Differential adaptations in nursery seedlings from diverse Chilean provenances of Peumus boldus Mol.
iForest - Biogeosciences and Forestry, Volume 9, Issue 3, Pages 409-413 (2016)
doi: https://doi.org/10.3832/ifor1893-008
Published: Jan 29, 2016 - Copyright © 2016 SISEF
Short Communications
Abstract
Seed germination, seedling growth and biomass allocation of the endemic species Peumus boldus Mol. (Boldo) were studied in four provenances (two northern and two southern provenances) from central Chile. Seeds collected from five different mother plants for each provenance were sowed in plastic pots and placed in an ambient nursery. Germinated seeds were transplanted to 130-mL containers and cultivated under nursery conditions during one growing season. Germination capacity, seed weight, morphological traits of seedlings (root collar diameter, height, number of leaves, foliar area, root length), their biomass allocation pattern (dry mass of leaves, shoots and roots) and survival were analyzed. Results showed significant differences among provenances and mother plants for most traits. Northern provenances showed slower germination, smaller size, higher root biomass, lesser leaf area, and higher survival, while seedlings from southern provenances were taller, with more body mass, larger leaf area and lower root biomass. We concluded that northern provenances of Peamus boldus are more tolerant to drought and therefore are suitable for ecological restoration of drought-prone Mediterranean sites, while the use of southern provenances must be restricted to restoration of more humid environments.
Keywords
Boldo, Habitat Differentiation, Adaptation, Seed Provenances, Seedling Growth, Survival
Introduction
In the last decades, several studies reported the increase of land degradation and desertification in Mediterranean ecosystems ([12], [23], [29]). Mediterranean plants are well adapted to summer drought, though such adaptation may not be sufficient to ensure plant regeneration under extreme land degradation scenarios (e.g., wildfires - [27]). Indeed, water stress is a major cause of failure in ecological restoration of Mediterranean ecosystems ([20]). According to climate change predictions, the frequency and intensity of drought is expected to increase in Mediterranean regions ([8], [14]). This calls for studies focused on the ecology of species and their intra-specific variability in order to better understand their adaptability to the predicted changes and their suitability for ecological restoration purposes.
Peumus boldus Mol. (Boldo) is a sclerophyllous evergreen shrub native to Chile between 30° to 41° S and growing in plant communities of the Mediterranean region in central Chile ([13]). This species has several characteristics that make it an interesting case study for ecological restoration protocols, namely, a higher resistance to drought compared with other Mediterranean species, surviving in sites with less than 200 mm of annual precipitation ([7]). Its perennial leaves are used in medicine for their digestive, choleretic, and liver-protection properties ([9]), and this has generated a high pressure over the native forests. Furthermore, Boldo seeds have low germination rates (19-44% - [7]) due to the dormancy imposed by the essential oils of the pericarp. Botti & Cabello ([6]) pointed out that if Boldo seeds are sown during fall or winter, they will only germinate during the winter of the next year, being the embryo still immature; therefore, seeds must be collected in spring and sown immediately.
Despite the strong geographical isolation of southern provenances from the northern ones ([7]), information about the genecological differentiation of Peumus boldus is scarce. Vogel et al. ([33]) analyzed alkaloid and essential oil production in three different Boldo populations from central Chile, finding that northern provenances had significantly higher essential oil content and alkaloid concentration. Moreover, the available information on the variability of germination and seedling growth among provenances is limited. Germination was reported to greatly vary among populations ([4]).
It is known that provenances growing in Mediterranean environments show reduced leaf area as one of the key traits to withstand water deficit ([1]). Contrastingly, provenances from more mesic environments have elongated and large leaves and are restricted to humid or subhumid sites mainly in mild coastal areas (e.g., the “ilex” morphotype of Quercus ilex - [19]). According to the theory of specialization ([18]), genotypes adapted to favorable environments present superior performances in these environments as compared with those adapted to drought, but this is reduced when conditions are limiting, which results in a high phenotypic plasticity. It is also known that germination requirements of different species are often related to specific adaptation to their habitat ([22]). As a consequence, seeds collected from different parts of the species’ range often vary in their germination requirements.
In Chile, a limited number of studies have been carried out in P. boldus on the effect of provenance and mother plant in seed germination and seedling development. The aim of the present study was to analyze the effect of seed provenance and mother plant on the germination and short-term development of P. boldus. The wide distribution of P. boldus and the heterogeneity of the environments colonized suggest a high phenotypic plasticity of the species in terms of morphology. We tested the hypothesis that seedlings from northern provenances are more adapted to xeric environments, while southern provenances have adaptation mechanisms suitable for more humid environments.
Material and methods
Site selection
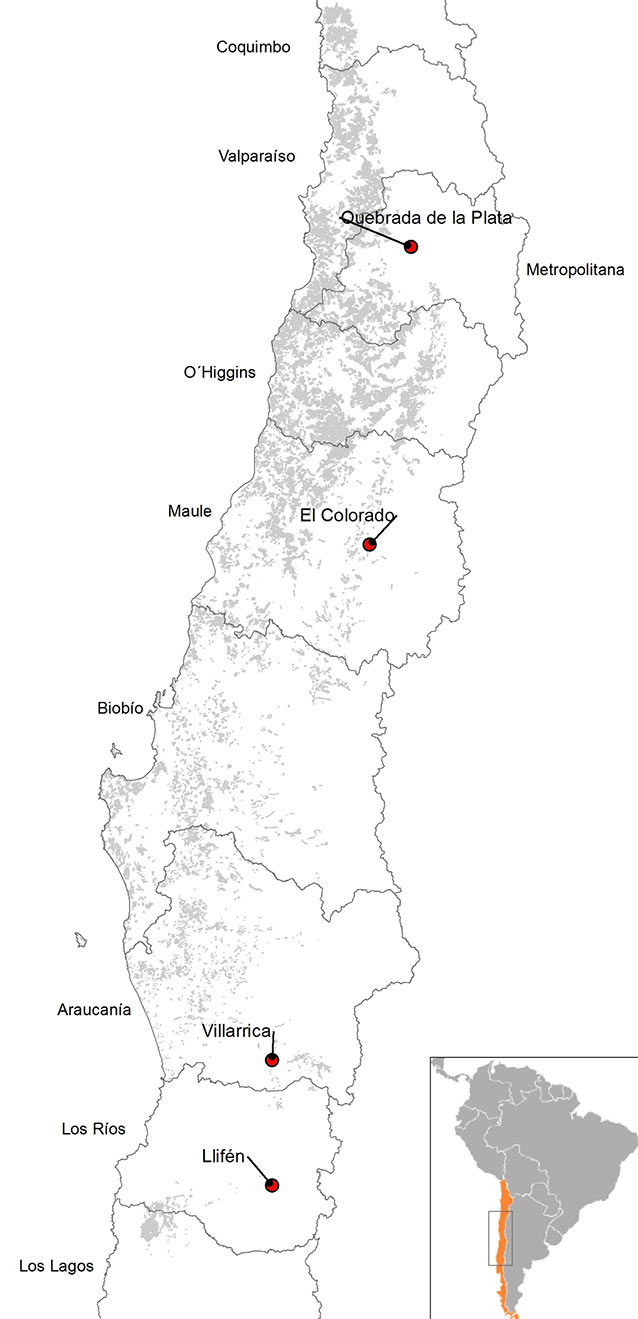
Four provenances of P. boldus were selected in mainland Chile, at distances of at least 150 km, along a latitudinal gradient (Fig. 1). Two seed sources from central Chile represented northern provenances (Quebrada de la Plata and Colorado), and two seed sources from the south represented southern provenances (Villarica and Llifén). A general description of the seed collection sites is given in Tab. 1. Ripe fruits of trees were harvested at the four collection sites between December 2006 and February 2007. The collection date varied since the rate of seed maturation differed between provenances (early December in the north, late February in the south). For each provenance, five dominant or co-dominant mother trees with clear bole, well developed crown, good health condition and abundant fruits were randomly selected at a minimum distance of 100 m. We harvested 500 fruits per mother tree, evenly distributed over the canopy. Fruits were then depulped, seeds were washed and sown immediately after collection, according to Botti & Cabello ([6]).
Fig. 1 - Location of the four provenances of Peumus boldus selected for seed collection (red circles).
Tab. 1 - Location, Köppen climate classification, mean annual temperature and mean total precipitation of the four collection sites.
| Provenance | Climate classification (Köppen) |
Latitude/ Longitude |
Altitude (m) |
Mean Annual Precipitation (mm) |
Mean Annual Temperature (°C) |
|---|---|---|---|---|---|
| Quebrada de la Plata (northern interior) |
Temperate Mediterranean with dry hot summer (Csb) |
33° 29′ 38″ 70° 53′ 45″ |
473 | 350 | 14.2 |
| El Colorado (northern pre-Andean) |
Temperate Mediterranean with dry hot summer (Csb) |
35° 35′ 44″ 71° 11′ 38″ |
738 | 676 | 8.1 |
| Villarrica (southern pre-Andean) |
Temperate, warm summers and cold winters (Cfb) |
39° 17′ 44″ 72° 12′ 18″ |
241 | 2000 | 12.1 |
| Llifén (southern pre-Andean) |
Temperate, warm summers and cold winters (Cfb) |
40° 12′ 00″ 72° 15′ 50″ |
286 | >2000 | 11.1 |
Seed germination variability
Seeds were soaked in distilled water for 24-48 h and those floating were discarded. Seeds were set to germinate in 1-liter plastic pots containing a mixture of sand and topsoil 1:1 (v/v). Four plastic pots were used as replicates and a total of 100 seeds per mother plant were sown in each pot. Seeds were then placed under nursery conditions (average temperature 23.5 °C, 60-80% relative humidity). The nursery was located at the University of Chile, Santiago (33° 34′ 05″ S, 70° 37′ 55″ W, 611 m. a.s.l) with climatic conditions similar to those of the Quebrada de la Plata provenance. Germination was monitored two times per week and recorded when the radicle emerged from the testa. The germination capacity (GC) was expressed as the proportion of the total number of germinated seeds out of sown seeds. Seed weight (SW) was estimated according to ISTA ([15]) and expressed as the average weight of 1000 seeds. As P. boldus has germination difficulties due the pericarp, seeds were sown immediately after harvesting, which led to different sowing dates. To avoid possible biases due to differences in sowing date, the number of days elapsed since sowing until full germination (radicle > 5 mm showing geotropism - DG) was registered for every provenance.
Seedling growth
The germinated seeds were transferred to 130 mL containers (BASF®, Santiago, Chile) filled with a mixture of sand and topsoil 1:1 (v/v). No fertilizer was added to the substrate. The experimental setup comprised four provenances, five mother plants per provenance, five replicates of 7 seedlings per replicate, totaling 700 seedlings (4×5× 5×7 = 700 seedlings). The seedlings were watered to field capacity and grown under nursery conditions during one growing season. Seedlings were protected by a 50% shading plastic mesh (Raschel®, Santiago, Chile).
Total height (H, cm), root collar diameter (D, mm) and number of leaves (NL) of all seedlings were recorded, as well as their survival in percentage (SUR). Survival was measured according to a categorical scale (1 = alive, 0 = dead). At the end of the first year of growth, seedlings were cut and separated into three fractions: leaves, stems and roots. The dry weight of each fraction was determined after oven drying at 65 °C for 48 h. The relative biomass was then determined and the following variables were recorded: leaf dry weight (LDW), stem dry weight (SDW), root dry weight (RDW) and total dry weight (TDW). Root weight ratio (RWR = RDW/TDW) and leaf weight ratio (LWR = LDW/TDW) were also determined. Foliar area of seedlings (FA, cm2) was also measured using a common desk-top scanner (Hewlett-Packard®, Cupertino, CA), and the surface of all leaves measured using a digital planimeter (Tamaya®, Tokyo, Japan). Roots length (RL, cm) was also measured as the distance from the root collar to the top of the tap root.
Statistical analysis
All traits were analyzed by 2-Way ANOVA using a nested design, i.e., provenance as a fixed factor and the mother plant as the random nested factor within provenance. The general linear model approach (GLM) was used, with type III sum of squares. Comparisons between groups for categorical variables (i.e., SUR) were done using a Chi-square test. Tukey’s HDS post-hoc tests were carried out when significant provenance and mother plant differences were detected (α = 0.05). The SPSS® v.18 software (IBM, New York, USA) was used for all statistical analyses.
Results
Variability in seed germination
The overall mean germination capacity (GC) of P. boldus seeds was low (Tab. 2). However, significant differences in GC among mother plants within provenances were found. For example, the mean germination capacity in the Colorado and Llifén provenances differed by more than 100%. Seed weight was different among both provenances and mother plant; however, the germination of small seeds (<650 mg) was similar to that of large seeds (>900 mg). Moreover, we found significant differences among provenances in the germination time (i.e., the number of days elapsed from sowing to the emergence of the radicle - DG). Indeed, northern provenances germinated 37 days later than southern provenances.
Tab. 2 - Main effects on seed parameters of P. boldus tested through 2-way ANOVA using provenance (Prov) as fixed factor and mother plant (MP) as a random factor nested within provenance. Means ± standard errors are reported. (GC): Germination capacity; (SW): average weight of 1000 seeds; (DG): days elapsed since sowing to radicle emergence. (*): p < 0.05; (ns): non-significant.
| Trait | Northern Provenances |
Southern Provenances |
Main effects of factors |
|||
|---|---|---|---|---|---|---|
| Quebrada de la Plata |
Colorado | Villarica | Llifén | Prov | MP(Prov) | |
| GC (%) | 3.66 ± 0.96 | 5.78 ± 1.66 | 3.78 ± 0.92 | 2.70 ± 0.64 | ns | * |
| SW (g) | 93.8 ± 16.4 | 62.0 ± 4.50 | 64.4 ± 2.30 | 77.8 ± 4.55 | * | * |
| DG (days) | 165 ± 3.2 | 167 ± 4.1 | 131 ± 2.6 | 126 ± 3.3 | * | ns |
Seedling development
Morphological traits and survival were different at the provenance and mother plant levels for most traits under consideration (Tab. 3). Seedlings from the southern provenances (Villarrica and Llifén) were taller and with thicker diameters than those from the northern provenances; however, the highest survival rate was found in the latter provenances (Colorado). Significant differences in leaf number and morphology were also found. Northern provenances showed smaller leaves as compared with southern ones, which had a lesser number of larger leaves. Regarding root anatomy, significant differences were also observed at population and mother plant levels. Seedlings from northern provenances (Quebrada de la Plata) exhibited longer roots as compared to southern provenances (Villarica and Llifén).
Tab. 3 - Main effects in morphological traits and survival of P. boldus tested through 2-way ANOVA using provenance (Prov) as fixed factor and mother plant (MP) as a random factor nested within provenance. Means ± standard errors are reported. (H): total seedling height; (D): root collar diameter; (NL): number of leaves; (SUR): survival; (RL): root length; (FA): foliar area. (*): p < 0.05; (ns): non-significant.
| Trait | Northern Provenances |
Southern Provenances |
Main effects of factors |
|||
|---|---|---|---|---|---|---|
| Quebrada de la Plata |
Colorado | Villarica | Llifén | Prov | MP(Prov) | |
| H (cm) | 6.65 ± 0.19 | 7.56 ± 0.19 | 9.15 ± 0.28 | 8.79 ± 0.26 | * | * |
| D (mm) | 1.65 ± 0.04 | 1.71 ± 0.04 | 1.81 ± 0.04 | 2.01 ± 0.05 | * | ns |
| NL | 10.94 ± 0.47 | 9.57 ± 0.26 | 8.28 ± 0.27 | 9.77 ± 0.33 | * | * |
| SUR (%) | 64.16 ± 7.18 | 71.18 ± 5.43 | 42.90 ± 5.76 | 54.70 ± 6.28 | * | * |
| RL (cm) | 5.56 ± 0.29 | 4.95 ± 0.21 | 4.80 ± 0.20 | 5.22 ± 0.30 | * | * |
| FA (cm2) | 10.80 ± 0.61 | 14.82 ± 0.75 | 15.89 ± 0.89 | 17.92 ± 1.02 | * | * |
Biomass allocation
In general, there was shift in biomass allocation from northern to southern provenances, with the northernmost provenance seedlings (Quebrada de la Plata) having less body mass (TDW) and allocating a higher proportion of the total mass to belowground organs (RWR). Contrastingly, the southernmost provenance seedlings (Llifén) were taller and allocated a larger proportion of the total mass to leaves (LWR - Tab. 4). No differences in SDW were observed among provenances.
Tab. 4 - Main effects in biomass traits of P. boldus tested through 2-Way Analysis of Variance (ANOVA) using provenance (Prov) as fixed factor and mother plant (MP) as a random factor nested within provenance. Mean ± standard errors are reported. (LDW): leaf dry weight; (SDW): shoot dry weight; (RDW): root dry weight; (TDW): total dry weight; (RSR): root to shoot ratio; (RWR): root weight ratio; (LWR): leaves weight ratio. (*): p < 0.05; (ns): non significant.
| Trait | Northern Provenances |
Southern Provenances |
Main effects of factors |
|||
|---|---|---|---|---|---|---|
| Quebrada de la Plata |
Colorado | Villarica | Llifén | Prov | MP(Prov) | |
| LDW (mg) | 0.14 ± 0.00 | 0.22 ± 0.01 | 0.20 ± 0.01 | 0.23 ± 0.01 | * | * |
| SDW (mg) | 0.05 ± 0.00 | 0.06 ± 0.00 | 0.07 ± 0.00 | 0.08 ± 0.00 | ns | * |
| RDW (mg) | 0.63 ± 0.05 | 0.88 ± 0.07 | 0.60 ± 0.04 | 0.83 ± 0.07 | * | * |
| TDW (mg) | 0.82 ± 0.02 | 1.16 ± 0.02 | 0.87 ± 0.02 | 1.14 ± 0.03 | * | * |
| RWR | 0.77 ± 0.00 | 0.76 ± 0.00 | 0.69 ± 0.00 | 0.73 ± 0.00 | * | * |
| LWR | 0.17 ± 0.00 | 0.19 ± 0.00 | 0.23 ± 0.00 | 0.20 ± 0.00 | * | * |
Discussion
Seed germination
In this study, the germination of P. boldus seeds was highly variable among mother plants within provenances, while its variation among provenances was not significant. The variability of seed germination is often interpreted as reflecting adaptations to specific ecological conditions ([11], [25]). Obviously, the environmental characteristics of the microsite occupied by a seed may strongly influence its probability of germination; however, the environmental conditions experienced by mother trees in the previous generation may also affect the germination of seeds ([28], [2]). Small differences in local site conditions, such as substrate type, soil humidity etc., cannot be excluded even when seeds are collected in the neighborhood. Several studies have shown a small-scale adaptation to such local habitat differentiation ([17], [3]), which may reduce the correlation between population differentiation and geographical distance.
In this study, we found no differences in seed germination capacity among different P. boldus provenances from a wide latitude gradient, while all the variation was attributed to differences among individual mother trees. Intra-population variability in germination is common in forest tree populations ([2]) and might correspond to genetic factors ([31]) as well as environmental variability during seed ripening ([21]). In addition, seeds collected from different individuals of the same population also display large differences in germination, which supports the hypothesis that individual mother tree genotype also plays an important role in seed germination. Indeed, an attempt was made to minimize microsite-specific environmental differences by collecting seeds only from healthy individuals growing under similar conditions in the field. Nonetheless, the observed differences in germination capacity may be interpreted as mainly due to maternal effects, i.e., to genetic differences of the sampled trees. However, a complete ecological understanding of the variation in P. boldus seed germination requires a multiple generation study.
Another possible explanation of the large within-population variation observed in seed germination could be related to differences in fruit ripening among mother trees. As a general rule, fruits should be collected only after the seeds have reached the full maturity ([5]). This aspect has been poorly investigated in P. boldus. According to Muñoz ([24]) the germination capacity of this species diminishes when the fruit is overripe; however, full seed maturity is not easily detectable, as the fruit color is widely used as an indicator of seed maturity. As mentioned above, we sowed P. boldus seeds immediately after fruit collection to avoid changes in their germination responses due to dry storage at room temperatures ([2]). However, differences in seed dormancy due to their different maturity cannot be excluded.
Different seed dormancy levels may also reflect differences in germination length, in terms of days elapsed since sowing until the radicle emerge (DG). In our study, significant differences in DG were found among geographically distinct provenances of the species. The northern Colorado provenance had the higher DG, which could be related to the higher elevation and lower mean annual temperature of the site (700-800 m and 8.1 °C, respectively). A longer delay in seed germination with increasing altitude and decreasing temperature may be the result of the increased susceptibility to unfavorable conditions during flowering and seed development. Moreover, seeds from this provenance were the smallest, indicating a lower endosperm content and thus lesser source of nutrient available for the embryo, which may defer the initiation of germination. Indeed, it has been reported that small seeds usually emerge more slowly ([30]). It is noteworthy to mention that the northernmost provenance (Quebrada de la Plata), characterized by large seeds sown three months earlier than southern provenances, showed a slower germination and yielded smaller seedlings with less body mass at the end of the experiment. This provenance seems to be adapted to the harsh conditions of the collection site, characterized by a low annual precipitation. Thus, it could be hypothesized that a delayed seed germination might represent an adaptation strategy to escape unfavorable conditions.
Seedling development and biomass allocation
We found a strong differentiation in seedling growth and biomass (both above- and belowground) among all the four provenances tested. The northernmost provenance site (Quebrada de la Plata) is characterized by a Mediterranean climate with low precipitation; accordingly, its seedlings showed the lowest foliar area (FA), which suggests a higher growth potential under low water availability, as a smaller transpiration surface may reduce the desiccation risk. Results of the present study are in line with findings of Doll et al. ([10]) who analyzed three provenances of P. boldus in central Chile and found that the Mediterranean provenance had more leaf rolling than Coastal provenances. Leaf rolling has been proposed to be an effective mechanism in decreasing the transpiration rate of plants experiencing water deficit ([26], [16]).
On the other hand, southern provenances showed a lower survival rate when growing in a Mediterranean climate (as at the nursery in Quebrada de la Plata). According to the theory of specialization ([18]), genotypes adapted to favorable environmental conditions exhibit superior performances in these environments, but the opposite occurs when conditions become limiting. In this study, seedlings from southern provenances showed performances consistent with this theory. In the xeric environment of the nursery, they grew more (higher H and TDW) and likely had an overall higher transpiration (as inferred from the superior FA, LDW and LWR), which clearly reduced survival. These provenances grow well in sites with more than 2000 mm annual precipitation, while in Quebrada de la Plata rainfall was only 350 mm.
Although based on a nursery experiment with no field validation, our results revealed some important consequences in the field of restoration ecology. The introduction of P. boldus southern provenances in sites with a more Mediterranean climatic characteristics, as in the northern part of the species’ range, may result in poor adaptation. Native plant species are routinely planted or sown in ecological restoration projects, but successful establishment and survival often depend on where and how seeds are collected ([32]). Our results highlights the importance of using seeds from locally adapted individuals of P. boldus in Mediterranean restoration projects, as local populations often show better performances as compared with non-local genotypes. Southern provenances of P. boldus showed several characteristics typical of genotypes non-tolerant to drought (i.e., taller, with more body mass, larger leaf area and less root biomass), thus their use in restoration projects in drought-prone Mediterranean sites should be avoided. This is particularly important in regions such as Chile, where the Mediterranean environments are expected to shift southward as a consequence of the global climate change.
Conclusions
Provenance played a significant role in the early development of seedlings of P. boldus, highlighting the importance of the choice of suitable seed sources in ecological restoration plans. Northern provenances showed higher survival in comparison with the southern provenances. In addition, seedlings of the provenance from the driest site allocated more biomass to roots and less leaf dry weight, which suggest their adaptation to drought-prone Mediterranean ecosystems. On the other hand, the southern provenance seems to be more adapted to humid environments.
Acknowledgements
The authors gratefully acknowledge Dr. Anne Bliss from the University of Colorado at Boulder (CO, USA) who provided helpful criticism of the manuscript.
CR conceived the study, organized the seed collection and reviewed the final manuscript; SE carried out the data analysis, and wrote an earlier draft and the final version of the manuscript; EG carried out the seed collection and the nursery trial; RS contributed with ideas and comments on the methodology of an earlier draft of the manuscript; AC commented the final draft of the manuscript.
References
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Emilio F Garrido
Departamento de Silvicultura, Facultad de Ciencias Forestales y de la Conservación de la Naturaleza, Universidad de Chile, Avenida Santa Rosa 11315, La Pintana, Santiago (Chile)
Rómulo E Santelices
Antonio M Cabrera
Centro de Desarrollo para el Secano Interior, Facultad de Ciencias Agrarias y Forestales, Universidad Católica del Maule, Avenida San Miguel 3605, Talca (Chile)
Corresponding author
Paper Info
Citation
Magni CR, Espinoza SE, Garrido EF, Santelices RE, Cabrera AM (2016). Differential adaptations in nursery seedlings from diverse Chilean provenances of Peumus boldus Mol.. iForest 9: 409-413. - doi: 10.3832/ifor1893-008
Academic Editor
Gianfranco Minotta
Paper history
Received: Oct 02, 2015
Accepted: Oct 20, 2015
First online: Jan 29, 2016
Publication Date: Jun 01, 2016
Publication Time: 3.37 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2016
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 49973
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 41848
Abstract Page Views: 2875
PDF Downloads: 3901
Citation/Reference Downloads: 40
XML Downloads: 1309
Web Metrics
Days since publication: 3688
Overall contacts: 49973
Avg. contacts per week: 94.85
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2016): 2
Average cites per year: 0.20
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Outplanting performance of three provenances of Quillaja saponaria Mol. established in a Mediterranean drought-prone site and grown in different container size
vol. 13, pp. 33-40 (online: 21 January 2020)
Research Articles
Tropical seedling performance under drought: a functional trait approach for species selection in restoration
vol. 19, pp. 9-17 (online: 10 January 2026)
Research Articles
Optimum light transmittance for seed germination and early seedling recruitment of Pinus koraiensis: implications for natural regeneration
vol. 8, pp. 853-859 (online: 22 May 2015)
Short Communications
Growth of Stone pine (Pinus pinea L.) European provenances in central Chile
vol. 10, pp. 64-69 (online: 29 August 2016)
Research Articles
Local adaptation at a small geographic scale observed in Juniperus excelsa populations in southern Turkey
vol. 14, pp. 531-539 (online: 24 November 2021)
Research Articles
Contrasting holm oak provenances show different field performance but similar resilience to drought events eight years after planting in a Mediterranean environment
vol. 11, pp. 259-266 (online: 29 March 2018)
Research Articles
Effect of restoration methods on natural regeneration in the Brazilian Atlantic Forest
vol. 18, pp. 23-29 (online: 15 February 2025)
Research Articles
Effects of cultural treatments, seedling type and morphological characteristics on survival and growth of wild cherry seedlings in Turkey
vol. 5, pp. 283-289 (online: 17 December 2012)
Research Articles
Matching seedling size to planting conditions: interactive response with soil moisture
vol. 12, pp. 220-225 (online: 25 April 2019)
Research Articles
The effect of seed size on seed fate in a subtropical forest, southwest of China
vol. 9, pp. 652-657 (online: 04 April 2016)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword