Analysis of growth of recruits of natural regeneration of Sorbus torminalis (L.) Crantz - a rare European forest tree species
iForest - Biogeosciences and Forestry, Volume 11, Issue 1, Pages 72-78 (2018)
doi: https://doi.org/10.3832/ifor2347-010
Published: Jan 25, 2018 - Copyright © 2018 SISEF
Research Articles
Abstract
We compared growth and survival of wild service tree (Sorbus torminalis [L.] Crantz) recruits of different origin (generative: seedlings; vegetative: root suckers) established in a fenced plot at the Forest District of Krzyz (north-western Poland). Total height, annual growth of the dominant shoot, stem diameter at root collar, number of first-order branches, and mortality were measured every year over the period 2011-2015 (5 years). In 2011, a total of 382 multi-age recruits originated both from seeds (212) and root suckers (170) were recorded. Five-year mortality was higher in the generative progeny (12.3% - only youngest seedlings) as compared with vegetative recruits (2.9%). The growth rate of individuals markedly increased with height as absolute values, but slightly decreased in terms of relative growth. Statistical analysis revealed that the effect of the recruit origin on growth was noticeably weaker than that of age, defined in terms of development (height) classes. The origin of recruits had a major effect on the annual growth of the dominant shoots and a minor (though significant) effect on stem diameter and the number of first-order branches. Overall, the analysis of growth rate showed that generative recruits grow faster than the vegetative ones. Our results highlight the importance of stimulating the generative regeneration and protecting seedlings as a conservation strategy for Sorbus torminalis.
Keywords
Sorbus torminalis, Regeneration, Growth, Mortality, Seedlings, Root Suckers
Introduction
The wild service tree Sorbus torminalis (L.) Crantz is a typical rare forest species with a scattered distribution ([29]). It is distributed across western, central and southern Europe, but also occurs in south-western Asia and north-western Africa. The species is a minor component of various oak and sometimes beech or pine-dominated forests, where it is usually found at low density (<30 trees ha-1 - [9]) and only exceptionally occurs in considerable densities ([23], [12], [28]).
The wild service tree forms fleshy, brown fruits, dispersed by birds and small mammals ([25], [29], [32], [28]). It reproduces both by seeds and root suckers, the latter especially at the distribution border in northeastern Europe ([13], [30], [31], [32], [14]). However, abundant regeneration by root suckers has also been observed in the centre of the species distribution, in Switzerland ([33]) and in Germany ([11], [34], [28]). The recruits are often browsed by deer, so their protection by fencing is recommended ([24], [4]). Vegetative reproduction could be particularly important under circumstances where natural growth of forest trees is difficult, e.g., near the distribution limit of the species, in dynamic habitats or under heavy shade ([18]). Furthermore, in case of Sorbus torminalis, root suckering may sometimes be the only possible way to reproduce and survive.
The wild service tree is often considered a light-demanding/semi-shade species, often out-competed by other hardwood species ([12], [9], [2], [3] [26]). On the other hand, some observations and studies show that the species is shade tolerant and can persist for many years under the canopy of other deciduous trees ([28]).
In Poland, Sorbus torminalis occurs rarely in deciduous and mixed forests in its western and southern part and the species has been protected by law since 1946. Recently, active protection measures have been implemented in Polish state forests to preserve this species. Initiation of natural regeneration and protection of recruits against browsing damage are among the basic tools of active protection of natural populations of the wild service tree ([2], [3], [4]).
Although knowledge of the development of natural regeneration is essential for properly planning conservation activities, there are no studies comparing the growth and survival of Sorbus torminalis recruits originated from seeds and root suckers in natural populations. Several studies investigated the growth and survival rate of wild service tree seedlings cultivated in nurseries or progeny-provenance plots ([10], [35]), but no data are available so far on the spontaneous regeneration of such species in the forest, with the exception of Schüte ([34]) who investigated the growth and shade tolerance of root suckers in a natural population in Germany.
In this study, we analysed the survival and growth of Sorbus torminalis recruits (both root suckers and originated from seeds) in the Forest District of Krzyz (north-western Poland). Fenced plots were established in the forest to investigate offspring under conditions of releasing from browsing and limitation of competition from hornbeam seedlings. We addressed the following questions: (1) does the survival rate of S. torminalis recruits depend on their age (expressed as age-related development classes of offspring) and origin (seedlings and root suckers)? (2) To what extent the growth rate of S. torminalis recruits does depend on their age and origin?
Materials and methods
Study area
The wild service tree occurs in only one site at the Forest District of Krzyz, namely in the moraine upland of the Drawa Valley, within the area of the Debina Forestry. The study area is a deciduous mesic forest habitat on cambisoils, represented by a low-density seed stand of pedunculate oak with scant admixture of hornbeam and beech. This forest is not under legal protection. The average annual precipitation in the region is approximately 544 mm and the annual average temperature amounts to 8.4 oC ([38]).
The local population of Sorbus torminalis comprises 23 adult trees and quite numerous recruits of natural regeneration. In spring 2011, lots of new seedlings were found in forest compartment 295 (52o 57′ 36.9″ N, 15o 58′ 38.3″ E; elevation 64 m a.s.l.), Three plots with abundant offspring were fenced to protect the recruits against browsing by deer. Moreover, hornbeam seedlings overgrowing Sorbus torminalis recruits were removed once a year by the local forest service.
Data collection
Data was collected within the largest plot (0.41 ha), which was chosen for the detailed study for two reasons: (i) because of the large number of recruits originated both from seeds and root suckers; (ii) because the light regime (semi-shade) was approximately the same for all recruits over the plot. All the individuals of S. torminalis within the plot were mapped using an electronic tachymeter GTS-229® (Topcon Ltd., Singapore). Each recruit was provided with label and sequential number. Three adult trees of the species growing within the fenced plot were also mapped. The plot was monitored each autumn since 2011. During the five subsequent years (2011-2015), each of the recruits were measured for the following characters: total height, annual growth of the dominant shoot, stem diameter at root collar and the total number of first order branches. Annual growth was defined as the length between the scars left on the stem by the last winter buds and the stem top. As we were not able to clearly pinpoint the age of recruits, we used age-development classes expressed in terms of offspring height (see “Data analysis”). In the case of seedlings, their initial age (in 2011) was estimated based on counting the height annual increments (about 1-10 years). Although we do not have knowledge about browsing episodes before fencing the plot in 2011, we have noted very few damaged specimens in the first year of observations. Moreover, the architecture of suckers did not suggest any former intensive grazing.
Data analysis
In order to analyse the effects of origin and age on the mortality and growth of Sorbus torminalis recruits, the individuals were separated into two categories: generative progenies (seedlings) and vegetative progenies (suckers) and into four age-development classes based on their total height: (CI) up to 15 cm; (CII): 16-49 cm; (CIII): 50-79 cm; (CIV): over 79 cm. The origin of recruits was determined on the basis of morphological features, mode of distribution and supported by a genetic analysis performed on the sample of 66 individuals (Burczyk J & Jankowska-Wroblewska S - unpublished data).
To analyse how the population of recruits changed over time, the mortality of vegetative and generative progenies over the five years was determined, together with the distribution of specimens in the different age-development classes in 2011 and 2015.
All analyses that concerned plant growth were based on the age-development classification carried out in 2011. First, multivariate and variate analyses of variance (MANOVA and ANOVA, respectively) were performed to compare the size of progenies of different age-development classes in the first and last years of observations. Next, hierachical multiple regression was used to estimate differences in growth rate among progenies of different age and origin. The age-development classes were used as the main predictor (model M1), and then the origin categories were included in the model (model M2). Statistical analysis were carried out including all the measured variables (annual growth, stem diameter and number of first order branches), except total height which was used to establish age-development classes and therefore excluded from both MANOVA and regression analyses. Annual differences between individuals of different origin and age were tested using a t-test. Prior to the statistical analyses all the variables were log-transformed to better approximate normality and homogeneity. Because some of the variables had a value of 0, the transformation was done according to the formula x = log (x+1). In MANOVA/ANOVA and regression analysis, the homoscedasticity was tested on scatter plots between the predictors and the residuals. Charts included in the paper show real (untransformed) values. All statistical analyses were carried out using STATISTICA® ver. 12 (StatSoft, Tulsa, OK, USA) and SPSS® ver. 19 (IBM Corp., Harmonk, NY, USA).
Results
Regeneration structure
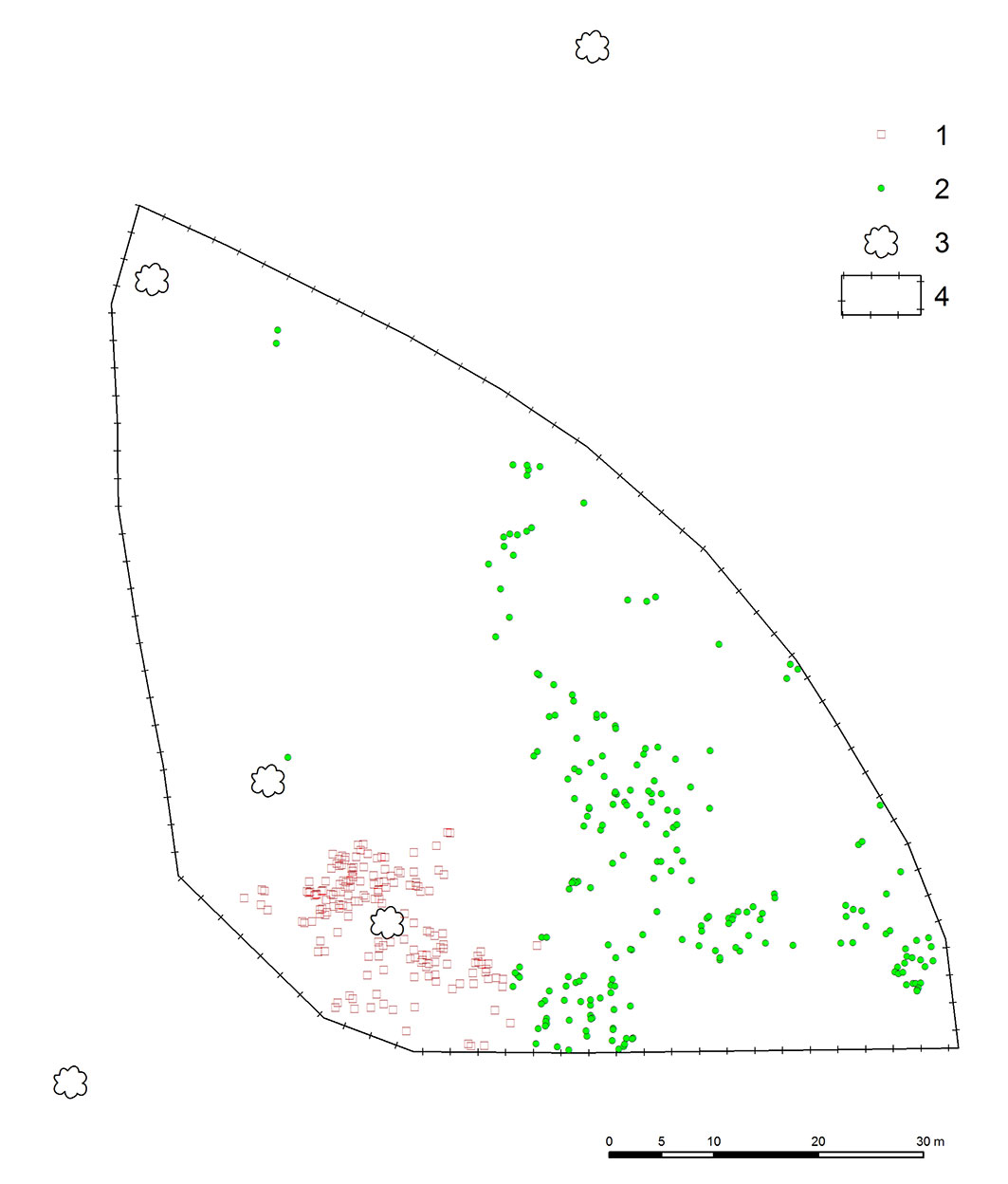
Overall, 382 multiage recruits of Sorbus torminalis originating both from seeds and root suckers were recorded in 2011, of which 212 were generative and 170 vegetative progenies (Fig. 1).
Fig. 1 - Spatial distribution of Sorbus torminalis generative (1, red squares) and vegetative (2, green circles) recruits within the experimental plot; (3): adult wild service trees; (4): fence.
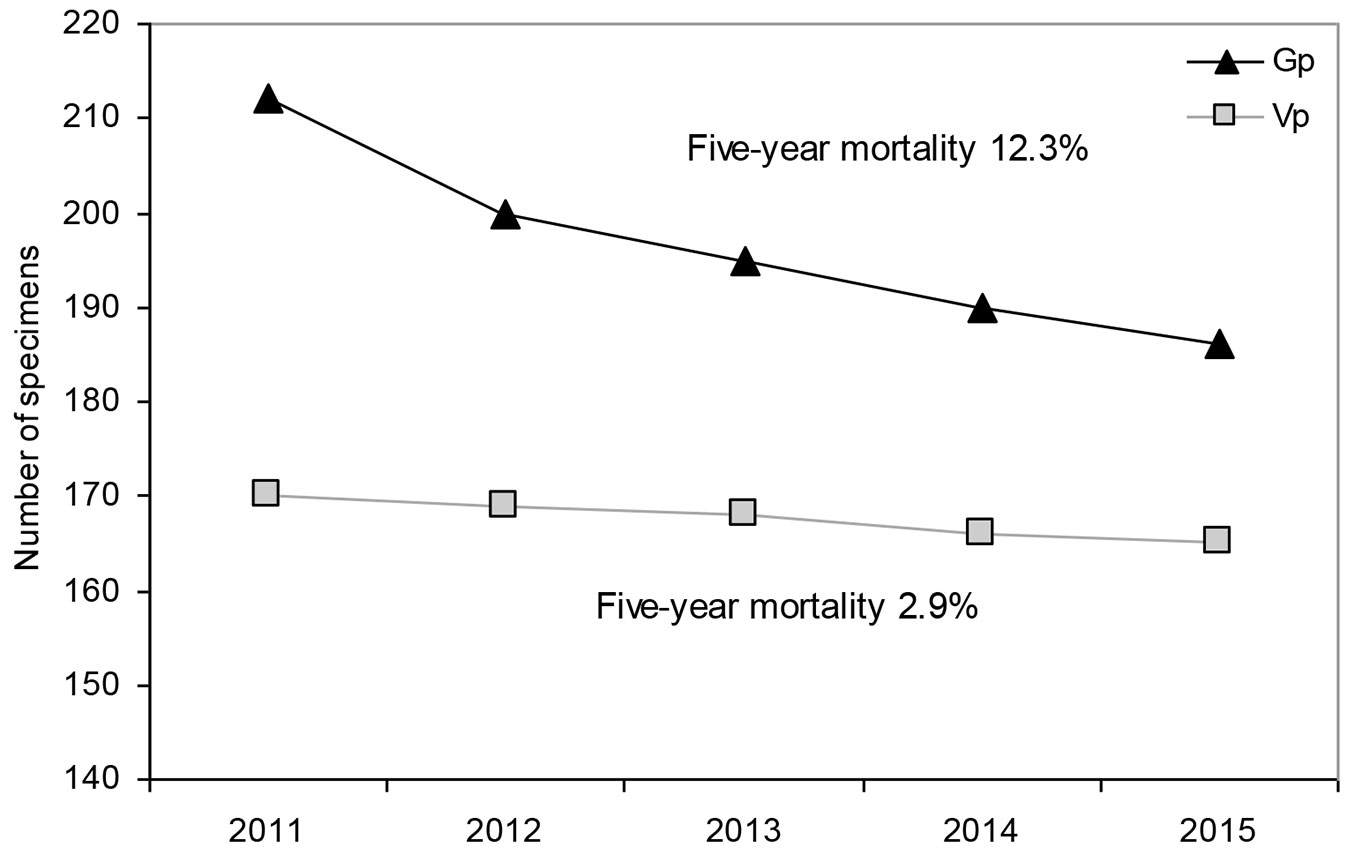
The number of individuals decreased to 313 in the subsequent five years: 31 died, 36 were missing and 2 were damaged. Five-year mortality was clearly higher in the generative progeny (26 specimens, ca. 12%) as compared to vegetative one (5 specimens, ca. 3% - see Fig. 2). All dead generative individuals were seedlings belonging to the age-development class CI, while dead vegetative recruits were from classes CI, CII and CIII.
Fig. 2 - Fig. 2 - Mortality of generative (Gp) and vegetative (Vp) recruits of Sorbus torminalis over the years 2011-2015.
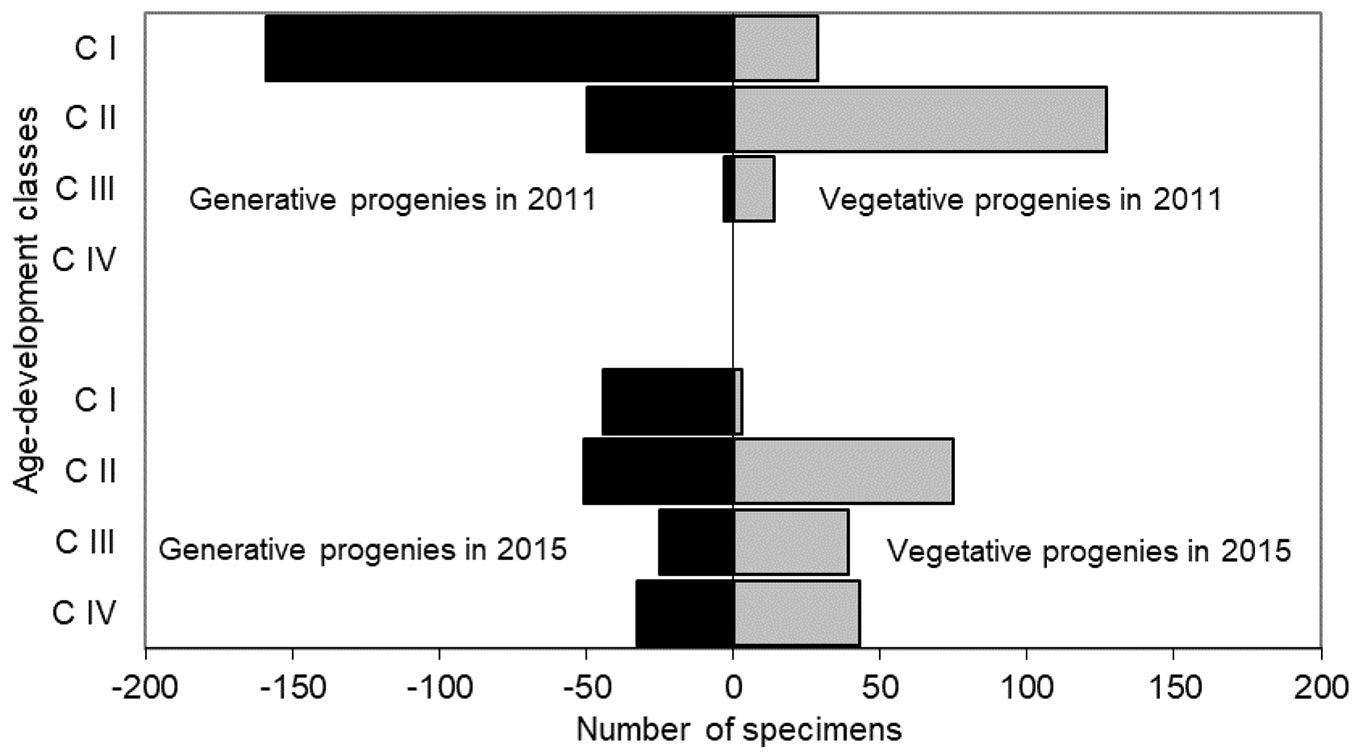
The age-development pyramid changed between 2011 and 2015. In the first year of the study, CI individuals dominated in the generative and CII among the vegetative progeny. CIV individuals were not observed. In 2015 all four age-development classes were observed and their share was more evenly distributed (Fig. 3).
Growth rate of recruits of different age
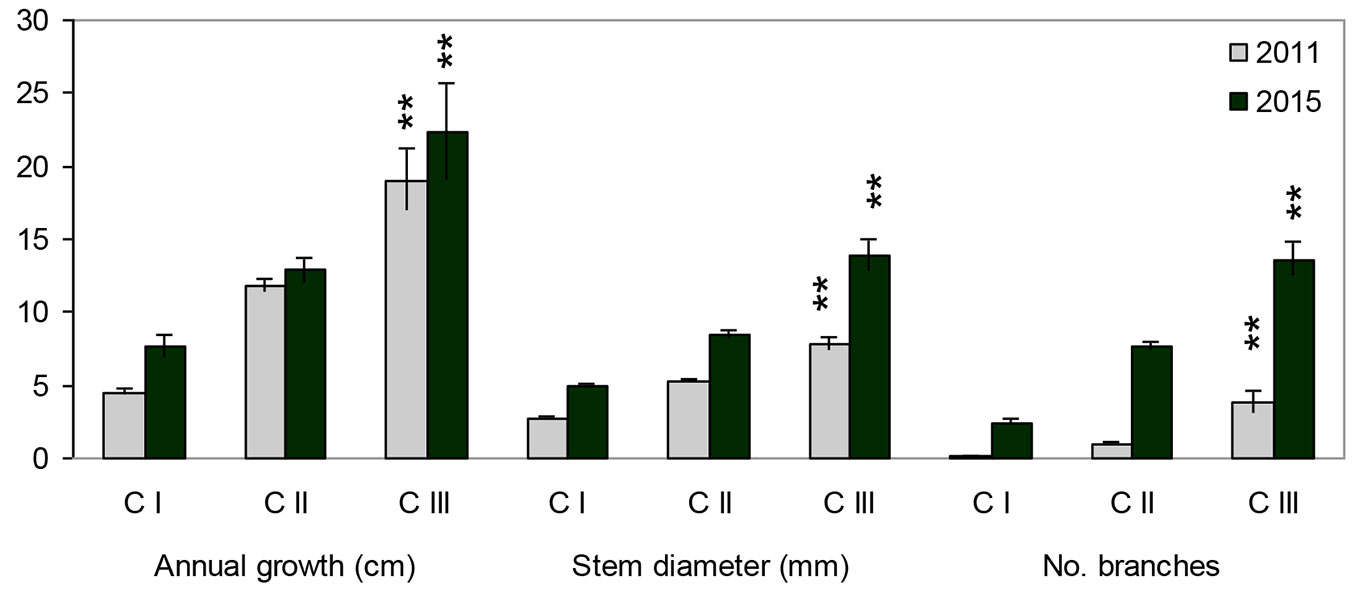
Individuals that belonged to different age-development classes in 2011 differed significantly four years later (2011: MANOVA Wilk’s λ=0.39, F[6;616]= 62.62, p<0.0001; 2015: MANOVA Wilk’s λ=0.55, F[6;616]= 35.46, p<0.0001). individuals of different age differed for all the tested variables (ANOVA, p<0.0001). The growth rate of recruits increases markedly with age, when we compare the direct values of the characters (Fig. 4), and slightly decreases when the relative growth is considered (Fig. 5).
Fig. 4 - Mean values of height growth in individuals belonging to different age-development classes (development classes by the state for the year 2011). Asterisks indicate significant differences (p<0.001) among classes in 2011 and 2015 after ANOVA. Error bars represent the standard error.
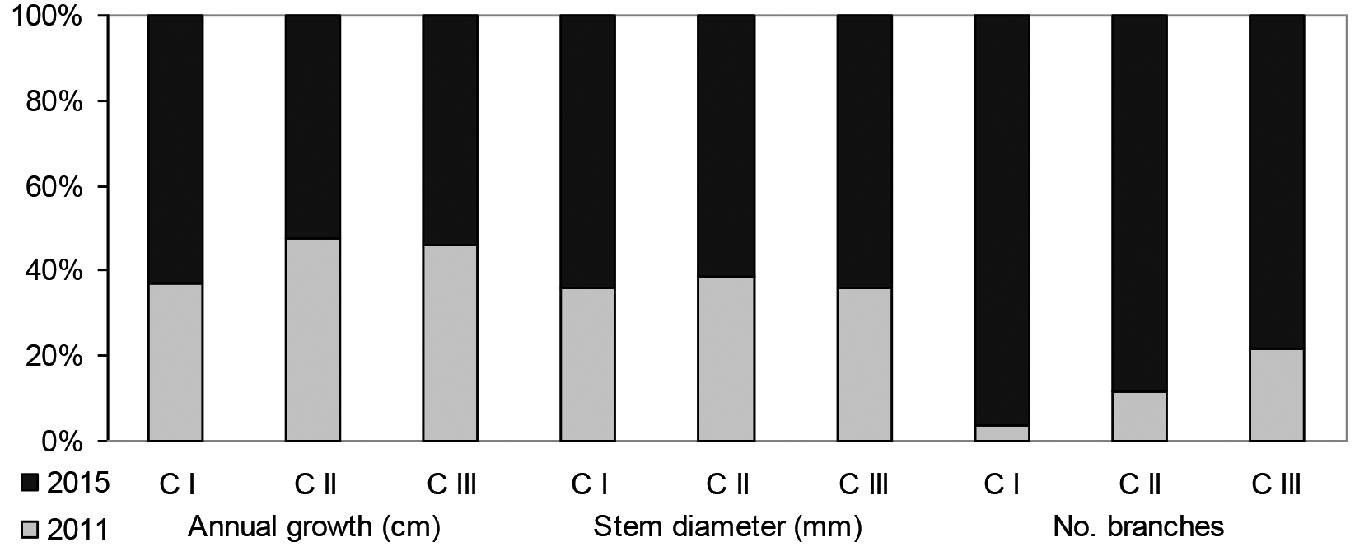
Fig. 5 - Relative values of growth characteristics in individuals belonging to different age-development classes (development classes by the state for the year 2011).
Growth rate of recruits of different age and origin
According to hierarchical multiple regression, age-development classes accounted for 20-32% of the variability in the growth of recruits. The predictor was highly significant in all derived M1 models (p<0.000). When the origin of recruits was included in the model (M2 models), the total explained variance slightly increased to 21-33% (Tab. 1). The impact of the origin of recruits on their growth was noticeably weaker (β<0.1) than the impact of the age-development class (β>0.5). The origin of recruits had a major effect on the annual growth of shoots (β=-0.09; t=-3.67; p<0.01), as well as a minor (though significant) effect on the number of branches (β=-0.06; t=-2.59; p=0.01) and stem diameter (β=-0.05; t=-2.08; p=0.04 - see Tab. 2).
Tab. 1 - Summary of the hierarchical multiple regression of the age-development classes and origin on Sorbus torminalis recruits growth. (a) M1 predictors: (Constant), age-development classes by the state for the year 2011; M2 predictors: (Constant), age-development classes by the state for the year 2011, origin. (SE): standard error; (df): degrees of freedom.
| Dependent variable |
Model (a) | R2 | Adj-R2 | SE estimate |
R2 | F | df | Prob. |
|---|---|---|---|---|---|---|---|---|
| Annual growth | M1 | 0.204 | 0.203 | 8.713 | 0.204 | 400.271 | 1;1563 | <0.001 |
| M2 | 0.211 | 0.210 | 8.679 | 0.007 | 13.476 | 1;1562 | <0.001 | |
| Stem diameter | M1 | 0.322 | 0.321 | 2.637 | 0.322 | 741.823 | 1;1563 | <0.001 |
| M2 | 0.326 | 0.325 | 2.634 | 0.002 | 4.341 | 1;1562 | 0.037 | |
| No. branches | M1 | 0.283 | 0.283 | 3.381 | 0.283 | 618.174 | 1;1563 | <0.001 |
| M2 | 0.286 | 0.286 | 3.375 | 0.003 | 6.691 | 1;1562 | 0.010 |
Tab. 2 - Tab. 2 - Coefficients of the hierarchical multiple regression of the age-development classes and origin on Sorbus torminalis recruits growth. Age-development classes by the state for the year 2011.
| Dependent variable |
Predictors | Unstandardized coefficients |
Standardized coefficients |
t | p-value | |
|---|---|---|---|---|---|---|
| B | SE | Beta | ||||
| Annual growth | constant | -1.977 | 0.669 | - | -2.954 | 0.003 |
| age-dev. class | 8.435 | 0.437 | 0.498 | 19.302 | <0.001 | |
| origin | -1.849 | 0.504 | -0.095 | -3.671 | <0.001 | |
| Stem diameter | constant | 0.733 | 0.203 | - | 3.610 | <0.001 |
| age-dev. class | 3.286 | 0.133 | 0.592 | 24.778 | <0.001 | |
| origin | -0.319 | 0.153 | -0.050 | -2.083 | 0.037 | |
| No. branches | constant | -2.436 | 0.260 | - | -9.361 | <0.001 |
| age-dev. class | 3.903 | 0.170 | 0.564 | 22.971 | <0.001 | |
| origin | -0.507 | 0.196 | -0.063 | -2.587 | 0.010 | |
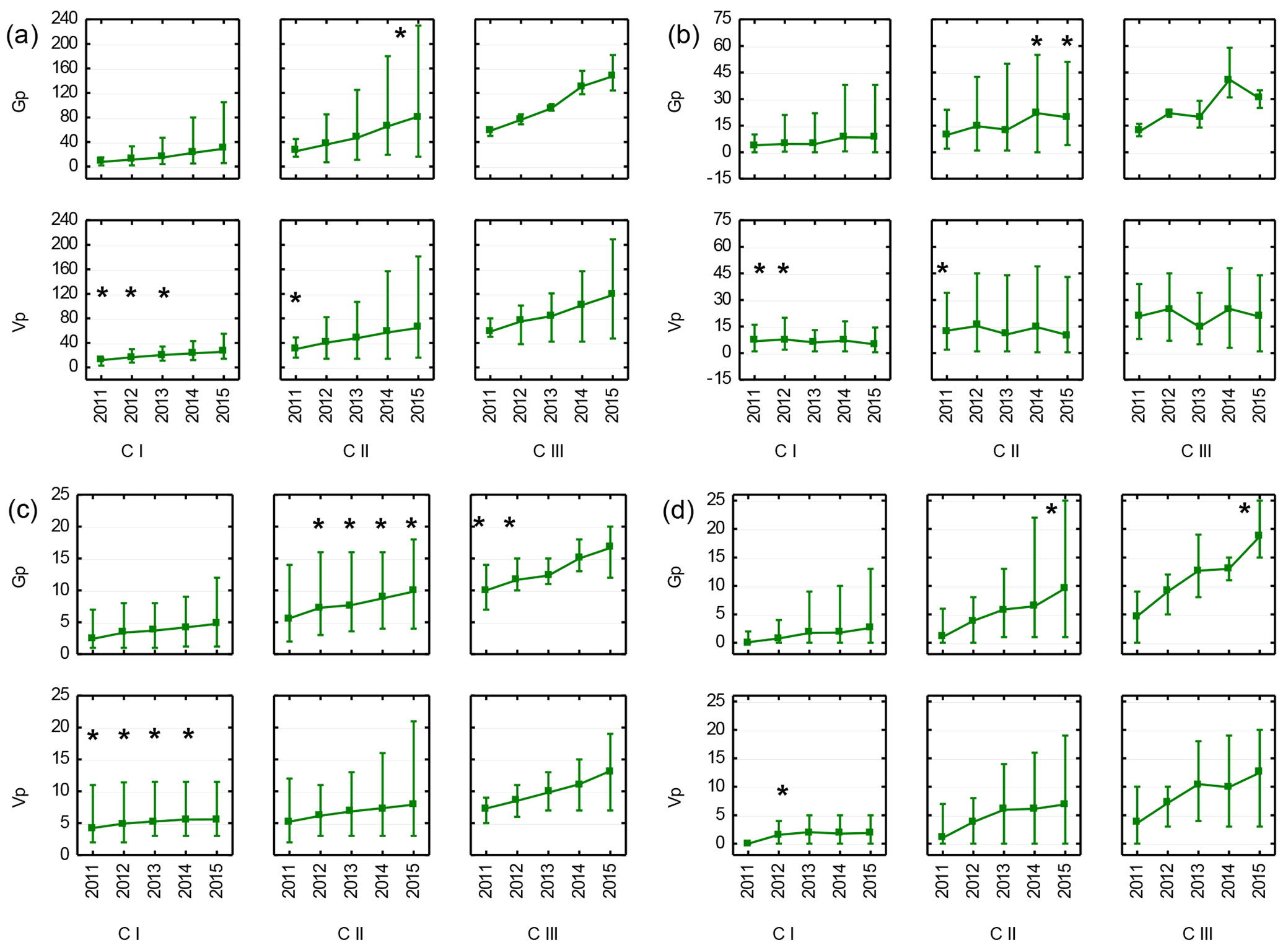
In the first year of the study, vegetative individuals from CI and CII classes were significantly taller, showed greater annual growth and had thicker stem diameter (only CI) as compared to generative ones (t-test, 0.0001<p<0.05). There were no significant differences in the number of lateral branches of suckers and seedlings from CI and CII groups. In 2011, the CIII root suckers and seedlings did not differ in any of studied variables. Over the next four years, the growth rate was higher for generative individuals. In 2015, the seedlings that had previously been classified as CI had smaller stem diameter as compared to vegetative individuals, and did not differ from them as for the other characters. CII Individuals were noticeably taller, had higher annual growth and a larger stem diameter than the stem of their vegetative counterparts. CII and CIII seedlings were also more branching than vegetative individuals (Tab. 3).
Tab. 3 - Means and t-test results for morphological characters of the generative (Gp) and vegetative progeny (Vp) of Sorbus torminalis in 2011 and 2015. Age-development classes by the state for the year 2011.
| Character | Class | 2011 | t-test (p-value) |
2015 | t-test (p-value) |
||
|---|---|---|---|---|---|---|---|
| Gp | Vp | Gp | Vp | ||||
| Total height (cm) | CI | 7.8 | 12.0 | <0.001 | 29.0 | 25.5 | 0.459 |
| CII | 25.0 | 30.1 | 0.001 | 81.5 | 64.5 | 0.007 | |
| CIII | 58.3 | 58.5 | 0.994 | 147.3 | 118.1 | 0.314 | |
| Annual growth (cm) | CI | 3.9 | 6.7 | <0.001 | 8.3 | 4.9 | 0.356 |
| CII | 9.7 | 12.6 | 0.013 | 19.7 | 10.2 | <0.001 | |
| CIII | 11.7 | 20.8 | 0.082 | 30.3 | 20.5 | 0.256 | |
| Stem diameter (mm) | CI | 2.4 | 4.3 | <0.001 | 4.8 | 5.6 | 0.056 |
| CII | 5.6 | 5.2 | 0.259 | 9.8 | 7.9 | <0.001 | |
| CIII | 10.0 | 7.3 | 0.065 | 16.7 | 13.2 | 0.249 | |
| No. branches | CI | 0.1 | 0.0 | 0.129 | 2.6 | 1.9 | 0.833 |
| CII | 1.0 | 1.0 | 0.851 | 9.6 | 6.9 | <0.001 | |
| CIII | 4.7 | 3.6 | 0.820 | 18.7 | 12.5 | 0.116 | |
Observations of recruits on a year-by-year basis indicated that the largest differences between generative and vegetative individuals emerged in the CI and CII age-development classes. Root suckers, which were larger in the CI stage were next overgrown by generative individuals, and these differences persisted in the CII and CIII stages. CIII individuals showed a very dynamic growth regardless of their origin. However, vegetative CIII individuals were, generally, less uniform than generative ones, as expressed by the greater discrepancy between the minimum and maximum values of annual growth, stem diameter and number of branches (Fig. 6a, Fig. 6b, Fig. 6d).
Fig. 6 - Mean (points), minimum and maximum values (vertical lines) of total height (a), annual growth (b), stem diameter (c) and number of branches (d) of generative (Gp) and vegetative (Vp) recruits of Sorbus torminalis in five subsequent years (2011-2015). Stars denote the significant differences (p<0.05) after t-test. Age-development classes by the state for the year 2011.
Discussion
Our study evaluates the impact of the origin and age of Sorbus torminalis recruits on their survival and growth rate in a natural population of the species. Both research aspects are novel for the wild service tree.
In the study area, the share of recruits originated from seeds and root suckers was very even. It appeared that five-year mortality was clearly higher in the generative progeny when compared to vegetative one. However, it also depended on the age of the offspring. All dead generative individuals were among the youngest seedlings (CI), while dead vegetative recruits were observed in CI, CII and CIII classes. The results of our studies also showed that the survival rate of Sorbus torminalis seedlings in the fenced plot is comparable with that that recorded for seedlings grown in cultivation ([35]). This supports the effectiveness of simple conservation activities, such as fencing and partial reducing of competition, on the survival of spontaneous regeneration. The low seedling survival at their earliest years has been previously described for various deciduous trees in the temperate zone ([16], [36], [41]). Zywiec & Holeksa ([39]) assumed that the Sorbus aucuparia individuals of vegetative origin could have higher survival rate than the seedlings. However, according to their study, the probability of survival of Sorbus aucuparia sprouts increases with age, which was not found in our study.
We found that the main factor affecting the growth of Sorbus torminalis recruits is their age and not their origin. The growth rate increases with height when the direct growth is considered, but subtly decreases when we compare relative growth. This may indicate the importance of a larger pre-existing biomass of recruits in observed growth rate. The role of larger pre-existing biomass in observed superior gross production has been shown for e.g. Robinia pseudoacacia and Ailanthus altissima ([6]).
Surprisingly, newly established seedlings of S. torminalis showed a very slow growth rate, not exceeding 10 cm in height after 3-4 years. As a comparison, the average height of 4-year-old seedlings of this species in progeny-provenance plots from two locations in Poland amounted to about 92 cm ([35]), whereas in Iran the height of 3-year old seedlings was 50 cm on average ([10]). However, it seems that the significant differences observed in the growth rate of the youngest seedlings (up to about 5-8 years) progressively flattens out during their development. For seedlings from the CIII class (50-79 cm), the average annual height increments attained ca. 30 cm in our study. These results are consistent with observations of height increments of young Sorbus torminalis trees (up to 20 years) by other authors ([24], [34], [28]). Indeed, the wild service tree can be regarded as a slow-growing species with shade-tolerant characteristics in the juvenile phase ([28]). A recent dendrochronological survey of the species in Poland showed that its mean radial growth is 1.44 mm year-1, and only 1.28 mm year-1 for the population at Krzyz Forest District ([7]). Slow growth rate of forest tree seedlings is commonly observed in natural stands, as a seedling bank is an important element of the reproductive strategy of many tree species ([22], [8], [5], [36], [1], [37], [40]). In the form of a seedling bank a new generation of trees is waiting for a long time for a canopy gap. When lighting conditions improve, most seedlings significantly accelerate their growth. Some recent studies also revealed that trees with early slow growth lived longer than fast-growing trees ([21], [15]).
In our study, the impact of the origin of Sorbus torminalis recruits on their growth was noticeably weaker than the impact of the age-development class. The analysis of growth rate showed that, generally, generative recruits have grown faster than vegetative individuals. Also, we found that the origin of recruits had a major effect on annual growth of shoots and a minor effect on the number of branches and diameter of stems. Initially, large-sized vegetative individuals were overgrown by generative individuals over the next four years. More intensive branching in generative recruits results in higher total leaf biomass and photosynthetic area. On the other hand, less dynamic growth of sprouts after four years, together with the higher variability of all analysed growth features may be related to the increasing overcrowding of individuals in a small area, which results directly from the nature of root suckering. As previously mentioned, the studied population was fenced and released from browsing pressure. This treatment allowed us to be sure that the slower growth of sprouts in CII and CIII classes was not a consequence of herbivory. According to Savill (1991 after [24]), the root suckers of S. torminalis are more shade tolerant than seedlings and can easily withstand the overshade in the first years. The root suckers grow quickly in height in the first 10-15 years and then their growth is expected to slow down. Schüte ([34]) reported that after 6-8 years root suckers are still not independent from the root system of the mother tree. This may explain the observation that suckers or sprouts are less susceptible to stress factors as shade or water deficit ([27]) and they have faster initial growth compared to seedlings. This finding is consistent with our observations. Also, in case of Robinia pseudoacacia which reproduces both sexually and asexually ([19], [17], [20]), some studies have shown that new horizontal roots are supplied with photosynthates from the mother plant or ramet for a few years only and that resource translocation between neighboring ramets within the same clone ceases after several years ([17]). This might explain the advantage of root sprouts in the initial period of their development.
Generative reproduction can be very important for ensuring the continuity of Sorbus torminalis natural populations, as for the higher shade tolerance of younger seedlings and their faster growth at later stages. As a conservation strategy for the species, the in situ promotion and protection of natural regeneration from seeds should be undertaken ([9], [2], [3]). In recent years, S. torminalis seedlings produced in the nursery have been planted in forest stands in Poland with the aim of increasing their biodiversity and contributing to the protection of this rare species. Although such activities are certainly commendable, in situ conservation measures should not be neglected.
Further studies on the reproductive strategy of Sorbus torminalis in different natural stands should be undertaken to acquire more comprehensive knowledge of this rare and valuable species of European forests.
Acknowledgements
This study was supported by the Ministry of Science and Higher Education, Warsaw, Poland (statutory funds no 508.641.00). We thank prof. J. Burczyk and Dr. S. Jankowska-Wroblewska (Jan Kazimierz University in Bydgoszcz, Poland) for re-sharing their unpublished molecular data. We are also grateful to J. Zwierzynski, M. Podsiedlik, L. Kaczmarek and J. Bednorz for help with field measurements.
LB planned the research, carried out the field measurements; RN performed the statistical analysis; LB and RN wrote the article.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Renata Nowinska
Department of Botany, Poznan University of Life Sciences, Wojska Polskiego 71C, 60-625 Poznan (Poland)
Corresponding author
Paper Info
Citation
Bednorz L, Nowinska R (2018). Analysis of growth of recruits of natural regeneration of Sorbus torminalis (L.) Crantz - a rare European forest tree species. iForest 11: 72-78. - doi: 10.3832/ifor2347-010
Academic Editor
Emanuele Lingua
Paper history
Received: Jan 04, 2017
Accepted: Oct 25, 2017
First online: Jan 25, 2018
Publication Date: Feb 28, 2018
Publication Time: 3.07 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 48114
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 40203
Abstract Page Views: 3046
PDF Downloads: 3795
Citation/Reference Downloads: 15
XML Downloads: 1055
Web Metrics
Days since publication: 2966
Overall contacts: 48114
Avg. contacts per week: 113.55
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2018): 3
Average cites per year: 0.38
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Clonal structure and high genetic diversity at peripheral populations of Sorbus torminalis (L.) Crantz.
vol. 9, pp. 892-900 (online: 29 May 2016)
Research Articles
Arbuscular mycorrhizal fungal symbiosis with Sorbus torminalis does not vary with soil nutrients and enzyme activities across different sites
vol. 8, pp. 308-313 (online: 03 September 2014)
Research Articles
Methods to inventory and strip thin in dense stands of aspen root suckers
vol. 8, pp. 590-595 (online: 22 April 2015)
Research Articles
Carbohydrate metabolism during new root growth in transplanted Larix olgensis seedlings: post-transplant response to nursery-applied inorganic fertilizer and organic amendment
vol. 10, pp. 15-22 (online: 22 September 2016)
Research Articles
Effects of nitrogen loading under low and high phosphorus conditions on above- and below-ground growth of hybrid larch F1 seedlings
vol. 11, pp. 32-40 (online: 09 January 2018)
Research Articles
Controlled-release fertilizers combined with Pseudomonas fluorescens rhizobacteria inoculum improve growth in Pinus halepensis seedlings
vol. 8, pp. 12-18 (online: 12 May 2014)
Research Articles
Near zero mortality in juvenile Pinus hartwegii Lindl. after a prescribed burn and comparison with mortality after a wildfire
vol. 12, pp. 397-402 (online: 31 July 2019)
Research Articles
Regeneration of Abies pinsapo within gaps created by Heterobasidion annosum-induced tree mortality in southern Spain
vol. 7, pp. 209-215 (online: 27 February 2014)
Research Articles
Forest litter as the mulch improving growth and ectomycorrhizal diversity of bare-root Scots pine (Pinus sylvestris) seedlings
vol. 8, pp. 394-400 (online: 20 August 2014)
Review Papers
Ensuring future regeneration success of Qualea grandiflora Mart. (Vochysiaceae) in neotropical savanna (cerrado) biomes by reviewing the available information and identifying research gaps
vol. 13, pp. 154-164 (online: 02 May 2020)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword