Seed trait and rodent species determine seed dispersal and predation: evidences from semi-natural enclosures
iForest - Biogeosciences and Forestry, Volume 8, Issue 2, Pages 207-213 (2015)
doi: https://doi.org/10.3832/ifor1185-008
Published: Aug 28, 2014 - Copyright © 2015 SISEF
Research Articles
Abstract
Seed traits affect seed dispersal by animals. However, the combined role of seeds and dispersers in determining seed dispersal is not well explored. We attempted to test how seed traits and predators determine seed dispersal and predation interaction in a rodent-mediated seed dispersal system. Semi-natural enclosure experiments were conducted to investigate seed dispersal and predation of five sympatric tree species with different seed traits, Juglans mandshurica, Quercus mongolica, Pinus koraiensis, Corylus mandshurica and C. heterophylla by three rodent species, Apodemus peninsulae, Tamias sibiricus and Clethrionomys rufocanus showing different body sizes, hoarding behaviors and activity rhythms. Our results demonstrated that seed species with thick coat were removed more slowly than thin-coated seeds in regardless of rodent species, reflecting a consistent negative effect of seed coat on seed dispersal. Seeds with thick coat were less likely to be eaten both in situ and after removal by small rodents. Seeds with high caloric value were more likely to be larder-hoarded, whereas seed traits showed no influence on scatter-hoarding. Rodent species with large body size tended to eat more seeds in situ, while small-sized rodents preferred to eat seeds after removal. Large-sized rodent species scatter-hoarded more seeds, however, small-sized rodents larder-hoarded more seeds. Seeds with thick coat showed high mutualism but low predation with rodents, while rodents with large size showed low mutualism but high predation with seeds. Our results indicate that both seeds and predators play important roles in determining seed dispersal and predation in the seed-rodent dispersal system.
Keywords
Introduction
Many plant species producing large-sized seeds rely substantially on animals for their seed dispersal ([18], [26], [46], [51], [39]). In the dispersal systems, animals depend on plant seeds as nutrition supplies for their survival, especially in food shortage periods, and future reproductive success ([53], [42]). Seed predators may also act as effective seed dispersers, provided that seeds are cached and not completely retrieved ([64], [32], [71]). Two kinds of seed hoarders can be distinguished according to their caching strategies: larder hoarders usually store food items in central caches; while scatter hoarders store food in spaced caches and invest little to defense stores and reduce pilferage ([53], [27]).
Seed dispersal can be affected by various properties of seeds (e.g., [38], [51], [33], [68]). Seed traits have been recognized as important factors affecting rodents’ final decision to manipulate food sources ([29], [68], [32]). Seed size ([37], [57], [60], [2], [4]), handling time ([28]), moisture content ([22]), energy and soluble carbohydrates ([29], [30], [58]), nutrients ([1], [25], [59]), secondary chemical compounds (e.g., tannins and other polyphenols - [49]), seed coat thickness ([68], [52]) as well as seed germination schedule ([60]) have been identified to show influences on seed removal and dispersal. Apart from the influence of seed traits, environmental factors such as habitat qualities and seasonal variations show great impacts on seed removal and dispersal ([23], [31], [54], [36]).
Sympatric rodent species often differ greatly for instance in body size, tooth morphology, and nutrition requirement, and they may differ in affecting seed fates in the field (e.g., [30], [40]). Large-sized rodent species may have strong abilities of opening hard seeds, defending food caches or predation, which may affect their seed hoarding strategies. It suggests that larder-hoarding is often adopted by dominant or stronger animals because they can defend their larders ([9]), while scatter-hoarding is generally considered as a strategy of subordinate animals to minimize the risk of catastrophic loss of hoarded food ([35], [44]). Therefore, body size of small rodents may be another important factor in affecting hoarding strategies (e.g., scatter- vs. larder-hoarding). Previous field studies usually evaluated the interaction between seeds and rodents at community levels, as it is impossible to evaluate the role of a single rodent species in seed dispersal of various tree species. Therefore, it is hard to discriminate specific behavioral response of one given rodent species to certain seed traits when selecting seeds ([41], [68]). Although seed trait plays an important role in determining the formation of mutualism and predation interactions among multiple tree and animal species ([68], [32], [55]), the effects of both seed and rodent traits on seed dispersal and predation have been not well evaluated ([8]).
The purpose of this study is to investigate how three sympatric rodent species with different body sizes affect seed dispersal and seed fates of five sympatric tree species in semi-natural enclosures in northeastern China. We predicted that: (1) thick-coated seeds will be removed more slowly than seeds with thin coat because thick coat usually increase the time for rodents to eat seeds ([68]); (2) thick-coated seeds will be less likely eaten in situ but more likely eaten after removal according to the handling-time hypothesis; (3) more seeds with high caloric value will be hoarded by rodents according to the optimal forage theory ([45]); (4) large-sized rodent species will eat more seeds in situ, while rodents with small size tend to eat seeds after removal; (5) large-sized rodents will larder-hoard more seeds than small-sized ones, while rodents with small size will scatter-hoard more seeds than large-sized rodents due to their lower ability to defend larders ([34], [44]).
Materials and methods
Study site
This study was conducted in the Dongfanghong Forestry Center (average elevation 750 m, located at 46° 50′ to 46° 59′ N, 128° 57′ to 129° 17′ E) in the Dailing district, Yichun city, Heilongjiang Province, north-eastern China. The climate of the experimental site is dominated by the north temperate zonal monsoon with severe and long winters and short summers. The annual average temperature is 1.4 °C with maximum at 37 °C and minimum at -40 °C. Average annual precipitation averages at 660 mm, with 80% of annual precipitation falls between May and September ([65]).
Experimental enclosures
Sixteen separate semi-natural enclosures (10 × 10 × 2.5 m) were constructed in an open area at the edge of the forests. The enclosures were built using bricks about 2.5 m above and 0.5 m below the ground. The walls of the enclosures were smoothed to prevent escape of small rodents. Grasses commonly found in the forests were distributed with an average coverage of 60%, while trees and shrubs were removed to prevent rodents from escaping by climbing. To prevent avian predators from entering the enclosures, the enclosures were covered with plastic nets on the top. An artificial nest constructed of bricks (H × W × L = 20 × 15 × 30 cm) and a plastic water bowl were placed at one corner of the enclosure to allow animals to rest and drink freely. A seed station of 0.5 m2 was established at the center of each enclosure.
Tested rodent species
The dominant rodent species in the study site are Apodemus peninsulae (Rodentia, Muridae), Clethtionomys rufocanus (Rodentia, Cricetidae) and Tamias sibiricus (Rodentia, Sciuridae). They were chosen for the present experiments because their significant role in the dispersal of large seeds of local tree species ([65]). A. peninsulae and C. rufocanus are small nocturnal species, while T. sibiricus is a larger diurnal rodent. T. sibiricus mainly scatter-hoards but occasionally larder-hoards seeds of local tree species, while A. peninsulae behaves differently, mainly larder-hoarding but sometimes scatter-hoarding seeds. Another nocturnal species, C. rufocanus, is pure seed predator and only larder-hoards seeds of local tree species.
During seed fall in early September 2010, steel frame live traps with a size of 9 × 10 × 25 cm (H × W × L), baited with peanuts and some carrots, were placed in the forests with a 5 m interval along four transects at 9:00 a.m. for animal trapping. We checked the traps every three hours to ensure safety of the captured rodents. All traps were removed at 6:00 p.m. and re-placed next day. Trapping stopped in bad-weather days, such as heavy rain. The target animals captured in each visit were transported by car to the laboratory housing room within no more than 30 minutes. In the laboratory, the animals were individually kept in steel frame cages (H × W × L: 40 × 50 × 90 cm) at outdoors conditions (15-25 °C, 14h of daylight). They were provided with carrots, peanuts, tree seeds and water ad libitum. No animal died during trapping and laboratory rearing. The Dailing Forestry Bureau of the Heilongjiang Province issued permits for the experimental animal trapping. Our behavioral trials and housing procedures were approved by the College of Agriculture, Henan University of Science and Technology. Four days after the experiments, all animals were released where they were captured.
Experimental seeds
The seeds of the five sympatric plant species Juglans mandshurica, Quercus mongolica, Pinus koraiensis, Corylus mandshurica and C. heterophylla were tested (Tab. 1). In the study region, C. mandshurica and C. heterophylla are dominant shrub species, while P. koraiensis, Q. mongolica and J. mandshurica are dominant tree species. Seeds of these five tree species were reported to be dispersed and predated by T. sibiricus, A. peninsulae, and C. rufocanus ([47]). Seeds of J. mandshurica are rich in fat and have the largest size and the thickest coat. C. heterophylla produces seeds of medium size and thick coat, while Q. mongolica produces medium-sized acorns with thinnest seed coat, low nutrition level and high level of tannins. C. mandshurica and P. koraiensis have the smallest seeds with thin coat and high nutrition level. During seed fall, seeds of the five species were collected from the ground under 10-15 trees. Thirty seeds of each tree species were randomly selected for the measurement of physical traits (seed mass, seed length, seed width and coat thickness by using electric vernier caliper and precision scale). Ninety seeds of each tree species were dried at 60 °C for 48 hours. The whole kernel of each seed was carefully collected and weighed. Then, thirty kernels of each species were mixed up into a sample for nutrition analyses, therefore a total of three samples were collected. Concentrations of crude protein, crude fat, crude starch and tannin of the seeds were measured by the Cereal Quality Supervision and Testing Centre, Ministry of Agriculture, China (No. 12 Southern Zhongguancun Road, Haidian District, Beijing). The caloric values of seeds were calculated by the average gross energy equivalents of protein, fat, and carbohydrates. Yang & Xiao ([61]) have calculated the corresponding values for the three organic matters, i.e., 17.2 kJ/g, 38.9 KJ/g, and 17.2 kJ/g, respectively. Caloric value per seed was calculated using mean kernel mass × caloric value of seeds ([68]). The caloric values per seed and per gram kernel were used to reflect the nutritional values of tree species. Seeds were then labeled with plastic tags according to Yi et al. ([67]) with minor modifications. A hole (0.3 mm in diameter) was drilled through the seed coat of each seed, without damaging the cotyledon and the embryo. A flexible plastic tag (2.5 × 3.5 cm, < 0.3 g) was tied through the hole in each seed using a thin 10 cm-long steel thread.
Tab. 1 - Morphological and nutritional traits of the five seed species (mean ± SD). These seeds were the same as those in the removal experiments. (*): Data are drawn from Yi & Zhang ([65]), Yi et al. ([67]), and Yang et al. ([62]).
| Seed traits | Seed species | ||||
|---|---|---|---|---|---|
| Juglans mandshurica | Corylus mandshurica | Corylus heterophylla | Quercus mongolica | Pinus koraiensis | |
| Seed size (length × width - cm) | 4.19 × 2.89 | 1.43 × 1.20* | 1.58 × 1.44* | 2.52 × 1.84 | 1.60 × 1.11* |
| Seed mass (g) | 13.61 ± 1.28 | 0.73 ± 0.08* | 1.18 ± 0.28* | 2.86 ± 0.21 | 0.73 ± 0.05* |
| Seed coat thickness (cm) | 0.32 ± 0.65 | 0.11 ± 0.01* | 0.24 ± 0.03* | 0.05 ± 0.01 | 0.11 ± 0.01* |
| Proportion of kernel mass (%) | 21.10 ± 0.86 | 38.57 ± 3.55 | 18.66 ± 4.29 | 85.75 ± 1.66 | 37.03 ± 1.82* |
| Tannin concentration (%) | 0.07 ± 0.01 | 0.25 ± 0.02* | 0.07 ± 0.02* | 4.33 ± 0.34 | 0.02 ± 0.01* |
| Protein (%) | 27.02 ± 0.71 | 20.30 ± 0.16 | 28.27 ± 0.11 | 7.40 ± 0.21 | 16.18 ± 0.25 |
| Fat (%) | 61.11 ± 0.25 | 47.09 ± 0.14 | 0.24 ± 0.03 | 1.76 ± 0.14 | 37.92 ± 0.46 |
| Starch (%) | 0.07 ± 0.07 | 1.13 ± 0.25 | 0.00 ± 0 | 38.27 ± 1.97 | 0.42 ± 0.06 |
| Caloric value per seed (KJ) | 81.61 ± 0.21 | 6.21 ± 0.02* | 4.30 ± 0.03* | 20.96 ± 0.97 | 7.44 ± 0.05* |
| Caloric value of seed species (KJ/g) | 41.63 ± 0.11 | 17.74 ± 0.05 | 13.45 ± 0.11 | 9.52 ± 0.44 | 29.77 ± 0.19 |
Assessment of seed selection and dispersal by rodents
Trapped animals were starved for 12 hours before being moved in the enclosures. At 7:00 a.m. one animal was singly introduced in each enclosure to test seed removal, seed predation and hoarding. Nine adult individuals of A. peninsulae (5 females, 4 males, body mass: 26.83 ± 5.64 g, mean ± SD), 7 C. rufocanus (3 females, 4 males, body mass 33.38 ± 3.57 g) and 7 T. sibiricus (3 females, 4 males, body mass: 104.80 ± 9.25 g) were randomly selected for the seed removal experiments. Ten intact tagged seeds of each tree species were supplied to each individual only once. The tagged seeds in the enclosures were checked in the morning (7:00 a.m.) and afternoon (16:00 p.m.) daily in the following 3 days (81 hours). The seed removal rate by small rodents was reflected by the proportions of intact seed remaining at the seed stations. Scatter-hoarded seeds were located easily because the attached tags are usually left on the ground after being buried by rodents.
Data analysis
Statistical Package for the Social Sciences (SPSS 16.0) was used for data analyses. Cox regression was used to identify difference in seed removal rates of the five seed species at seed stations. Two-way ANOVA was used to test the effects of plant and rodent species on the number of seeds remained at seed stations (IIS), eaten in situ (EIS), intact after removal (IAR), eaten after removal (EAR), scatter-hoarded (SH) and larder-hoarded (LH), respectively.
Results
Seed removal rate according to plant species
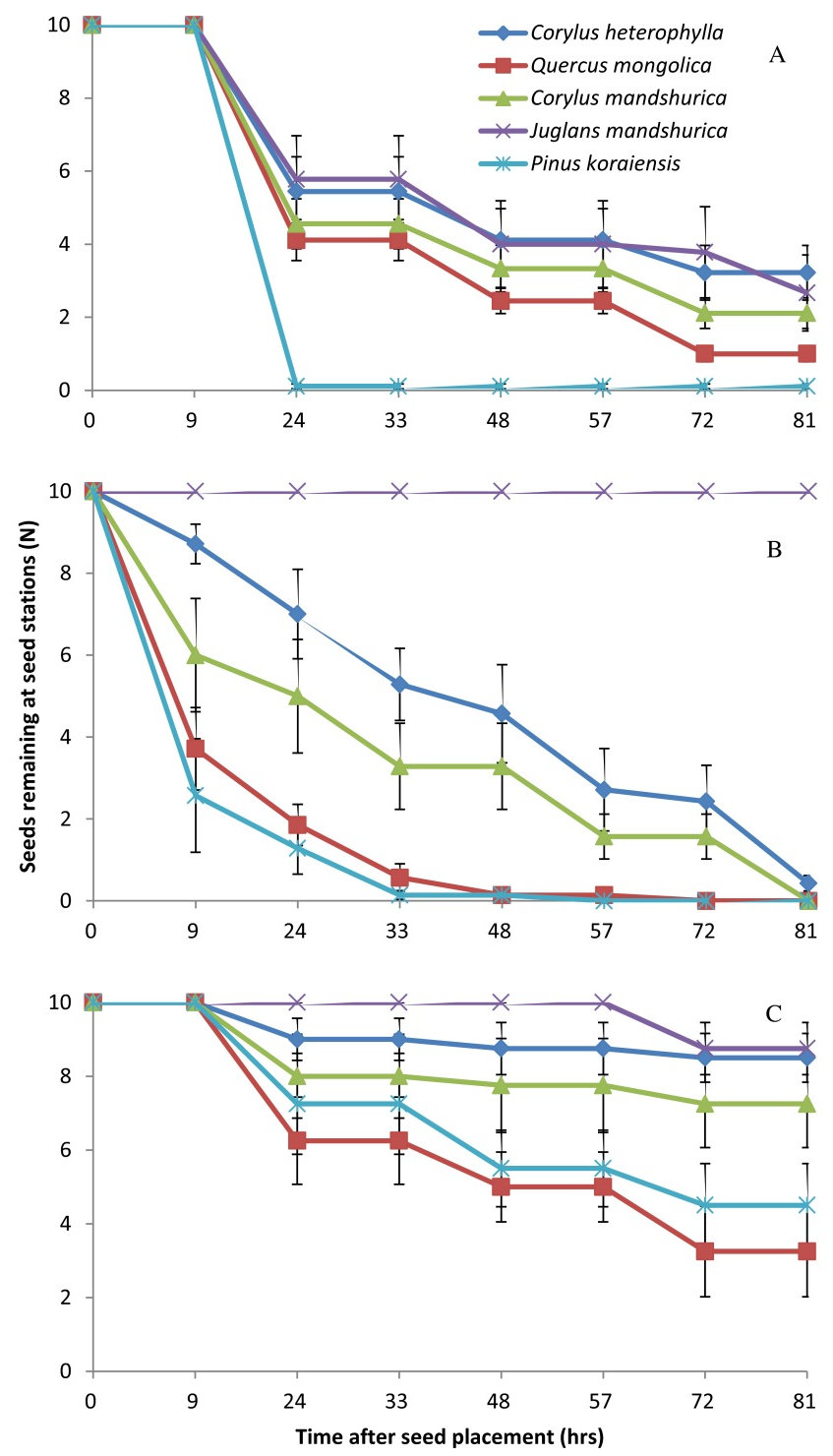
Seed removal rate by A. peninsulae was marginally different among the five tested plant species (Wald = 9.34, df = 4, P = 0.053), following the order: P. koraiensis > Q. mongolica > C. mandshurica > C. heterophylla > J. mandshurica (Fig. 1A, Fig. 2A). As in A. peninsulae, also T. sibiricus showed significant differences in seed removal rates among the plant species (Wald = 19.01, df = 4, P = 0.001 - Fig. 1B, Fig. 2B). Finally, significant differences between plant species in seed removal rates were found in C. rufocanus (Wald = 20.73, df = 4, P < 0.001 - Fig. 1C), but with a different preference order: Q. mongolica > P. koraiensis > C. mandshurica > C. heterophylla > J. mandshurica.
Fig. 1 - Seed removal of five sympatric seed species from seed stations by individual of each rodent species in semi-natural enclosures. (A): Apodemus peninsulae (n = 9); (B): Tamias sibiricus (n = 7); (C): Clethrionomys rufocanus (n = 7). Data are expressed as mean ± SE.
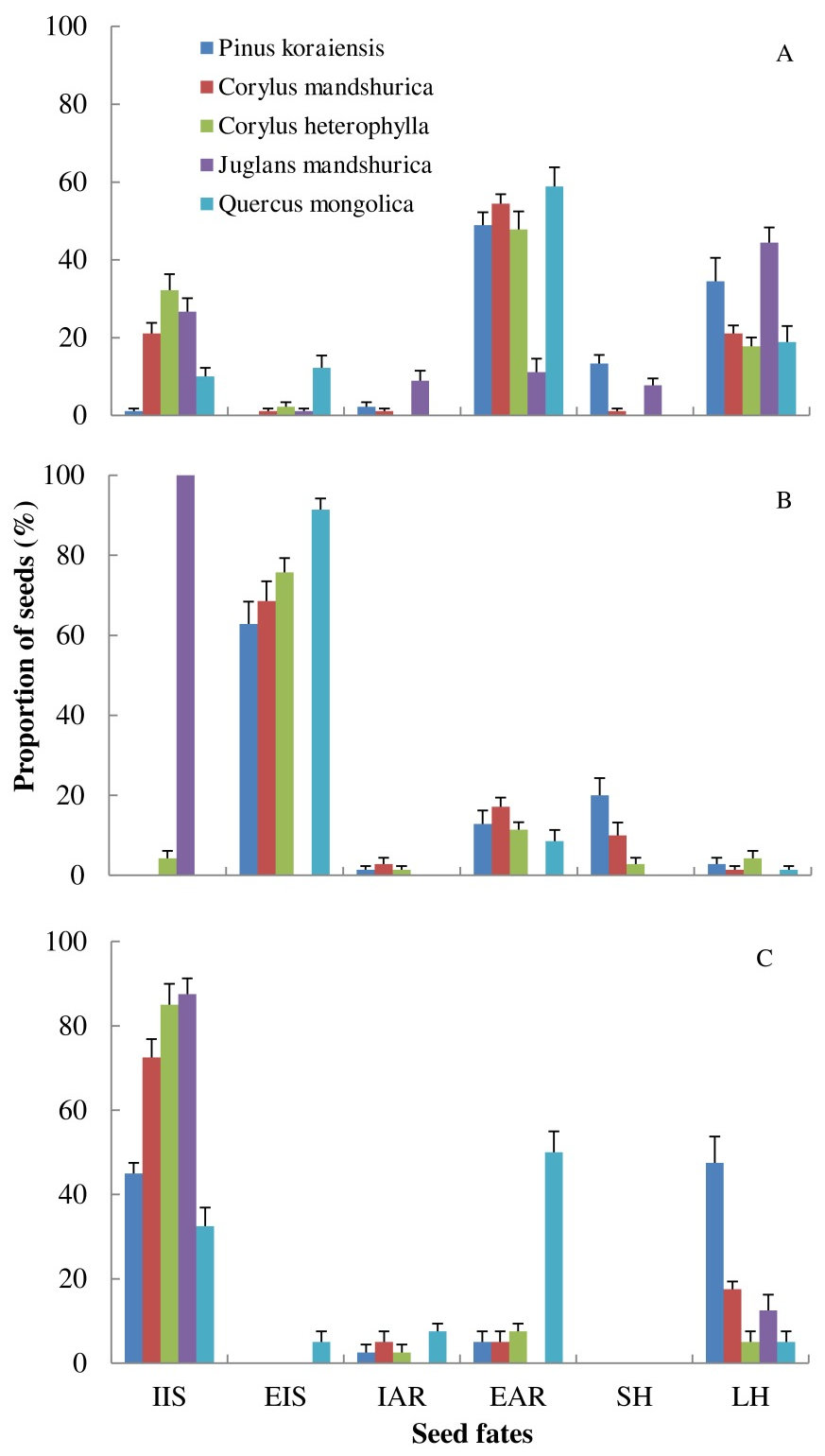
Fig. 2 - Seed fates of five sympatric seed species manipulated by individual of each rodent species in semi-natural enclosures. (A): Apodemus peninsulae (n = 9); (B): Tamias sibiricus (n = 7); (C): Clethrionomys rufocanus (n = 7). (IIS): intact in situ; (EIS): eaten in situ; (EAR): eaten after removal; (IAR): intact after removal; (SH): scatter-hoarded; (LH): larder-hoarded. Data are expressed as mean ± SE.
Seed fates according to plant species
More seeds of P. koraiensis and Q. mongolica were removed from seed stations than those of C. mandshurica, C. heterophylla and J. mandshurica (F = 99.639, df = 4, P < 0.001- Fig. 2, Tab. 2, Tab. 3). EIS and EAR seeds were significantly affected by seed species (EIS: F = 40.224, df = 4, P < 0.001; EAR: F = 22.124, df = 4, P < 0.001), with seeds of Q. mongolica, with the thinnest coat, showing the highest EIS and EAR rates (Tab. 2). Seeds of P. koraiensis were instead more likely to be scatter-hoarded than the other four seed species (F = 8.18, df = 4, P < 0.001). Larder-hoarding was also affected by seed species, i.e., small rodents larder-hoarded more seeds of P. koraiensis and J. mandshurica than those of Q. mongolica, C. mandshurica and C. heterophylla (Fig. 2, Tab. 2, Tab. 3), indicating that seeds with high caloric value were more likely to be hoarded by rodents.
Tab. 2 - The effects of seed and rodent species on seed fates of the five sympatric tree species. Data are seed numbers and expressed as mean ± SD. Different letters in the same column indicate significant difference at P < 0.05 among seed or rodent species. Seed fates in the table were defined as: intact in situ (IIS), eaten in situ (EIS); eaten after removal (EAR), intact after removal (on surface - IAR), scatter-hoarded (SH) and larder-hoarded (LH).
| Type | Species | IIS | EIS | IAR | EAR | SH | LH |
|---|---|---|---|---|---|---|---|
| Seed species | Pinus koraiensis | 0.95 ± 1.85 a | 2.20 ± 3.24 a | 0.20 ± 0.41 a | 2.75 ± 2.27 a | 1.30 ± 1.30 a | 2.60 ± 2.39 a |
| Corylus mandshurica | 2.40 ± 2.82 b | 2.45 ± 3.44 a | 0.25 ± 0.44 a | 3.15 ± 2.30 a | 0.40 ± 0.82 b | 1.35 ± 1.14 b | |
| Corylus heterophylla | 3.30 ± 3.25 b | 2.75 ± 3.70 a | 0.10 ± 0.31 a | 2.70 ± 2.30 a | 0.10 ± 0.31 c | 1.05 ± 0.94 c | |
| Juglans mandshurica | 6.45 ± 3.68 b | 0.05 ± 0.22 c | 0.40 ± 0.82 a | 0.50 ± 1.00 b | 0.35 ± 0.59 b | 2.25 ± 2.38 a | |
| Quercus mongolica | 1.10 ± 1.45 a | 3.85 ± 4.12 b | 0.15 ± 0.37 a | 3.95 ± 2.72 c | 0.00 ± 0.00 c | 1.00 ± 1.49 c | |
| Rodent species | Apodemus peninsulae | 1.82 ± 1.66 a | 0.33 ± 0.77 a | 0.24 ± 0.61 a | 4.42 ± 2.21 a | 0.44 ± 0.72 a | 2.73 ± 1.84 a |
| Tamias sibiricus | 2.09 ± 4.02 a | 5.97 ± 3.39 b | 0.11 ± 0.32 a | 1.00 ± 0.94 b | 0.66 ± 1.16 a | 0.20 ± 0.41 b | |
| Clethrionomys rufocanus | 6.45 ± 2.46 b | 0.10 ± 0.31 a | 0.35 ± 0.49 a | 1.40 ± 2.00 b | 0.00 ± 0.00 b | 1.80 ± 1.80 a |
Tab. 3 - Two-way ANOVA summary of seed fates correlated with seed and rodent species.
| Seed Fates |
Source | Sum of Squares |
df | Mean Square |
F value | P value |
|---|---|---|---|---|---|---|
| IS | Rodent species | 327.169 | 2 | 163.585 | 166.928 | < 0.001 |
| Seed species | 390.573 | 4 | 97.643 | 99.639 | < 0.001 | |
| EIS | Rodent species | 742.469 | 2 | 371.234 | 487.364 | < 0.001 |
| Seed species | 122.558 | 4 | 30.639 | 40.224 | < 0.001 | |
| IAR | Rodent species | 0.756 | 2 | 0.378 | 1.813 | 0.169 |
| Seed species | 0.353 | 4 | 0.088 | 0.424 | 0.791 | |
| EAR | Rodent species | 270.262 | 2 | 135.131 | 104.642 | < 0.001 |
| Seed species | 114.278 | 4 | 28.570 | 22.124 | < 0.001 | |
| SH | Rodent species | 5.513 | 2 | 2.757 | 6.532 | 0.002 |
| Seed species | 13.820 | 4 | 3.455 | 8.186 | < 0.001 | |
| LH | Rodent species | 126.600 | 2 | 63.300 | 46.032 | < 0.001 |
| Seed species | 48.490 | 4 | 12.123 | 8.816 | < 0.001 |
Seed fates according to rodent species
The three rodent species showed significantly different manipulations of seeds in term of total number of seeds remaining at seed stations (F = 166.92, df = 2, P < 0.001); more seeds were left by C. rufocanus than by A. peninsulae and T. sibiricus, respectively (all P < 0.001 - Fig. 2, Tab. 2, Tab. 3). T. sibiricus, with large body size, ate more seeds at seed stations than small-sized A. peninsulae and medium-sized C. rufocanus (F = 487.36, df = 2, P < 0.001). However, more seeds were eaten after removal (EAR) by A. peninsulae than by C. rufocanus and T. sibiricus (F = 104.64, df = 2, P < 0.001). T. sibiricus and A. peninsulae scatter-hoarded more seeds than C. rufocanus (F = 6.53, df = 2, P < 0.001). Finally, more seeds were larder-hoarded by A. peninsulae and C. rufocanus than by T. sibiricus (F = 46.03, df = 2, P < 0.001 - Fig. 2, Tab. 2, Tab. 3).
Correlation between seed traits and seed fates
Regression of the proportion of IIS vs. seed coat thickness indicated that seed species with thick coat were more likely to be left at seed stations (r = 0.909, P < 0.05 - Tab. 4). No significant relationship was found between the proportion of EIS and seed coat thickness (r = -0.827, P = 0.084); however, the proportion of EIS was negatively correlated with the caloric value of seeds (r = -0.942, P < 0.05). We also found that the proportion of EAR was negatively correlated with both coat thickness and caloric value of seeds (r = -0.905, P < 0.05; r = -0.892, P < 0.05, respectively). Seed caloric value was positively and marginally correlated with the proportion of larder-hoarded seeds (r = 0.876, P = 0.051), but not with scatter-hoarded seeds (r = 0.499, P > 0.05).
Tab. 4 - Linear regression results between seed fates and seed traits. Seed fates in the table were defined as: intact in situ (IIS), eaten in situ (EIS); eaten after removal (EAR), intact after removal (on surface) (IAR), scatter-hoarded (SH) and larder-hoarded (LH).
| Seed fates |
Seed mass (g) |
Seed coat thickness (cm) |
Tannin content (%) |
Caloric value per seed (KJ) |
Caloric value (KJ/g) |
|---|---|---|---|---|---|
| IIS | r = 0.867 P = 0.057 |
r = 0.909 P = 0.033 |
r = -0.427 P = 0.462 |
r = 0.823 P = 0.083 |
r = 0.644 P = 0.241 |
| EIS | r = -0.809 P = 0.098 |
r = -0.827 P = 0.084 |
r = 0.649 P = 0.236 |
r = -0.792 P = 0.110 |
r = -0.942 P = 0.017 |
| IAR | r = 0.829 P = 0.083 |
r = 0.520 P = 0.369 |
r = -0.335 P = 0.582 |
r = 0.844 P = 0.073 |
r = 0.850 P = 0.068 |
| EAR | r = -0.855 P = 0.065 |
r = -0.905 P = 0.035 |
r = 0.598 P = 0.287 |
r = -0.823 P = 0.083 |
r = -0.892 P = 0.042 |
| SH | r = -0.184 P = 0.767 |
r = -0.067 P = 0.915 |
r = -0.483 P = 0.410 |
r = -0.158 P = 0.800 |
r = 0.499 P = 0.392 |
| LH | r = 0.375 P = 0.534 |
r = 0.317 P = 0.603 |
r = -0.519 P = 0.370 |
r = 0.396 P = 0.509 |
r = 0.876 P = 0.051 |
Discussion
High level of defense (e.g., thick seed coat) to predators usually reduces seed removal rates from seed stations by seed dispersers ([68]). Our results support the first prediction suggesting that thick-coated seeds are removed more slowly than thin-coated seeds by small rodents because they may require greater efforts to handle. Rodents have to spend more time to eat seeds with thick coat, which may increase predation risk ([20]); selecting thin-coated seeds may reflect their feeding strategies. Previous studies suggest that large-sized seeds are more likely to be cached by rodents ([56], [57], [59], [5]). In the three investigated rodent species, we fail to detect any apparent relationship between seed mass/size and number of seeds eaten or hoarded. More Q. mongolica acorns, the richest in tannins, were eaten either in situ or after removal than those of the other four plant species, inconsistent with the high tannin hypothesis ([49]). This suggests that tannin contents in seeds are unimportant in determining seed hoarding by rodents in our study region ([70]). Early germinating Q. mongolica acorns were eaten instantly rather than hoarded, in agreement with the perishability hypothesis ([16], [50], [60]). We propose that in Q. mongolica instant consuming rather than acorn caching is probably related to their higher perishability ([48], [15], [51]). A seed coat thinner than those of other seed species might be an alternative explanation for instant consumption of Q. mongolica acorns by small rodents. Therefore, the effect of seed mass and tannin on seed fates might be masked by other prominent seed traits, e.g., seed coat ([43]). The significant and negative correlations between seed removal rates and seed coat thickness reflect the crucial role of seed coat in determining seed selection and seed dispersal ([68]).
No significant relationship was found between seed coat thickness and the proportion of EIS. Our second prediction that thick-coated seeds are less likely to be eaten in situ but more likely to be eaten after removal is not fully demonstrated in this study. Although feeding thick-coated seeds after moving them away would be a safer way to prevent rodents from predation risk ([20]), our results show a significant and negative relationship between the proportion of EAR and seed coat thickness. These results indicate that seeds with extreme thick coat (e.g., J. mandshurica) prevent small rodents to disperse and eat them, generally reflecting the negative effect of thick seed coat on seed dispersal ([68]).
Also the prediction that seeds with high caloric value are more hoarded by rodents is verified in this study. Seeds of P. koraiensis and J. mandshurica with high caloric value were more likely to be larder-hoarded, but less likely to be eaten by small rodents, in agreement with previous observations reporting that small rodents prefer to cache seeds with high nutritional value ([11], [12], [3], [10]). The significant and positive relationship between the proportion of larder-hoarded seeds and the caloric value of seeds also indicates the important role of energy reserves in seeds in determining food hoarding by animals ([26]). More seeds of P. koraiensis were scatter-hoarded than those of J. mandshurica, although the latter shows higher caloric value per seed, reflecting the trade-offs between nutrition rewards and efforts in handling the thick coat of J. mandshurica seeds.
Animals with different body size may display different seed disposal abilities, and therefore show different preferences for seeds with contrasting traits ([40], [68]). In our study, the large T. sibiricus ate more seeds in situ, while the medium-small A. peninsulae and C. rufocanus ate more seeds after removing them to safe places (e.g., corners and nests), supporting our fourth prediction. Actually, body size cannot always indicate seed handling ability, as T. sibiricus refuse to select seeds of J. mandshurica.
Larder-hoarding animals usually store food in their nests and invest more efforts to defend them, while scatter-hoarders bury one or several seeds in dispersed caches and space them far apart ([27]). Although it has been suggested that scatter-hoarding is due to poor ability of animals defending food caches ([34], [44]), A. peninsulae and C. rufocanus, with small body mass, larder-hoarded more seeds than T. sibiricus did, while T. sibiricus scatter-hoarded more seeds than A. peninsulae and C. rufocanus. Contrasting with the last prediction, our results suggest that body size is not linked with the evolution of food hoarding strategies (scatter-hoarding and larder-hoarding). Caching strategies may represent trade-offs between cache defense maximization and cache pilferage minimization ([24], [14], [9]).
We acknowledge that many environmental factors may influence seed selection, seed removal and dispersal, and ultimately seed fates in the field ([17], [19], [13], [36]). Although the same-sized enclosures have been applied to investigate caching behavior of chipmunks and other rodent species ([69], [6], [7], [21]), the semi-natural enclosures cannot completely mimic the field conditions. Lack of competition and unnatural presentation of seeds are supposed to alter the behavior of rodents in the enclosures. Although previous studies show that the average dispersal distances of seeds of P. koraiensis, C. mandshurica, C. heterophylla, and J. mandshurica are less than 4 m in the field ([66], [65], [63]), the enclosures may not be large enough to allow the expression of rodents’ desired seed dispersal range.
Our results shed light on the interaction and coevolution between rodents and plants bearing large seeds. On one hand, plants need to increase seed size and nutrition contents to attract potential dispersers. On the other hand, plants have to avoid predation through developing various physical and chemical defense systems ([49], [68]). Trade-offs between attractive and defensive traits of plants regulates dispersers’ decision to remove, consume, and hoard seeds. From the animal’s point of view, trade-offs between costs and rewards of manipulating seeds may shape their different abilities of handling, consuming, and hoarding a seed. Thus, the interactions between plant seeds and rodents are often complex and diffuse at community level. The combined effects of seeds and rodents appear to play an important role in determining seed dispersal and predation in the seed-rodent dispersal system.
Acknowledgments
Funding for this study was partially supported by the National Natural Science Foundation of China (No. 31470113), the State Key Laboratory of Integrated Management of Pest Insects and Rodents (ChineseIPM1404), and the Program for New Century Excellent Talents in University (NCET-12-0693).
References
Gscholar
Online | Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Guoqiang Liu
College of Agriculture, Henan University of Science and Technology, Luoyang 471003 (China)
Corresponding author
Paper Info
Citation
Yi X, Wang Z, Liu C, Liu G (2015). Seed trait and rodent species determine seed dispersal and predation: evidences from semi-natural enclosures. iForest 8: 207-213. - doi: 10.3832/ifor1185-008
Academic Editor
Massimo Faccoli
Paper history
Received: Nov 20, 2013
Accepted: Jun 24, 2014
First online: Aug 28, 2014
Publication Date: Apr 01, 2015
Publication Time: 2.17 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 57174
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 46267
Abstract Page Views: 5255
PDF Downloads: 4251
Citation/Reference Downloads: 22
XML Downloads: 1379
Web Metrics
Days since publication: 4206
Overall contacts: 57174
Avg. contacts per week: 95.15
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 17
Average cites per year: 1.55
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
The effect of seed size on seed fate in a subtropical forest, southwest of China
vol. 9, pp. 652-657 (online: 04 April 2016)
Short Communications
Evidence of Alectoris chukar (Aves, Galliformes) as seed dispersal and germinating agent for Pistacia khinjuk in Balochistan, Pakistan
vol. 14, pp. 378-382 (online: 22 August 2021)
Research Articles
Dispersal and hoarding of sympatric forest seeds by rodents in a temperate forest from northern China
vol. 7, pp. 70-74 (online: 18 November 2013)
Research Articles
Inter- and intra-annual patterns of seed rain in the black spruce stands of Quebec, Canada
vol. 10, pp. 189-195 (online: 13 December 2016)
Research Articles
Seed germination traits of Pinus heldreichii in two Greek populations and implications for conservation
vol. 15, pp. 331-338 (online: 24 August 2022)
Research Articles
The effectiveness of short-term microwave irradiation on the process of seed extraction from Scots pine cones (Pinus sylvestris L.)
vol. 13, pp. 73-79 (online: 13 February 2020)
Review Papers
Soil seed banks of pioneer tree species in European temperate forests: a review
vol. 11, pp. 48-57 (online: 25 January 2018)
Research Articles
Influence of mother plant and scarification agents on seed germination rate and vigor in Retama sphaerocarpa L. (Boissier)
vol. 7, pp. 306-312 (online: 08 April 2014)
Short Communications
Towards an optimal sampling effort for paternity analysis in forest trees: what do the raw numbers tell us?
vol. 5, pp. 18-25 (online: 27 February 2012)
Research Articles
Is there an effect of storage depth on the persistence of silver birch (Betula pendula Roth) and rowan (Sorbus aucuparia L.) seeds? A seed burial experiment
vol. 14, pp. 224-230 (online: 06 May 2021)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword