lesions from girdled branches expanded onto the stems. The dieback of the infected tops was slightly weaker, as 20% (5% in June, 15% in July) and 25% (in August) of the EL and NL, respectively, girdled tree tops, causing their dieback. Five of the observed 28 tree top lesions expanded onto the stem.
Health condition of trees
The growing number of lesions and the area they covered decreased the overall health condition of saplings; their health grade increased from 1.6, 1.6, and 1.7 in June to 2.7, 3.2, and 2.9 at the end of the observation period (September) for the Limbaži, Bauska, and Aizpurve stands, respectively. Nevertheless, mortality of the studied saplings was low, as only one sapling in the Aizpurve stand died in August. That sapling was strongly mechanically damaged in July and had an extensive lesion area (215 cm2).
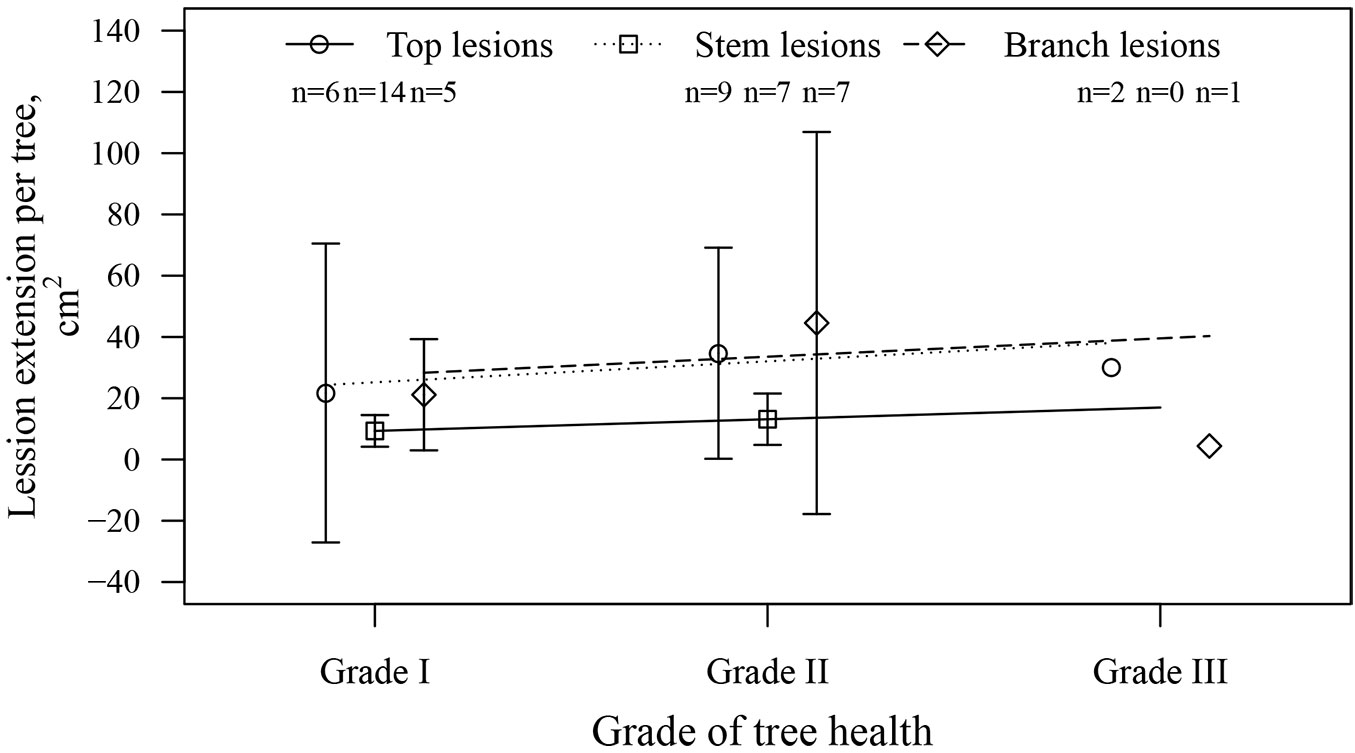
There were no significant relationships between the health grade at the beginning of the observation period and the expansion rate of EL or NL, nor between health grade and the number of lesions, although slight positive tendencies were visible (Fig. 6). There was no significant relationship between the number of EL and NL, suggesting a similar probability of a lesion emerging, irrespective of preceding infection. The morphometric parameters showed no effects on the development of lesions, as the correlations between the total lesion area per sapling in September and height, as well as between total lesion area and diameter of the saplings, were not significant (r = -0.09 and 0.11, p-value = 0.55 and 0.47, respectively). Health condition class in June was negatively affected by tree diameter (τ = -0.21, p-value = 0.01), but not by tree height (τ = -0.02, p-value = 0.79).
Fig. 6 - The mean increase in the lesion area (extension) per tree during the observation period according to the health grade of saplings in June 2015 (beginning of the observation period). Whiskers indicate standard error (where applicable). (n): number of trees.
Discussion
Agent and occurrence of lesions
The isolation of H. fraxineus confirmed its involvement in the formation of lesions on ash saplings. The proportion of positive samples was intermediate (35%) in comparison to that found in Sweden ([2]) and lower than that in nurseries in Germany ([29]), suggesting the involvement of other agents ([8]). As this study focused on H. fraxineus, other agents were not quantified. Alternatively, the low occurrence of the pathogen in samples might be explained by seasonal ([20], [2]) and tree-vice ([29]) variation in the number of propagules, or by intense surface sterilisation of samples prior to incubation.
Most of the observed lesions were located on stems (Fig. 3), which were the largest parts of the trees, and hence featured most EL, including those previously occurring on tops. Considering that propagules of H. fraxineus ([29]) infect their host through leaves and shoots ([4]), most NL were found on branches (twigs) and tops (Fig. 3). The occurrence of NL on the lower parts of stems suggests that infection may have occurred through an alternative pathway (e.g., via lenticels or fine roots - [8], [5]), but genotyping would be necessary to validate this hypothesis. Nonetheless, there was no visible discoloration of wood below EL on stems leading towards shoots, as observed by Bengtsson et al. ([2]), although young hyphae may not be visible ([29]).
Lesion activity
Although sporulation of many pathogenic fungi is synchronised with the biological cycles of their host, H. fraxineus has an extended period of spore release ([11]), indicating a high possibility of infection throughout the season. Most NL emerged in June and July (Fig. 3), however, implying a seasonal pattern, which might be explained by the maturation of leaves and shoots ([29], [33]). The majority of NL appeared on branches (Tab. 2), where most leaves, which are the primary infected organs ([11], [13]), occurred.
The development of lesions explicitly differed among the stands, as indicated by the number and activity of lesions (Fig. 4). These differences might be related to the structure of the stands, as well as to the connectivity between them ([18]), and hence also to the abundance of propagules. The moister microclimate of a denser stand is considered to facilitate development of the pathogen ([33]), hence the occurrence of lesions. Although Bengtsson et al. ([2]) and McKinney et al. ([20]) suggested a weak effect of temperature on the development of lesions, differences among the stands (Fig. 4) still might be related to local climate. The highest and lowest numbers of active lesions was observed in the Bauska and Limbaži stands, where summers were the warmest and coolest, respectively. The effect of temperature was also supported by the seasonal pattern of lesion development, as the highest activity, particularly for NL, occurred in July (Tab. 2), when the temperature was the warmest (ca. 15-18 °C). Previously, the highest activity of H. fraxineus has been observed when the mean temperature is close to 20 °C ([15], [33]). The sharp decrease in lesion development after August (Fig. 3, Tab. 2) might be linked to shortening of the photoperiod and initiation of cold hardening, which ceases the development of lesions ([20]). It remains difficult, however, to overtly distinguish the influence of temperature on fungal and tree life-cycles ([2]).
Lesion extension
The extension of lesions, which could be considered the main process affecting the overall condition of saplings ([20]), differed between EL and NL, as well as amongst the affected parts of trees, and were modulated by local factors (Fig. 4). The extension of lesions mostly decreased as the season advanced (Fig. 4), similarly to the emergence of NL. Nonetheless, higher rates of extension of NL indicated that after emergence, lesions rapidly grew to a certain minimum size within the first month. Apparently, a month was necessary for ash to partially compartmentalise the development of the pathogen ([22]), slowing extension of the lesion. Alternatively, this might be connected to the higher activity of younger strains of fungus ([19]). The opposite was observed for EL on tree tops in two of the three stands, which exhibited maximum extension in August (Fig. 4). This could likely be related to slower compartmentalisation of the pathogen at the cessation of the growing period, when formation of callus is slower ([22]). In the Bauska stand, however, EL on tree tops were the most active in June, presumably due to the moister microclimate of the denser stand.
At the end of the observation period, the largest lesions were observed on tree tops, which were the largest shoots. The fastest spread of the pathogen within its host occurs in the stem pith, which is closest to the surface of shoots ([29]). In addition, the bark on shoots is thinner, facilitating the extension of lesions ([8]). Larger lesions on top shoots might also be related to microclimatic conditions, as apical shoots receive more atmospheric moisture during the night (dew), particularly in dense stands, as suggested by NL in the Bauska stand (Fig. 4). In the older parts of a tree, hyphae must apparently penetrate wood ray parenchyma ([29]), which limits their development, explaining the slower extension of lesions on stems (Fig. 4). The opposite was observed by Bengtsson et al. ([2]) in Sweden, likely due to differences in climate and/or tree genetics ([25], [26]). Nevertheless, the actual size of lesions (under bark) was larger for EL (Fig. 5), implying some latent extension, as observed by Schumacher et al. ([29]) and McKinney et al. ([20]). The actual and visible sizes of NL were more similar (Fig. 5), likely because of the rapid extension (Fig. 4).
Health condition of trees
The intermediate proportion of NL (33% of the accounted) and low mortality of the affected parts of trees (ca. 22%) indicated chronic formation of lesions, suggesting a certain resistance to the dieback ([26]). The presence of latent EL suggested that trees were able to sufficiently compartmentalise the pathogen ([22]). In addition, biological limitations (e.g., inactive physical defence) of development ([2]) were observed, as most of the lesions stopped expansion when reaching the shoot base or the main stem. This might be related to the anatomical properties of wood, such as differences in vessel size and lateral connectivity, or pith diameter between the transitions of height increments of consecutive years ([30]). Regarding shoots, this might also be linked to differences in the thickness of primary and secondary bark compared to stem diameter ([8]). Nevertheless, previous studies have shown that the mortality of affected trees increases drastically in subsequent years ([12], [25], [26], [2]).
Although the overall health condition of the saplings decreased differently during the observation period, the non-significant relationships between initial health condition and the number and extension of lesions (Fig. 6) suggested comparable infection pressure for all saplings, regardless of previous infection. This was supported by the non-significant relationship between the number of EL and NL in September, indicating the influence of stochastic processes or microclimate. The opposite was observed by McKinney et al. ([20]) in Denmark, where susceptibility to the disease appeared to be prevailingly controlled by deterministic factors, such as genetics. Such differences could be related to the milder and more humid climate in Denmark that facilitates development of H. fraxineus and extension of lesions ([15], [33]), enhancing the tree-vice differences in susceptibility ([29]). Nevertheless, relationships between morphometrics (diameter) and health grade were observed, suggesting that the largest trees were also the healthiest.
Conclusion
As hypothesised, the development of lesions followed a seasonal pattern that could be linked to meteorological and phenological conditions. The emergence, activity, and extension of lesions was greatest during June and July, when the plant tissue had not yet matured and ambient temperature was close to the optimum for H. fraxineus development. Accordingly, the most rapid extension of the lesions was observed on top shoots, the youngest parts of trees, suggesting that height growth would be notably affected. Differences in the development of lesions amongst the stands indicated effects of climate, as well as local factors (e.g., microclimate); hence, alterations in lesion development might be expected with the changing climate. The extension of newly-emerging lesions was faster than that of existing ones, particularly during the first month of observation, suggesting that approximately one month was necessary for trees to compartmentalise the infection. The development of lesions was not related to the health condition of trees, and a relationship between the number of existing and emerging lesions did not exist, indicating that infection was stochastic. Biological limitations of lesion development were observed, as most lesions remained in the initially-infected parts of trees. The proportion of emerging lesions exceeded the proportion of latent lesions, indicating a progressive decrease in the overall health condition of trees; however, both this proportion and tree mortality appeared to be lower than in other studies. Nonetheless, a longer observation season involving a wider spectrum of sites is required to evaluate the annual cycles in development of the infection, as well as to allow the assessment of secondary infections.
Acknowledgements
This study was financially supported by the Latvian State Forest project “Dieback and regeneration of ash stands in Latvia” (No 5.5.-5.1_0017_101_14_28) and by the Forest Sector Competence Centre project “Methods and technologies for increasing forest capital value” (No L-KC-11-0004)”. We are grateful to anonymous reviewers for their comments and constructive advices.
References
Gscholar
Gscholar
Gscholar
Online | Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Roberts Matisons
Kristine Kenigsvalde
Talis Gaitnieks
Natalija Burneviča
Latvian State Forest Research Institute Silava, Rigas str. 111, Salaspils, LV-2169 (Latvia)
Corresponding author
Paper Info
Citation
Matisone I, Matisons R, Kenigsvalde K, Gaitnieks T, Burneviča N (2018). Seasonal development of lesions caused by Hymenoscyphus fraxineus on young Fraxinus excelsior trees in Latvia. iForest 11: 17-23. - doi: 10.3832/ifor2283-010
Academic Editor
Alberto Santini
Paper history
Received: Nov 16, 2016
Accepted: Oct 23, 2017
First online: Jan 09, 2018
Publication Date: Feb 28, 2018
Publication Time: 2.60 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 47811
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 40553
Abstract Page Views: 2905
PDF Downloads: 3243
Citation/Reference Downloads: 12
XML Downloads: 1098
Web Metrics
Days since publication: 2953
Overall contacts: 47811
Avg. contacts per week: 113.33
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2018): 2
Average cites per year: 0.25
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Lenticel infection in Fraxinus excelsior shoots in the context of ash dieback
vol. 12, pp. 160-165 (online: 04 March 2019)
Research Articles
Temporal development of collar necroses and butt rot in association with ash dieback
vol. 10, pp. 529-536 (online: 05 May 2017)
Research Articles
Genetic variation of Fraxinus excelsior half-sib families in response to ash dieback disease following simulated spring frost and summer drought treatments
vol. 9, pp. 12-22 (online: 08 September 2015)
Research Articles
Application of fungicides and urea for control of ash dieback
vol. 8, pp. 165-171 (online: 13 August 2014)
Review Papers
Linking patterns of forest dieback to triggering climatic and weather events: an overview on Mediterranean forests
vol. 17, pp. 309-316 (online: 30 September 2024)
Technical Reports
Means of combating forest dieback - EU support for maintaining forest health and vitality
vol. 2, pp. 38-42 (online: 21 January 2009)
Research Articles
Effect of origin and morphological characteristics of sessile oak (Quercus petraea) seedlings on the development of Cryphonectria parasitica
vol. 18, pp. 16-22 (online: 15 February 2025)
Research Articles
Impact of rotation length of Eucalyptus globulus Labill. on wood production, kraft pulping, and forest value
vol. 15, pp. 433-443 (online: 20 October 2022)
Research Articles
Verticillium wilt of Ailanthus altissima in Italy caused by V. dahliae: new outbreaks from Tuscany
vol. 13, pp. 238-245 (online: 19 June 2020)
Research Articles
Evaluating the impact of Hymenoscyphus fraxineus in Trentino (Alps, Northern Italy): first investigations
vol. 10, pp. 871-878 (online: 06 November 2017)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword