Ensuring future regeneration success of Qualea grandiflora Mart. (Vochysiaceae) in neotropical savanna (cerrado) biomes by reviewing the available information and identifying research gaps
iForest - Biogeosciences and Forestry, Volume 13, Issue 3, Pages 154-164 (2020)
doi: https://doi.org/10.3832/ifor2684-013
Published: May 02, 2020 - Copyright © 2020 SISEF
Review Papers
Abstract
Qualea grandiflora Mart. (Vochysiaceae) is one of the most widespread species within the cerrado formation, which counts amongst the most threatened ecosystems worldwide. Understanding the regeneration ecology of Q. grandiflora is a central requirement for the success of conservation measures and silvicultural management strategies. Exhaustive investigation was carried out into each of the development stages, and the connected processes within the regeneration cycle, to provide a better understanding of the main factors influencing the regeneration ecology and the recruitment dynamics of the species. For this purpose, we analysed 92 different sources of information in this review, divided into two groups (n = 41 with “basic species information” and n = 51 with “specific information about regeneration stages and processes”) relevant for regeneration and silviculture. Our literature review showed the high proportion of studies addressing the processes flowering, pollination and fruiting, whereas the subsequent processes like seed dispersal, seed storage, germination and seedling development are almost entirely lacking. This also applies for spatial information about environmental conditions and the related regeneration processes in Q. grandiflora. This knowledge is important for management, for example, knowledge of the critical distances between flowering and seed producing trees to ensure genetically diverse regeneration and the identification of safe sites for seedling establishment. Most of the practical suggestions in relation to increasing densities or growth of Q. grandiflora seedlings and saplings made in the literature are linked to less intensive fire management strategies adopted at certain times. The use of selective herbivory to reduce the increasing competition pressure exerted by invasive grasses and hampering Q. grandiflora seedlings is also cited. In this study we highlight the need for more complex species-specific information following the development stages and processes of the regeneration cycle so as to prepare a continuous strategy with a range of management approaches.
Keywords
Cerrado Formations, Environmental Influences, Regeneration Cycle, Silvicultural Management Strategies
Introduction
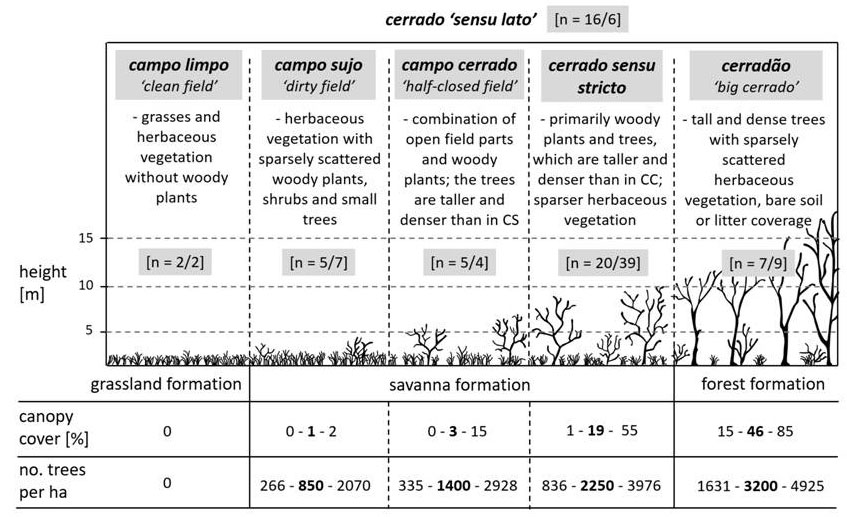
Qualea grandiflora Mart. is a typical tree species of the cerrado (neotropical savanna), a highly-threatened biome and one of the 25 “hotspots” of biodiversity worldwide ([56]). The cerrado encompasses different vegetation types, and is technically termed cerrado sensu lato (Fig. 1).
Fig. 1 - Overview of the cerrado sensu lato vegetation types according to Eiten ([25]) and Ruggiero et al. ([82]). Depending on the environmental characteristics, a series of vegetation types occurs in the cerrado sensu lato (CSL) savanna formation, henceforth referred to simply as cerrado, ranging from open grasslands (campo limpo, CL) to dense woodlands (cerradão, CD), with three intermediate vegetation types. These three intermediate vegetation types are: campo sujo (CS), described as grassland with a scattering of shrubs and small trees; campo cerrado (CC), where there are more shrubs and trees but still a large proportion of grassland; and cerrado sensu stricto (CSS), where trees and shrubs dominate but with a fair amount of herbaceous vegetation ([62]). Meanings of the numbers in the grey boxes: first number - publications including general information about Q. grandiflora, Vochysiaceae, cerrado, savannas or about the regeneration of other tree species typical of the cerrado biome; second number - publications specifically concerning the regeneration phases of Q. grandiflora or Qualea spp. investigated in this study, or the proportion of Q. grandiflora in the different formations; multiple responses possible.
The cerrado is one of the largest biomes in Brazil, with parts situated in Paraguay and Bolivia ([89] - Fig. S1 in Supplementary material). In the year 2004, cerrado biomes covered approximately 2 million km2, mostly in the central region of Brazil ([25]). Studies have estimated that between 40% to 55% of cerrado formations have been cleared and that a high degree of fragmentation has occurred ([62], [38]). Around 44% of the flora is endemic to this biome, and the total species richness is very high ([38]), as well as the risk of extinction of cerrado species ([90]). The corresponding climate is seasonally tropical with a dry winter. Maximum monthly temperatures can reach 40 °C. The minimum values are close to 0 °C during May, June and July. Frost events are also common in the cerrado. The annual precipitation lies between 1200 mm and 1800 mm and is concentrated in spring and summer (October to March), which make up a rainy season with warm temperatures ([74], [25]). Between May and September the levels of precipitation are greatly reduced and the mean temperatures fall ([40]). The long dry periods are characterised by the limited availability of soil nutrients and frequent burning, caused mainly by man ([35]). Therefore, the environmental conditions in the cerrado can be classified as stressful, and especially difficult for tree regeneration.
Q. grandiflora attains high frequencies, reaching proportions of up to 85% on the 376 analysed cerrado sites in Brazil ([73] - Fig. 2a, Fig. 2b). Its presence decreases from the cerradão (dense woodland) to the campo sujo ([25]). It is restricted to Central and South America, occurring in rain forests and in the cerrado ([47], [13], [57]). The tree species Q. grandiflora is a deciduous and anemochorous species ([50]). It is usually found from 700 m to 1100 m a.s.l., on dry and well drained sites occupied by dense woodlands and savanna formations ([16], [57]).
Fig. 2 - Pictures of structures typical of (a) the open cerrado formations and (b) the cerradão where Q. grandiflora is one of the most common tree species.
Q. grandiflora is a light-demanding, selective xerophytic tree species classified as a pioneer tree species due to its occurrence in the early stages of succession on dry and poor soils. Adult trees reach heights of between 7 m to 12 m, and 30 m to 40 cm in diameter. The tree traits of Q. grandiflora (e.g., thick, rough and irregularly fissured bark - [48]) are typical of species adapted to fire as the main disturbance factor occurring in their ecosystem ([8]). Apart from its ecological importance, the wood of Q. grandiflora is used locally, for example, as timber, charcoal and firewood ([92], [70]). The species is also important for the restoration of degraded areas and as an ornamental tree along roadsides and in urban areas due to its conspicuous flowers and fruits ([23], [69]). Medicinal substances are extracted from the bark for use as antiseptics for the treatment of external wounds and for their anti-ulcerogenic properties ([31], [33]), and also from leaf extracts, which have analgesic effects and can potentially act as an anti-convulsion medicine ([31]). Although Q. grandiflora is considered to be one of the most widespread and variably used tree species in the cerrado ([25], [16]), there exists no strategy for the silvicultural or landscape-oriented management of this tree species ([76]). This means in particular that there is no strategy with regard to its regeneration. The current lack of knowledge concerning the species’ regeneration ecology poses difficulties in developing conservation strategies with practical relevance for the species. For all forest ecosystems worldwide, it is accepted that an understanding of the species-specific regeneration ecology is important to guaranteeing the continued presence of tree species as an important part of the vegetation community ([39], [43]). Fundamental information on different processes occurring within the regeneration cycle is necessary in order to develop species-specific conservation strategies ([6]). The success of seedling establishment, in general, depends strongly on environmental factors during the different development stages. This is all the more obvious for tree species regenerated under harsh environmental conditions such as those found in the cerrado formations ([29]). Factors such as seed predation, fire, water stress and a low availability of nutrients may have an existential effect on the regeneration capacity of Q. grandiflora ([34]). It is important to identify the main bottlenecks in the regeneration cycle of Q. grandiflora, and to analyse whether and how they can be influenced or buffered by specific silvicultural measures ([51]).
We approached the problem through the following steps: (i) we analysed the current knowledge of the development stages and specific processes linked to the regeneration cycle of Q. grandiflora by means of an extensive literature review; (ii) we identified the main factors influencing regeneration; (iii) we formulated possible protection and silvicultural measures for all regeneration stages; and finally (iv) we highlighted the most important gaps in the knowledge with relevance for future research. One of the main objectives behind the study was to use the information that emerged from the literature review to identify the gaps in the information that still exist in relation to the regeneration cycle of Q. grandiflora and to formulate questions for future research. The overarching purpose of this study was to scrutinise current management strategies and to suggest new management approaches that should be considered to achieve the in situ conservation of Q. grandiflora within the cerrado biome.
Methods
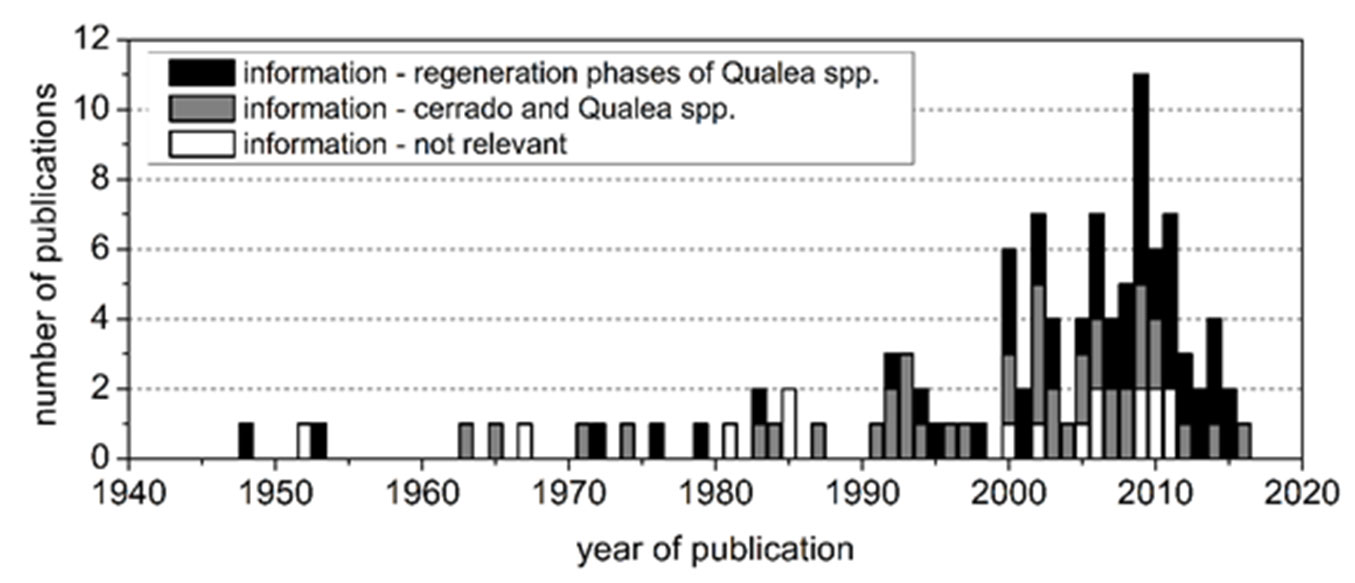
For the purpose of the literature review, the following literature databases were searched: Web of Science, Digital Book Information System (DBIS), CAB Abstracts, BIOSIS Previews, Google Scholar, the Catalogue of the Saxony State and University Library in Dresden (SLUB) and the Journal Storage Database (JSTOR). The search was undertaken for publications in the languages English, Portuguese and Spanish. Publication media such as scientific books, international peer-reviewed scientific articles, conference papers, reports and theses were integrated into the search. “Grey literature” was also included because unfortunately many relevant facts concerning the species were not to be found in the peer-reviewed publications. The choice of keywords was based on terms typically directly linked with the main development stages and processes in the regeneration cycles ([43]) of tree species. This common approach facilitated a systematic analysis and provided for better comparability with regeneration studies carried out for other tree species. For the literature search the name of the target species, Q. grandiflora, or of the associated family, Vochysiaceae, was combined with the main regeneration processes (flowering, pollination, fruiting, seed dispersal, seed storage, germination, growth of seedlings and saplings) and stages (flowers, pollen, fruits, seeds, germinants, seedlings, saplings - for detailed information see Tab. S1 in Supplementary material). Additional terms were used to define and describe the corresponding environmental conditions such as savanna formations, micro-sites, biotic and abiotic factors. A total of 108 publications related to the cerrado biome and to the Vochysiaceae family was filtered as a first step of the analysis (Fig. 3). After an initial review 16 publications were directly rejected for insufficient information content. The remaining 92 publications were subjected to a more intensive review.
Fig. 3 - Publications (n = 108) presenting information on dry forests, cerrado formations and the tree species Q. grandiflora in the period 1948 to 2016. (white): publications excluded from further analysis for a lack of relevance to the topic (n = 16); (grey): publications of general relevance to the topic of the cerrado biome and the regeneration of tree species (n = 41); (black): publications of specific importance for the regeneration cycle of Q. grandiflora or Qualea spp. (n = 51).
Results
Sixty of the 92 relevant publications were written in English, 31 in Portuguese and only 1 in Spanish. Distinguished on the basis of publication media, relevant information was found in journals (n = 79), books and monographs (n = 4), theses (n = 4), conference papers (n = 3) and grey literature (n = 2). Most publications (n = 64) were published in the period 2000 to 2016.
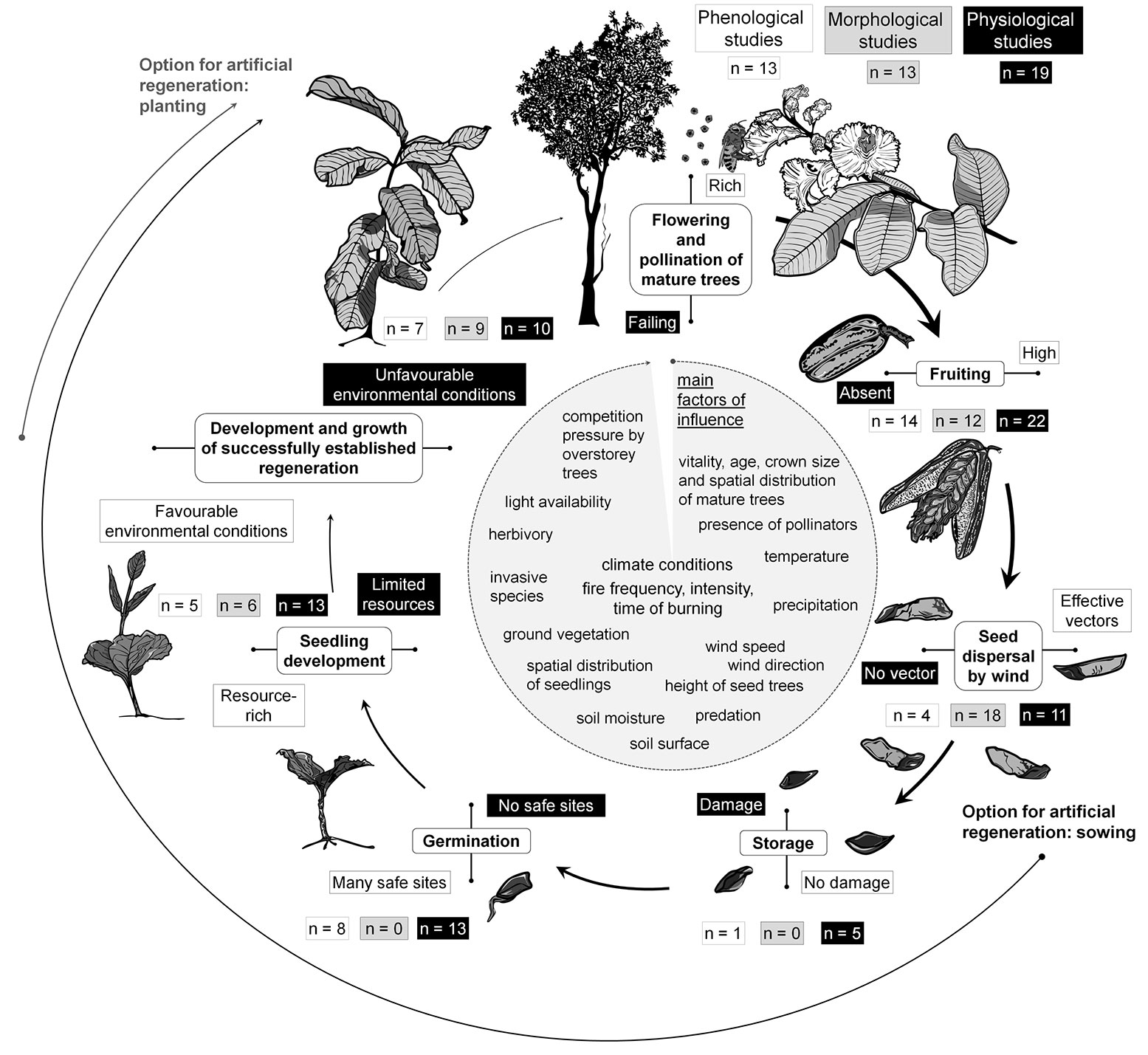
Based on the 92 publications, a first group of 41 publications was separated containing basic information connected with cerrado formations and Q. grandiflora. Publications in this group primarily contained information about Q. grandiflora as part of plant communities of different savanna formations and the climatic and soil conditions (Fig. 1). The second group of 51 publications directly addressed different aspects of the regeneration cycle of Q. grandiflora. This second group of studies was used for the following main data analyses. The contents of the second group of publications were distributed unequally between the different stages and processes in the regeneration cycle of Q. grandiflora. A high proportion of these used only descriptive methods ([60], [97]). Fig. 3 shows the three different categories of relevance of the publications (1: lack of relevance; 2: general relevance; 3: high relevance) relative to the regeneration aspects of Qualea spp. generally and Q. grandiflora specifically.
Almost all of the studies of the cerrado formations relevant for this review, and containing location coordinates, were carried out in Brazil (Fig. S2 in Supplementary material). This underlines the importance of the cerrado formation in Brazil, with coverage of approximately 25% of the territory ([53]). Of those studies identified as being relevant for regeneration, 6 were related to unspecified cerrado sensu lato vegetation, characterised by the presence of Qualea spp. (Fig. 1). Most of the publications (n = 39) were related to the cerrado sensu stricto formation. A low proportion of the published studies concerned study sites in the cerradão (n = 9). As is shown in Fig. 4, the investigations into the Q. grandiflora regeneration cycle concentrated on physiological aspects of flowering (n = 19) and fruiting (n = 22).
Fig. 4 - Regeneration cycle of Qualea spp. and Q. grandiflora differentiated according to the main development stages and connected processes (reference is a total of 51 publications, but multiple responses were possible for a single publication).
Aspects of seed morphology, and corresponding seed dispersal mechanisms, were described and analysed in 12 and 18 publications, respectively. Information concerning seed storage was scarce. Studies examining the germination process (n = 13) and seedling development (n = 13) mostly focused on phenological details (Fig. 4). Of the relevant studies, 12 were entirely descriptive with no additional information on measurements. In 16 publications individual aspects with practical relevance for silvicultural management were presented, but no study was directly related to an overall species-specific regeneration management strategy or practical measures such as those presented for tropical tree species by Martinez-Ramos et al. ([51]).
Flowering
The publications related to the flowering of Qualea spp. or Q. grandiflora described the species as monoecious and highly dependent on pollinators, particularly hawkmoths (Sphingidae) but also other moths and wild bees ([4], [64], [93], [5], [61], [85]). The longevity of flowers is only two days ([61]). Silberbauer-Gottsberger ([86]) reported 70 flowering Q. grandiflora trees per hectare during a one-year period, representing 38% of all mature Q. grandiflora trees. Over a period of three years the individual tree-based study carried out by Potascheff et al. ([69]) documented mean annual flower numbers of 109, 635 and 695 per tree. Flowering occurs in the dry period or during the transition between the dry and humid periods ([5], [45], [67]), and usually the flowering peak is reached quickly and is often synchronised between individual trees ([45]). These characteristics can be considered a strategic adaptation to several biotic and abiotic factors ([93], [1]), determined by intrinsic genetic traits ([78]) allowing the species to reproduce and survive in its native ecosystem. The effect of fire on the flowering of Q. parviflora was shown by Palermo & Miranda ([63]), who observed that no inflorescence was produced one year after fire, suggesting that the species may need more than one year before the normal reproductive cycle can recover. Consequently, fire intensity and the related damage determine the time until regeneration processes such as flowering return to a more regular state. This must be taken into consideration when using fire as a management tool (refer to the discussion). Another study showed flowering to be negatively correlated with precipitation ([67]). This suggests that flowering is induced by rehydration, due to the reduced transpiration brought about by leaf shedding ([9]). The available information concerning the periods of flowering and fruiting differed between the literature sources (Tab. 1).
Tab. 1 - Phenologically influenced frequency of flowering and fruiting processes in Q. grandiflora according to various sources. (FL): main time of flowering; (T): transition time of flowering and fruiting; (FR): main time of fruiting; (*): dry season with precipitation per month < 60 mm (according to [74]).
| Authors | Lat S (°) |
Long W (°) |
Altitude (m a.s.l.) |
Jan (1) | Feb (2) | Mar (3) | Apr (4) | May (5) | June (6) | July (7) | Aug (8) | Sept (9) | Oct (10) | Nov (11) | Dec (12) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Batalha & Mantovani ([5]) | 21 | 47 | 660-730 | FL | FL | - | - | FR | FR* | FR* | FR* | T | T | T | T |
| Kutschenko ([41]) | 15 | 47 | 1025-1150 | T | T | T | FR | FR* | FR* | FR* | FR | FR* | FL* | FL | FL |
| Negrelle ([57]) | 25 | 52 | 730-1100 | FL | T | FR | FR | FR | FR | - | - | - | FL | FL | FL |
| Pirani et al. ([67]) | 15 | 52 | 562 | T | FR | FR | FR | FR* | FR* | FR* | FR* | - | T | FL | FL |
| Santos & De Melo Ferreira ([85]) | 10 | 48 | 270 | FL | T | T | FR | FR* | FR * | FR * | FR* | FR | - | FL | FL |
| Ribeiro & Borghetti ([77]) | 15 | 47 | 1100 | FR | FR | FR | FR | FR* | FR* | FR* | FR* | FR* | * | - | FR |
| Silberbauer-Gottsberger ([86]) | 22 | 48 | 550-700 | FL | FL | - | - | FR | FR | FR* | FR* | - | - | - | FL |
| Silvério & Lenza ([88]) | 14 | 52 | 340 | FL | - | - | - | FR* | FR* | FR* | * | * | - | FL | FL |
| Lenza & Klink ([45]) | 15 | 47 | 1045-1146 | FL | - | - | - | * | * | FR* | FR* | FR* | T | FL | FL |
| Paulilo ([64]) | 22 | 47 | 760 | FL | FL | - | - | - | - | - | - | T | FL | FL | FL |
Pollination
The pollination process appears highly successful, given the almost annual flowering ([67]), and a high viability of the pollen of between 70% to 97% ([54], [10]). The dry period favours insect activity, and exemplifies the close plant-insect interaction. Fischer & Gordo ([30]) and Oliveira et al. ([61]) showed that in the case of Q. cordata and Q. grandiflora it is important to know how close the plant-pollinator interactions are. The authors could prove that the territorial behaviour of pollinators is strongly influenced by the total number of flowers and the distribution of flowering trees. Borges et al. ([10]) demonstrated that isolated flowers on an individual tree not visited by pollinators failed to develop fruits, and Barbosa ([4]) demonstrated auto-incompatibility in Q. grandiflora. The only study including spatial information about the pollination of Q. grandiflora indicated mean effective dispersal distances of 525 m for a cerrado landscape in Brazil, with an average distance between mother trees of 566 m ([69]). The same study showed that outcrossing rates were high, and that mating amongst relatives and selfing correlations were low.
Fruiting
According to Borges et al. ([10]), trees start fruiting at an average diameter at breast height (DBH) of 22 cm. Based on the very limited dendrochronology research to date, it can be assumed that a Q. grandiflora tree with this diameter is approximately 25 to 30 years old ([44]). However, Q. grandiflora trees of smaller dimensions have also been seen bearing fruit (personal observation, [19]). In the study by Borges et al. ([10]), a weak positive correlation was revealed between DBH and the number of fruits produced per tree. It may be assumed, therefore, that individuals of a higher diameter produce more fruits. The number of Q. grandiflora trees with ripe fruits during the year may reach 20 trees per hectare, or 11% of all mature trees ([86]). Observing the initial processes of fruiting in Q. grandiflora, Barbosa ([4]) recorded a relationship between flowers/ buds of 32.1%, compared to 0.8% for fruits/ flowers. Silberbauer-Gottsberger ([86]) documented a fruit/flower relationship of 28.6%. The rhythm of fruiting in the cerrado is more strongly linked to the seasonal climate than is the case for flowering and pollination ([86]). Oliveira et al. ([61]) described the amount of fruiting as highly variable between years. They observed high fructification rates for Q. grandiflora, with two years of fruit production followed by a year with almost no fruit. The production of fruit by anemochorous species in the cerrado formations is significantly negatively correlated with precipitation but significantly positively correlated with temperature ([67]). This phenomenon was described for Q. grandiflora and other species, especially wind dispersed tree species ([93]). Most of the anemochorous species (e.g., Kielmeyera coriacea, Cochlospermum religiosum, Dalbergia miscolobium) of the cerrado produce fruits during the dry period, reaching their peak in August and September ([5], [45], [67], [84]). Most of the studies mentioned that fruiting takes place during the period April to September (Tab. 1). Q. grandiflora has fruits that protect seeds from burning ([17]), but fire is not necessary for the opening of fruits and seed release.
Seed characteristics and dispersal
The literature review revealed that studies conducted on Q. grandiflora seeds are rare ([23]). Most such studies (Fig. 4) were concerned with descriptions of morphological seed characteristics ([27], [41], [77]). The studies showed damage to between 20% and 60% of seeds, caused mainly by Buprestidae and Hymenoptera ([18]). The available information on seed dispersal in Q. grandiflora is limited to the written description of anemochorous seed dispersal mechanisms ([60], [42], [49], [37], [72]). Typically, seed dispersal takes place during the dry period, with low air humidity, increasing wind velocity and decreasing leaf cover ([5], [94]).
According to Augspurger ([3]), the seeds of Q. grandiflora are comparable in size and weight to those of the species Cedrela odorata (Meliaceae), and consequently the estimated dispersal distances are 103 m to 410 m. Salazar et al. ([84]) documented a mean seed density of 0.9 seeds per m2 in a campo sujo formation, but the authors collected no seeds in the cerrado sensu stricto formation. A detailed analysis of Q. grandiflora seed dispersal distances using seed traps was performed by Da Ponte ([19]) within the Mbaracayú Nature Forest Reserve in eastern Paraguay, where mean dispersal distances of between 11 and 62 m were determined.
Seed storage
According to De Melo et al. ([22]) and Dousseau et al. ([23]), seeds of Q. grandiflora possess dormancy. Germination was observed to increase to 60% after seed storage, compared to 10% without storage. Rizzini ([79]) mentioned the importance of seed storage in the cerrado biomes to bridge periods of harsh conditions, including arid soil surfaces, prolonged periods of drought and, above all, fire. Ribeiro & Borghetti ([77]) demonstrated the high tolerance of Q. grandiflora seeds to heat shocks of between 80 to 140 °C. This resistance to high temperatures is necessary because of the occurrence of air and soil surface temperatures of up to 350 °C during fire, depending on the fuel load ([53]). However, fire can also promote germination by breaking seed dormancy ([7], [77]). The observed seed attributes and seed mass, therefore, increase seeds’ tolerance to environmental stress and are likely to contribute to the recruitment of the tree species in the harsh environments of the cerrado ([8], [77]). Assessments of the effects of seed predation revealed that more that 95% of Q. grandiflora seeds and fruits may be damaged by parrots or insects in open and closed woodland savannas ([20], [83]).
Germination and development of early seedling stages
The germination process of Q. grandiflora is assigned to the phanecotylar-epigeous type of germination ([27]). This means that the cotyledons are lifted above the soil surface. To increase the likelihood of successful germination in cerrado formations, and to survive the first seedling stages, tree species like Q. grandiflora disperse their seeds during the dry period, but shortly before the transition to the humid season begins. Q. grandiflora seedlings that emerge at the beginning of the rainy season develop more successfully due to the optimal soil moisture conditions (Tab. 1). Ribeiro & Borghetti ([77]) demonstrated the great tolerance of Q. grandiflora seeds to extremely high temperatures and desiccation, achieving high germination rates of 63% to 71%. Experimental studies by Bilio et al. ([7]) and Dousseau et al. ([23]) showed that temperatures of 25 to 30 °C lead to the highest germination rates under controlled conditions, irrespective of the presence or absence of light ([97]). In order to survive, seedlings must develop deep roots before the next dry season starts ([79], [83]). The rate of shoot growth is typically slow compared to the fast root growth occurring during the first months of seedling development ([79]). This may provide an explanation for the supposedly lower seedling and sapling establishment potential of Q. grandiflora, as it might suggest higher competitiveness of other, in some cases invasive species. Although Q. grandiflora has a high level of tolerance in terms of irradiation and soil nutrients as these affect seed germination and initial seedling growth, Klein ([40]) revealed that seeds of Q. grandiflora often achieve germination rates of less than 20% under natural conditions in the cerrado. Costa & Franco ([15]), by contrast, found higher germination rates in cerrado formations covered by ground vegetation comprising herbs and shrubs, which facilitate the germination process by providing small-scale shelter effects. Litter, ground vegetation and canopy cover exert an influence on germination and early seedling development, yet these factors are also affected by fire ([8]). Fire also results in an immediate flush of nutrients, while in the long-term leading to losses of phosphorus, nitrogen and sulphur.
Development and growth of established young trees
According to Eiten ([25]), the growth of established Q. grandiflora seedlings may be influenced primarily by adaptation features developed in order to survive in the harsh conditions of the cerrado rather than under sheltered conditions beneath the canopy. Paulilo et al. ([65]) emphasised the importance of water availability for further growth, biomass and leaf area development in Q. grandiflora. As a deciduous tree, Q. grandiflora can shed its leaves under water stress during the dry season, with new shoots emerging again later on during the wet season ([75]). Adaptations of young Q. grandiflora to limited water and nutrient availability in the cerrado ecosystem are the early development of deep taproots and the reduction of stomatal conductance ([25], [65]). This deep taproot development and the high capacity for re-sprouting (vegetative reproduction) are also crucial to compensate for damage caused by fire as demonstrated in various studies of the genus Vochysia and other savanna tree species ([79], [8]). Whereas Q. grandiflora can reach higher regeneration densities in unburned than burned areas, mortality as a consequence of intraspecific competition does not appear to be an important aspect in the dynamic of this species ([16]). Studies by Lima-Ribeiro ([46]) and Costa & Santos ([16]) revealed that the abundance of Q. grandiflora ranges between 80 to 147 and 36 to 638 individuals per hectare, respectively.
In a typical cerradão formation the relative light availability ranges between 20% to 30% ([35]). Although this level of light availability is not directly relevant for the survival of established young Q. grandiflora plants, it does affect the absolute growth rate of young plants, biomass partitioning (resource allocation), carbon assimilation rates, morphological plasticity and, finally, their interspecific competitiveness as demonstrated by Felippe & Dale ([26]). A high degree of plasticity has been described for Q. grandiflora in terms of the different formations in the cerrado biome (from the campo cerrado to the cerradão).
The below-ground biomass of tree species is typically very high in cerrado ecosystems ([74], [64], [55], [28]), but more than 80% of the root biomass occurs in the upper 30 cm of the soil ([79]).
Only three studies addressed the question of the spatial distribution characteristics of tree species in the cerrado biome. Studies carried out in different municipalities in Brazil revealed that individuals of Q. grandiflora with a DBH greater than 3 cm have a tendency towards spatially aggregated distributions ([24], [81]). Costa & Santos ([16]) observed a trend in the patterns of spatial distribution for Q. grandiflora dependent on the different cerrado formations. Q. grandiflora would appear to follow a more aggregated distribution pattern in open cerrado formations (Fig. 1), whereas in dense forest formations young and adult Q. grandiflora trees show a greater range of variation, from random spatial patterns to aggregated ([16]).
Discussion
After a summary of the available process- and stage-specific information concerning the regeneration cycle of Q. grandiflora, the following section contains a discussion of those aspects that can be included in strategies for the silvicultural management or conservation of the species (Tab. 2). It is particularly useful to follow again the chronology of the regeneration cycle in order to critically scrutinise the available information and to highlight which additional information would be helpful to enhance the regeneration success of Q. grandiflora in the cerrado biome.
Tab. 2 - Summary of the ecological bottlenecks, research gaps and suggestions for silviculture for each reproduction stage.
| Regeneration stage | Identified ecological bottlenecks | Research gaps within the regeneration cycle | Suggestions for silviculture |
|---|---|---|---|
| Flowering | extremes in rainy seasons (El Nino) intensive crown fires |
age, size and vitality related flowering information for individual trees, effects of drought and fire spatial information (spatial distribution patterns, distances between trees) |
promotion of potential seed trees by continuous crown tending regulation of the spatial distribution of flowering trees on local and regional scale to guarantee genetic exchange |
| Pollination | low number of mature trees in local populations missing pollinators intensive crown fires |
range of pollinator species and numbers of individuals detailed information about habitats, lifecycles and behaviour of pollinator species |
ensure an adequate number and distribution of mature trees support pollinating moths, bees and hummingbirds by establishing areas with dense sub-canopy structures as refuges protect endemic flowering tree species |
| Fruiting | calamities of seed predating insects intensive crown fires |
quantity of fruits and seeds damaged by fire events age, size and vitality related fruiting information for individual trees |
preservation of a sufficient number of seed producing trees regulation of the spatial distribution of seed trees seed collection in regions with high quantities of seed and for known provenances to implement direct seeding or plant propagation |
| Seed dispersal | heavy rainfall events high proportions of diaspore damage |
mean and maximum dispersal distances and densities related to individual tree parameters quantification of direct wind effects (e.g., wind speed, wind direction) |
long-term determination of seed source locations (see above) aggregate or regular seed distributions can be controlled by combining seed tree positions and wind parameters |
| Seed storage | calamities of seed predating insects intensive and frequent ground fires |
seed storage under field conditions (predation, influence of fire, depth of soil layer) causes of viability loss of stored seeds |
preparation of suitable surface conditions (e.g., low intensity fire, soil scarification) before seed dispersal, concentrated on potential deposition sites surrounding seed trees |
| Germination | calamities of seed predating insects intensive and frequent ground fires invasive species as competitors |
fire effects on seed germination safe site characteristics, competitors and facilitation plants |
break dormancy of deposited seeds by low intensity surface fires reduce competing ground vegetation, especially grasses (surface fire, soil scarification, canopy cover or light grazing) |
| Seedling establishment and development | intensive ground fires extremely long-term drought invasive species as competitors intensive herbivory |
temporal and spatial documentation of seedling establishment (density, distribution patterns) and growth (above- and below-ground) | direct (e.g., low intensity surface fires, mulching, mowing or light grazing) or indirect (suppression of light demanding competitors by regulating the shelter of canopy trees) regulation of interspecific competition, especially of invasive species |
| Development and growth of successfully established young trees | continuous influence of competitive invasive species extremely long drought periods continuous and intensive herbivory |
age and diameter frequency distributions documentation of growth parameters under different conditions (light availability, inter- and intraspecific competition) options for regulation of competition exerted by invasive species, e.g., using fire |
regulation of interspecific competition cuttings within the overstorey tree layer to regulate light availability and to reduce water stress low intensity surface fires guarantee re-sprouting of established regeneration |
It was shown that the fruit/flower relationship is characterised by extremely variable production of fruits relative to the numbers of flowers. The presence of pathogens responsible for damage to flower buds could be one explanation for the aforementioned high flower/bud ratio, whereas the low ratio of fruits/flowers may be explained by the absence of pollinators (see above, [10]). Considering the close interaction between insects and the success of pollination of Q. grandiflo- ra ([4], [86]), indirect measures taken to support the specific habitat requirements of pollinating insects are conceivable (e.g., moths, wild bees or hummingbirds). Studies focused on flowering and pollination are of practical relevance as information at the individual tree level (e.g., age-dependent flowering or fructification, tree vitality, crown and stem dimensions) is rare ([86]). Recent studies either focused only on individual growth parameters and age determination ([44]) or on genetic parameters within the regeneration cycle of Q. grandiflora ([69]). Although these studies represent a very important contribution to the field of species-specific regeneration in the cerrado formations, the combination of both aspects should be undertaken.
Another important aspect connected with pollination mechanisms at different spatial scales is the question of distance-dependence and the spatial distribution of flowering trees. Some initial findings have been produced by means of genetic analyses, an important tool for use in future studies to enhance the current knowledge of pollination and flowering ([2]). The case study by Potascheff et al. ([69]) conveys a first impression of pollen distances and related genetic traits. By analysing spatial information, we may be able to identify the critical distances between flowering trees necessary to ensure the successful regeneration of Q. grandiflora within different cerrado formations or across different spatial scales ([37], [11]). This information on maximum allowable distances between vital, mature Q. grandiflora trees is necessary for the development of successful conservation management strategies. Replicated documentation of local and regional flowering and fruiting frequencies, their success and the main factors of influence (biotic or abiotic) can provide an idea of the feasibility and possible limitations of proposed measures; for example, application of optimal fire management strategies to avoid fragmentation ([80]). Fire may affect important pollinators, either direct fire-induced mortality or indirectly by limiting flower resources ([32]). The global analysis of the effects of fire on pollinators published by Carbone et al. ([12]) revealed that wildfires and prescribed fires have different effects on different pollinator groups. Whereas habitat specialists such as butterflies and bird species fall in abundance, other pollinator groups such as Hymenoptera or Diptera are positively affected. But the authors stated that publications of these pollinator groups related to Q. grandiflora are particularly limited. The timing of fire and the burn frequency may have different impacts on pollinator guilds and should, therefore, be explored. Invasive plants (e.g., Melinis minutiflora, Brachiaria decumbens or Pteridium aquilinum) and their future management would appear to exert a great influence on pollination interactions and associations in cerrado biomes ([36]), because of the displacement of flowering endemic species, absent functional floral traits and the related interruption of plant-pollinator interactions ([14]).
The preservation of a sufficient number of potential seed trees of high vitality is one means to guarantee regeneration, but preliminary information about the natural ecosystem structure is needed to decide on sustainable measures appropriate within the current situations prevailing in cerrado formations ([39]). Although the density of trees is low in most cerrado formations, one option might be to consider whether mature individual trees of Q. grandiflora in dense cerradão formations would benefit from a reduction of inter- or intraspecific competition caused by other tree species or crown-enveloping species such as vines and lianas ([62]).
Important information about seed dispersal, a key process with implications for, e.g., the deposited seed densities, and dispersal distances and directions, is only infrequently available for Qualea spp. generally and Q. grandiflora in particular ([84]). Such information about distance-dependent seed densities and directions may be considered basic ecological information. This information would help silviculturists and local managers to estimate the probability of regeneration, and the subsequent spatial distribution of Q. grandiflora. In combination with empirical sampling of annual seed production at local scales, this information would make possible inferences about the long-term development of species-specific seed production. Should continuously low dispersal rates or locally isolated seed dispersal be observed in cerrado formations, additional controlled input of Q. grandiflora seeds or planting of known provenances could be undertaken as part of silvicultural management activities ([66]). Appropriate locations must be selected or additional measures to push back the competing ground vegetation must be examined (see below). Provisional results of the first direct seeding experiments revealed low success for Q. grandiflora ([66]). Regular tests of local and regional seed quality can help to identify the main reasons for seed damage and give an idea of the species-specific potential to germinate successfully. Given the seed dormancy-breaking function of fire, silvicultural activities have to adapt fire management to allow surface fires with low intensities ([96]). The information on seed dispersal and the safe sites required for germination suggest it would be expedient to concentrate these low intensity burns around mature trees or tree groups of the respective target species, for example, Q. grandiflora. The seeds of flat-seeded species such as Q. grandiflora should not be buried. Mulching appears the best site preparation as seedling emergence is higher on mulched sites than where seeds are uncovered ([87]).
The transition process from seed to seedling is one of the highest risk periods in the lifecycle of most plants, and the capacity of a seed to tolerate stress conditions is fundamental for germination and recruitment under harsh environmental conditions ([28]). There is a high degree of small-scale heterogeneity in soil moisture, which is critical for germination and to the success of the earliest development stages of seedlings ([28], [66]). Surface fires have been described as ecological bottlenecks, reducing seedling density, but preserving saplings ([8], [58]). To enhance the success of Q. grandiflora during the process of germination and seedling development, the positive effects of surface fires (reduction of competing ground vegetation cover, increase of direct irradiation and temperatures) can be used, but the timing of fire initiation appears to be the most crucial management factor beside fire intensity. Periodic burns implemented as a silvicultural management strategy may help to maintain plant diversity, protect plants from disease and allow perennial grasses and herbaceous plants promoting the establishment of Q. grandiflora seedlings ([95]). Another option to regulate competition from invasive grasses is light grazing by livestock with a preference for invasive species (e.g., African grass, Melinis minutiflora - [36]).
Little is known about the capacity of Q. grandiflora to re-sprout and the potential application of management employing coppice systems as an adaptation strategy to stimulate and increase the above-ground development and competitiveness of trees ([94]). However, it has been suggested that, compared to other cerrado species, Q. grandiflora is sensitive to disturbances, especially fire ([21]), as only the above-ground parts of young plants (e.g., shoots) are able to re-sprout ([52]). The regeneration of Q. grandiflora in cerrado formations could be enhanced by prescribed wet-season fires with the purpose of burning small areas or patches that may contribute to the creation of a mosaic of low-fuel patches and function as natural firebreaks reducing the spread of subsequent fires ([71]) and at the same time avoid the continuous spread of invasive species ([68]). In terms of traditional silvicultural management, the relatively low tree densities do not necessitate measures of intraspecific competition regulation. Intensive interspecific competition can be assumed to be more relevant for this developmental stage of cerrado tree species, especially in the cerradão formation ([91]).
Although the influence of shelter trees has been described for Q. grandiflora ([25]), no growth parameters directly related to the canopy cover provided by shelter trees or to light measurements are available. Therefore, silvicultural measures in cerradão formations where the number of Q. grandiflora seedlings is underrepresented but sufficient seed trees are present, should be oriented towards selective felling of individual trees or the felling of small groups within the dominant tree layer. The establishment of small canopy gaps is also expedient where a dense layer of naturally regenerated Q. grandiflora is present, but these plants show obvious poor growth. Low vitality and growth can be caused by low light availability or severe water stress, influenced by directly competing overstorey trees. Although Ferreira et al. ([28]) observed a positive relationship between water availability and the structural attributes of the vegetation, seedlings and juvenile plants of Q. grandiflora are exposed to high competition pressure by larger trees.
There are multiple reasons for species or age specific differences in the spatial distribution patterns of cerrado trees, yet none have been investigated. A lot of traditional silvicultural management systems are based on spatial information regarding the stages and processes within the regeneration cycle of tree species. As already mentioned, planning and practical management of natural regeneration in silviculture necessitate spatial information about seed trees, seed dispersal, predation and seedling establishment ([59]). Almost all ecological processes in regeneration ecology are determined by spatial and temporal factors, and can only be controlled and assessed by silvicultural measures adapted to time and space.
Conclusions
Using the intensive literature review as a first analysis step, it was determined that a lot of important details concerning the regeneration cycle of cerrado tree species such as Q. grandiflora are available. In the second step we identified the main factors influencing the success of regeneration and pointed out the stage-specific risks. The influence of the fire regime would appear to be very important for all regeneration stages and processes in Q. grandiflora. Given the long history of human impact, in the form of rising population densities, increasing farming activities and fragmentation of the cerrado biome and surrounding areas, it is difficult to determine the natural fire regime ([38]). It has been proven that Q. grandiflora and Qualea spp. are more sensitive to fire than other woody species in the cerrado biome, and that their abundance decreases in intensively burned areas ([52], [21]). Small fires during the wet season are advantageous for the successful regeneration of tree species such as Q. grandiflora ([71]). Although there has been a lot of research related to the fire regime in the cerrado biome, species-specific and goal-oriented field experiments are still missing ([71]). Any such experiments should consider the different key variables of the fire regime and the regeneration stages of tree species such as Q. grandiflora (e.g., fire and vegetation dependent storage and seeding experiments) to develop concrete strategies with practical relevance. Prescribed burning measures to promote regeneration of Q. grandiflora should consider the time of year, the time since the last rainfall event and the amount of accumulated fuel to ensure success ([71]). Many important aspects of the life stages and processes remain unknown or wholly theory-based ([23]). Where it to be available, information on features such as age or diameter frequency distributions could be used to derive a characteristic quantitative relationship between adult trees and the younger developmental stages of healthy populations in a typical cerrado formation. This information is needed as a proxy for whether a tree species needs active management intervention or not. One such example was presented for the cerrado biome by Oliveira & Marquis ([62]), at the level of cerrado formations and functional groups of species rather than on a species level. By comparing tree species in this way, and considering, for example, different local climate and soil conditions or fire frequencies, it is possible to characterise the species’ behaviour as controlled by abiotic environmental factors or interspecific competition, especially in the context of increasing competition from invasive species in neotropical savanna biomes.
Funding information
This literature review was carried out within the framework of a PhD study funded with a research grant provided by the DAAD (German Academic Exchange Service). A special thanks goes to our proof reader, David Butler Manning.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Online | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Franka Huth
Sven Wagner 0000-0003-3796-3444
Institute of Silviculture and Forest Protection, Department of Forest Sciences, Faculty of Environmental Sciences, TU Dresden, P.O. Box 1117, 01735 Tharandt (Germany)
Corresponding author
Paper Info
Citation
Da Ponte G, Huth F, Wagner S (2020). Ensuring future regeneration success of Qualea grandiflora Mart. (Vochysiaceae) in neotropical savanna (cerrado) biomes by reviewing the available information and identifying research gaps. iForest 13: 154-164. - doi: 10.3832/ifor2684-013
Academic Editor
Paola Mairota
Paper history
Received: Nov 14, 2017
Accepted: Feb 12, 2020
First online: May 02, 2020
Publication Date: Jun 30, 2020
Publication Time: 2.67 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2020
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 42105
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 35755
Abstract Page Views: 3010
PDF Downloads: 2582
Citation/Reference Downloads: 2
XML Downloads: 756
Web Metrics
Days since publication: 2109
Overall contacts: 42105
Avg. contacts per week: 139.75
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2020): 3
Average cites per year: 0.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Effects of gap size and within-gap position on seedlings establishment in silver fir stands
vol. 1, pp. 55-59 (online: 28 February 2008)
Research Articles
Modelling natural regeneration of Oak in Saxony, Germany: identifying factors influencing the occurrence and density of regeneration
vol. 16, pp. 47-52 (online: 16 February 2023)
Research Articles
Methods for predicting Sitka spruce natural regeneration presence and density in the UK
vol. 12, pp. 279-288 (online: 23 May 2019)
Short Communications
Multi-aged micro-neighborhood patches challenge the forest cycle model in primeval European beech
vol. 13, pp. 209-214 (online: 06 June 2020)
Review Papers
Problems and solutions to cork oak (Quercus suber L.) regeneration: a review
vol. 16, pp. 10-22 (online: 09 January 2023)
Research Articles
Modelling the moisture status of habitats by using NDVI on the example of the Cerrado and Atlantic Forest biomes borderland (Brazil)
vol. 18, pp. 375-381 (online: 16 December 2025)
Research Articles
Density and spatial distribution of beech (Fagus sylvatica L.) regeneration in Norway spruce (Picea abies (L.) Karsten) stands in the central part of the Czech Republic
vol. 9, pp. 666-672 (online: 12 March 2016)
Research Articles
Differences of fire activity and their underlying factors among vegetation formations in Greece
vol. 6, pp. 132-140 (online: 08 April 2013)
Research Articles
Short- and long-term natural regeneration after windthrow disturbances in Norway spruce forests in Bulgaria
vol. 11, pp. 675-684 (online: 23 October 2018)
Research Articles
Regeneration of Abies pinsapo within gaps created by Heterobasidion annosum-induced tree mortality in southern Spain
vol. 7, pp. 209-215 (online: 27 February 2014)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword