Identification and allelochemical activity of phenolic compounds in extracts from the dominant plant species established in clear-cuts of Scots pine stands
iForest - Biogeosciences and Forestry, Volume 10, Issue 1, Pages 309-314 (2017)
doi: https://doi.org/10.3832/ifor1791-009
Published: Feb 23, 2017 - Copyright © 2017 SISEF
Research Articles
Abstract
Dominant plant species established in the understory of clear-cuts may have a strong biochemical influence on pine regeneration process, with important consequences for reforestation management. We evaluated and compared the total phenolic content and the allelopathic activity of acqueous extracts from both roots and shoots of dominant plant species established in 1-yr-old and 2-yr-old clear-cuts of Scots pine (Pinus sylvestris L.) stands in Lituania. The highest total content of phenolic compounds was detected in the lingonberry (Vaccinium vitis-idaea L.) shoots from 1-yr-old and 2-yr-old clear-cuts, as well as in the common heather (Calluna vulgaris [L.[ Hull) shoots from 1-yr-old clear-cuts. High-performance liquid chromatography (HPLC) was used to identify and quantify the allelochemicals present in the active fraction to determine their possible role in allelopathy. The highest variety and content of phenolic compounds were observed in shoot extracts of the dominant species from both 1-yr-old and 2-yr-old clear-cuts. Scots pine seed germination and seedling growth were significantly and negatively correlated with ρ-coumaric acid and sinapic acid content, while Scots pine seedling growth was significantly and negatively correlated with ferulic, caffeic and hydroxycinnamic acids contents. The highest contents of these phenolic acids were determined in aqueous extracts of C. vulgaris from 1-yr-old clear-cuts and Rumex acetosella L. of 2-yr-old clear-cuts, which exerted a strong phytotoxicity on Scots pine seed germination. Moreover, morphometric parameters of Scots pine seedlings were most sensitive to aqueous extracts of V. vitis-idaea shoots from both 1-yr-old and 2-yr-old clear-cuts and R. acetosella shoots from 2-yr-old clear-cuts.
Keywords
Phenolics Identification, Allelopathy, Dominant Species, Germination
Introduction
Fraenkel ([11]) first recognized that secondary plant metabolites are not simply plant waste products, but play an important role in the plant defense against insects and herbivores. Since then, our understanding of the ecological roles of these compounds has greatly increased. Nowadays, it is well known that secondary plant metabolites can provide defence against a broad range of herbivores and pathogens, can mediate the interactions among competitors and mutualists, and may help ameliorating abiotic stress ([14]). Many thousands of plant secondary metabolites have a possible role in allelopathy and may inhibit both seed germination and seedling growth of nearby species ([16]).
The presence of allelochemicals able to affect forest tree growth and development seems to be widespread in all ecosystem types ([27]). Allelopathy is one of the factors involved in conifer regeneration failure in the presence of dense ericaceous understory as a consequence of forest harvesting or fire ([22]). The dominant species, mostly ericaceous, in the understory of clear-cuts in Scots pine (Pinus sylvestris L.) stands produce and secrete a range of phenolic compounds that are inhibitory to conifer seed germination (mainly primary root growth) and ectomycorrhizal growth. Phenolics are the main allelopathic compounds that inhibit seed germination, plant growth and other physiological processes, thus leading to changes in the floristic composition of plant communities and dominance of one plant species over others ([5]).
Allelochemicals are released through processes such as volatilization, root exudation, leaching, and decomposition of plant residues, and may be present in all plant organs, including leaves, flowers, fruits, roots, rhizomes, stems and seeds, some of which can store these compounds. However, the quantities and release pathways vary across species ([20]). Numerous authors have shown that the tissues of herbaceous plants, shrubs and trees of the understory contain different phenolic compounds ([9], [20]). Typically, the allelopathic inhibitory effect is the result of the concerted action of multiple allelochemicals that mutually affect different physiological processes, thus altering the growth patterns of plants ([25], [16]). The action of allelochemicals can affect respiration, photosynthesis, enzyme activity, water regulation, stomatal opening, hormone levels, mineral availability, cell division and elongation, and the structure and permeability of cell membranes and walls ([26], [20]). Alterations to cell membranes are one of the primary effects of allelochemicals, and these effects may then exert secondary effects ([4]).
Nonetheless, little is known about the phenolic compounds of the dominant understory species which are able to influence the process of reforestation in clear-cuts of Scots pine stands in boreal forests.
The objectives of this work were to evaluate and compare under laboratory conditions the identified content of phenolic compounds (IPC) and allelopathic activity of aqueous extracts produced from both shoots and roots of the dominant species growing in 1-yr-old and 2-yr-old clear-cuts of Scots pine stands (Vaccinio myrtilli-Pinetum). High-performance liquid chromatography (HPLC) was used to identify and quantify the allelochemicals present so as to ascertain their possible role in allelopathy, specifically in terms of Scots pine seed germination and the morphology of pine seedlings.
Materials and methods
Study area and dominant species record
We studied the phytocenoses of herbaceous successional stages in 1-yr-old and 2-yr-old clear-cut areas of Vaccinio myrtilli-Pinetum ([10]) forests during June-July 2010. In total, 17 clear-cuts in Central and South-Eastern Lithuania (forests of Kuras, Ropeja and Zeronys) were included in the survey (Tab. 1). Plant diversity, abundance (%) and projection cover (%) were estimated for herbaceous species in order to identify the dominant species. The analyses were carried out in 1 m2 replicated subplots (n=25) established along transects every 5 m at each site ([6]). Species were classified into a dominant phytosociological group for each site according to the projection coverage based on abundance proportion. The dominant species served as the source of aqueous extracts for assessing the impact of their allelochemicals on seed germination and seedling growth inhibition in Scots pine.
Tab. 1 - The location of the studied clear-cuts within Scots pine stands in Lithuania.
| Forest district |
Year of clear-cut |
Forest block no. |
GPS Coordinates |
|---|---|---|---|
| Kuras | 1 | 6 (46) | 54° 56′ 20.4″ N, 23° 34′ 53.9″ E |
| Ropeja | 1 | 90 (11) | 54° 31′ 29.9″ N, 25° 03′ 38.9″ E |
| 1 | 114 (1) | 54° 31′ 20.4″ N, 25° 03′ 31.1″ E | |
| Zeronys | 1 | 307 (1) | 54° 28′ 10.4″ N, 24° 52′ 27.9″ E |
| 1 | 307 (9) | 54° 28′ 15.6″ N, 24° 52′ 24.7″ E | |
| 1 | 343 (3) | 54° 26′ 55.4″ N, 24° 48′ 19.4″ E | |
| 1 | 241 (3) | 54° 27′ 41.1″ N, 24° 47′ 38.7″ E | |
| 1 | 74 (3) | 54° 31′ 52.8″ N, 24° 51′ 26.1″ E | |
| Ropeja | 2 | 90 (4) | 54° 31′ 44.5″ N, 25° 03′ 52.2″ E |
| 2 | 125 (8) | 54° 31′ 53.4″ N, 25° 08′ 57.1″ E | |
| 2 | 125 (12) | 54° 31′ 49.9″ N, 25° 08′ 58.6″ E | |
| 2 | 113 (3) | 54° 31′ 18.6″ N, 25° 02′ 56.4″ E | |
| 2 | 46 (7) | 54° 32′ 29.6″ N, 25° 03′ 59.6″ E | |
| 2 | 47 (14) | 54° 32′ 25.5″ N, 25° 04′ 33.8″ E | |
| 2 | 61 (10) | 54° 31′ 42.8″ N, 25° 01′ 23.8″ E | |
| 2 | 92 (1) | 54° 31′ 54.0″ N, 25° 04′ 51.6″ E | |
| 2 | 145 (4) | 54° 31′ 24.5″ N, 25° 07′ 34.3″ E |
Germination bioassay
Germination tests were carried out in the Laboratory of Ecology at the Institute of Forestry, Lithuanian Research Centre for Agriculture and Forestry.
Allelopathic activity of aqueous extracts of the dominant species was estimated based on seed germination bio-screening ([2]). For the preparation of aqueous extracts, shoots and roots were chopped into 0.5 cm long pieces before extraction. Fifty grams of each piece were immersed in a 15 × 20 × 5 cm plastic tray containing 250 ml of distilled water. Containers were closed with glass plates and kept at 5 °C in an incubator. After 12 h, the aqueous extracts were filtered through Whatman no. 1 filter paper and diluted to 0.2% (w/v) concentration and used for germination assays. The germination of pine seed was assessed according to the ISTA technique ([15]). One hundred Scots pine seeds were placed on filter paper in each 9-cm diameter Petri dish. Five milliliters of 0.2% extract was added to each Petri dish. Three replications were used per treatment. Seeds sown in distilled water served as a control. All Petri dishes were kept at 20 °C/29 °C and a 16/8-h light/dark photoperiod. Percentage germination and the length of the radicle and hypocotyls were recorded after 21 days. The values were expressed as relative (%) to the control.
Identification and quantification of allelopathic compounds
For the preparation of methanolic extract, 500 mg of dry shoot and root material of each dominant species was soaked in 10 ml of 75% methanol with shaking for 24 h at room temperature using a VWR Mini Shaker (J&M Scientific, JAV). Extracts were filtered through 0.22-μm low-ash filter paper.
Identified phenolics composition (IPC) was carried out using a reversed-phase HPLC. Two chromatograms were recorded simultaneously. The upper chromatogram was obtained by registering the UV absorbance of the effluent at 265 nm prior to the reaction, a mirror chromatogram was obtained by recording the absorbance at 517 nm after reacting the effluent with 2.2-diphenyl-1-picrylhydrazyl radical (DPPH) solution in the reaction coil. The mobile phase was supplied to the column at a flow rate of 0.75 ml min-1. Samples (extracts) of 10 μl were injected onto the HPLC system. A reversed-phase LiChroCart 12.5×0.4 cm column and a 0.5×0.4 cm pre-column filled with LiChroSpher RP-18e 5-μm I.D. packing material was used for separation. DPPH reagent was prepared by dissolving 0.01M DPPH in sodium acetate buffer, pH 7.6, methanol and acetonitrile (50/25/25, v/v). For gradient elution, solutions A (double-distilled water and 0.05% trifluoroacetic acid, TFA) and B (methanol and 0.05% TFA) were used as the component mobile phase. The phenolic compounds were eluted using the following gradient of mobile phase: 10% B at 0 min, 95% B at 30-33 min, 10% B at 37 min, 10% B at 48 min and 10% B at 58 min. Ten µl injection, 0.75 ml min-1 flow rate and λ= 254 nm, λ=517 nm detection zone were used. The identified phenolics were valued via the linear regression equation of the standards calibration curves.
Statistical analysis
The confidence limits of the data were based on Wilkin’s-λ test and Student’s theoretical criterion (t). Standard deviations (SD) and Pearson’s correlation coefficients (r) were calculated at the level of statistical significance α = 0.05. The allelopathic effect of extracts was tested using the package STATISTICA (StatSoft Inc., Tulsa, OK, USA). Cluster analysis was used to group dominant species. Differences in germination values between extract-treated seeds and control seeds were tested using the ANOVA post-hoc LSD test, while differences in the lengths of hypocotyls and radicles were tested using the Kruskal-Wallis test.
Results and discussion
Determination of dominant species in clear-cuts
One-year-old clear-cuts
Clear-cutting in pine forests causes drastic abiotic changes (e.g., irradiance, moisture) to the habitat of pine forests, leading to an abrupt change in the abundance of the understory species.
The phytosociological survey carried out in this study allowed the identification of the dominant species in the 1-yr-old clear-cuts, namely the feather moss (Pleurozium schreberi) and lingonberry (Vaccinium vitis-idaea), which were detected in 97% and 95% of the experimental plots, respectively. The common heather (Calluna vulgaris) was the third most abundant species in the clear-cuts understory (87% of the experimental plots). Numerous studies have been carried out on the natural productivity and medicinal uses of V. vitis-idaea and C. vulgaris ([19], [24]), and many were focused on the inhibitory effect of the Vaccinium canopy on confifer germination and natural regeneration ([23], [22]). Feather moss is an ever-present component of the boreal forest floor ([12]). While the ecology of this bryophyte within boreal forests has been widely analyzed, its contribution to plant-plant interaction has not been fully studied.
Two-year-old clear-cuts
The average abundance of the dominant species decreased by about 3% in the 2-yr-old clear-cuts, compared to the 1-yr-old clear-cuts, possibly due to the increased light exposure. Nonetheless, the projection cover of V. vitis-idaea increased by 2%, up to 12% across the examined 2-yr-old clear-cuts. Mean abundances of 44% and 68% were detected for bushgrass (C. epigejos) and sheep’s sorrel (R. acetosella), respectively, and were classified as dominants due to their vigorous spreading (generative and vegetative), thus structurally altering the phytocenoses in the clear-cut areas. Previous studies showed that the rapid vegetative propagation of C. epigejos through stolons favor its rapid population expansion, determining a consumption of nutrients which hampers pine seedling growth in Lithuanian clear-cuts ([17]). Moreover, its canopy shading suppresses neighboring herbaceous species or tree seedlings. Intensive generative propagation by small and abundant anemophilous seed is a specific characteristic of the acidophilous species R. acetosella, providing strong allelopathic potential in clear-cuts.
Inhibition of germination and seedling parameters
The phytotoxicity of aqueous extracts on acceptor-seed germination and hypocotyl and radicle length was dependent on the dominant species, plant part, age of the clear-cut, types of phenolic compounds present, and variation in phenolic concentration. Germination results and seedling parameters relative to control values are presented in Tab. 2.
Tab. 2 - Effect of the application of shoot and root acqueous extracts from the dominant species in the understory on seed germination and seedling early growth in Scots pine. Data are mean ± standard deviation (n=6) and the proportion relative to controls (=100%) is indicated. (*): p<0.05; (**): p<0.01; (***): p<0.001.
| Clear-cuts | Extracts | Dominant species | Germination | Hypocotyl length | Radicle length | |||
|---|---|---|---|---|---|---|---|---|
| Initial, % | Relative to Control, % |
cm | Relative to Control, % |
cm | Relative to Control, % |
|||
| 1-yr-old | Shoot | Vaccinium vitis-idaea | 68 ± 3 | 81 ± 3* | 1.5 ± 0.1 | 40 ± 7*** | 1.7 ± 0.1 | 35 ± 7 |
| Calluna vulgaris | 66 ± 1 | 78 ± 5* | 1.7 ± 0.1 | 44 ± 4 | 1.9 ± 0.1 | 39 ± 7 | ||
| Pleurozium schreberi | 70 ± 3 | 83 ± 2* | 1.7 ± 0.0 | 45 ± 4 | 2.0 ± 0.6 | 41 ± 10 | ||
| Root | Vaccinium vitis-idaea | 69 ± 2 | 82 ± 5* | 1.8 ± 0.0 | 48 ± 6** | 2.0 ± 0.6 | 43 ± 7 | |
| Calluna vulgaris | 68 ± 3 | 81 ± 5* | 1.7 ± 0.3 | 45 ± 4** | 1.9 ± 0.0 | 40 ± 7** | ||
| 2-yr-old | Shoot | Vaccinium vitis-idaea | 67 ± 2 | 80 ± 6* | 1.4 ± 0.2 | 37 ± 6** | 1.7 ± 0.0 | 35 ± 3*** |
| Calamagrostis epigejos | 68 ± 1 | 81 ± 1* | 1.8 ± 0.0 | 46 ± 6 | 1.8 ± 0.0 | 37 ± 13 | ||
| Rumex acetosella | 59 ± 5 | 70 ± 12* | 1.3 ± 0.1 | 40 ± 4** | 1.5 ± 0.0 | 28 ± 9* | ||
| Root | Vaccinium vitis-idaea | 72 ± 2 | 86 ± 7* | 1.8 ± 0.3 | 48 ± 8* | 2.2 ± 0.0 | 46 ± 5 | |
| Calamagrostis epigejos | 71 ± 4 | 84 ± 7* | 1.7 ± 0.2 | 46 ± 8** | 2.0 ± 0.1 | 41 ± 7** | ||
| Rumex acetosella | 69 ± 1 | 82 ± 5* | 1.8 ± 0.1 | 47 ± 3** | 1.9 ± 0.0 | 40 ± 9** | ||
| Сontrol | 84 ± 2 | 100 | 4.8 ± 0.3 | 100 | 3.8 ± 0.3 | 100 | ||
Extracts of C. vulgaris and R. acetosella exhibited the strongest phytotoxicity and inhibition on germination of Scots pine seeds, possibly due to differences in accumulation of phenolic compounds. Shoot and root aqueous extracts of C. vulgaris from the 1-yr-old clear-cuts reduced the mean seed germination by 78% and 81%, respectively, as compared with control seeds (100%), while shoots and roots extracts of R. acetosella of the 2-yr-old clear-cuts reduced mean germination by 70% and 82%, respectively (Tab. 3 and Tab. 4).
Tab. 3 - Content of identified phenolic compounds (IPC) in shoot extracts of dominant species in the undestory (mean ± standard deviation, n=6). Different letters in the same column indicate significant differences between means after one-way ANOVA analysis followed by a t criterion (p<0.05).
| Clea-cuts | Species | IPC content, mg g-1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gallic acid |
Chloro- genic acid |
Caffeic acid |
Trans p-coumaric acid |
Ferulic acid |
Trans- sinapic acid |
Syringic acid |
Hydroxy- cinnamic acid |
Couma- rine |
Total | ||
| 1-yr-old | P. schreberi | - | - | 0.04±0.00a | - | - | 0.19±0.02b | 0.03±0.01a | 0.29±0.05b | 0.37±0.03b | 0.92±0.15 |
| V. vitis-idaea | - | 3.57±0.16b | 6.26±0.53c | 0.37±0.01a | 0.39±0.03a | 0.77±0.01a | 1.48±0.25a | 9.68±0.03e | 8.17±0.10d | 30.69±3.74 | |
| C. vulgaris | - | 6.68±0.43c | 1.78±0.11b | 0.10±0.02a | 0.18±0.02a | 2.00±0.13b | - | 2.90±0.26b | 8.11±0.48d | 21.75±3.12 | |
| 2-yr-old | V. vitis-idaea | - | 1.37±0.16b | 4.35±0.07b | 0.76±0.03a | 0.84±0.01a | - | - | 6.09±0.15d | 6.65±0.13d | 20.06±2.70 |
| C. epigejos | 0.23±0.00a | 0.43±0.01a | - | - | 1.46±0.05b | - | - | - | 0.23±0.06a | 2.35±0.59 | |
| R. acetosella | - | - | - | 1.03±0.05b | 0.14±0.01a | 4.09±0.20c | - | 0.14±0.01a | 0.16±0.01a | 5.56±1.71 | |
Tab. 4 - Content of identified phenolic compounds (IPC) in root extracts of dominant species in the undestory (mean ± standard deviation, n=6). Different letters in the same column indicate significant differences between means after one-way ANOVA analysis followed by a t criterion (p<0.05).
| Clear-cuts | Species | IPC content, mg g-1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gallic acid |
Chloro- genic acid |
Caffeic acid |
Trans p-coumaric acid |
Trans- sinapic acid |
Syringic acid |
Hydroxy- cinnamic acid |
Couma- rine |
Total | ||
| 1-yr-old | V. vitis-idaea | - | 0.49±0.13c | 0.11±0.07a | 0.18±0.13a | - | 0.11±0.08a | - | - | 0.89±0.18 |
| C. vulgaris | - | - | - | 0.14±0.05a | - | - | 0.13±0.04a | 0.33±0.08a | 0.60±0.11 | |
| 2-yr-old | V. vitis-idaea | - | 0.11±0.07a | 0.08±0.00a | 0.39±0.03b | - | - | - | - | 0.58±0.17 |
| C. epigejos | - | 0.34±0.04b | - | 0.05±0.01a | - | - | - | - | 0.49±0.13 | |
| R. acetosella | 0.17±0.00a | - | - | 0.31±0.01a | 0.19±0.0a | - | 0.52±0.13a | - | 1.19±0.16 | |
The weakest phytotoxicity and inhibition was observed for aqueous extracts of P. schreberi shoot and V. vitis-idaea root sampled from the 1-yr-old clear-cuts, as well as C. epigejos shoot and V. vitis-idaea root from the 2-yr-old clear-cuts, and may be attributed to different phenolic contents. In general, the observed inhibition of shoot extracts was stronger than that of root extracts, although not statistically significant. This is consistent with previous studies which demonstrated the presence of different allelochemicals and concentrations in the shoots and roots of different dominant species ([9]).
Radicle growth of pine seedlings was more strongly inhibited than their hypocotyl growth by extracts of the tested dominant plants from both 1-yr-old and 2-yr-old clear-cuts. This finding is consistent with those of previous studies on different species, and suggests that seedling roots are more sensitive than shoots to allelochemicals ([25], [3]).
The analysis of seedling morphology revealed that V. vitis-idaea and C. vulgaris shoot extracts from the 1-yr-old clear-cuts had a stronger inhibition in term of hypocotyl and radicle growth than their root extracts. Contrastingly, P. schreberi shoot extracts and V. vitis-idaea L. root extracts from 1-yr-old clear-cuts showed a lesser impact on the same morphological variables (Tab. 2).
Hypocotyl length was similarly strongly supressed by applying V. vitis-idaea shoot and C. epigejos root extracts from the 2-yr-old clear-cuts. However, it was less inhibited by C. epigejos shoot extract compared to V. vitis-idaea shoot extract, possibly due to the different identified phenolic content between tested species. Radicle length was severely reduced by R. acetosella shoot and root extracts. Nonetheless, it was less suppressed by C. epigejos shoot and V. vitis-idaea root extracts.
Tab. 5 displays the results of the correlation analysis between the phenolic compounds identified in the extracts and their effects on Scots pine seedling growth. However, it has been proposed that the different responses of seedlings to shoot and root extracts might depend on evolutionary differences in resistance to allelopathic compounds among the acceptor species ([28]).
Tab. 5 - Effect of the phenolic compounds identified in acqueous extracts of dominant species in the understory on early growth parameters of Scots pine. Pearson’s correlation coefficients are shown (n=“?). (*): p<0.05.
| Parameter | Total phenolic content |
Trans p-coumaric acid |
Ferulic acid |
Chloro- genic acid |
Gallic acid |
Caffeic acid |
Trans- sinapic acid |
Syringic acid |
Hydroxy- cinnamic acid |
Couma- rine |
|---|---|---|---|---|---|---|---|---|---|---|
| Seed germination | 0.3 | -0.4* | 0.1 | 0.2 | 0.01 | 0.3 | -0.5* | 0.2 | 0.3 | 0.3 |
| Seedling radicle length | -0.01 | -0.4* | -0.1 | 0.1 | 0.1 | -0.02 | -0.3* | -0.04 | -0.02 | 0.1 |
| Seedling hypocotyl length | -0.3* | -0.5* | -0.5* | 0.04 | 0.3 | -0.4* | -0.1 | -0.2 | -0.3* | -0.2 |
Content and identified phenolic compounds
Phenolic compounds are the most important and common plant allelochemicals ([11]). Phenolics play a major role in ecosystem functionality as they mediate many interactions between the plant and its biotic and abiotic environments. Moreover, phenolic compounds are a striking example of metabolic plasticity, enabling plants to adapt to changes in their biotic and abiotic environments ([5]). Additionally, phenolics are particularly important in seed germination, development, and plant resistance to various stresses. Nonetheless, their content and composition are likely to differ depending on plant species, tissues and cells during ontogenesis and under the influence of various environmental stimuli ([16]).
We found that IPC composition and content varied depending on dominant species and cut-age, though in all cases an inhibitory effect on germination and seedling growth was observed. The highest IPC content was found in V. vitis-idaea shoot extracts from the 1-yr-old and the 2-yr-old clear-cuts and in C. vulgaris shoot extracts of the 1-yr-old clear-cuts (Tab. 3). IPC content was found significantly lower in P. schreberi of the 1-yr-old clear-cuts, and in C. epigejos and R. acetosella shoot extracts of the 2-yr-old clear-cuts, possibly due to species-specific biological peculiarities to synthesize and accumulate phenolics.
The largest content and the largest variety of IPC was determined in V. vitis-idaea shoot extracts from both the 1-yr-old (chlorogenic, caffeic, trans-ρ-coumaric, ferulic, trans-sinapic, syringic, hydroxycinnamic acids and coumarin) and the 2-yr-old clear-cuts (all the aforementioned compounds, except trans-sinapic and syringic acids). These properties explain the highest phytotoxicity and inhibition of the species extracts on pine seeds and seedlings. Chlorogenic, caffeic, trans-ρ-cumaric, ferulic, trans-sinapic, hydroxycinnamic acids and coumarine were also found in aqueous extracts of C. vulgaris from the 1-yr-old clear-cuts.
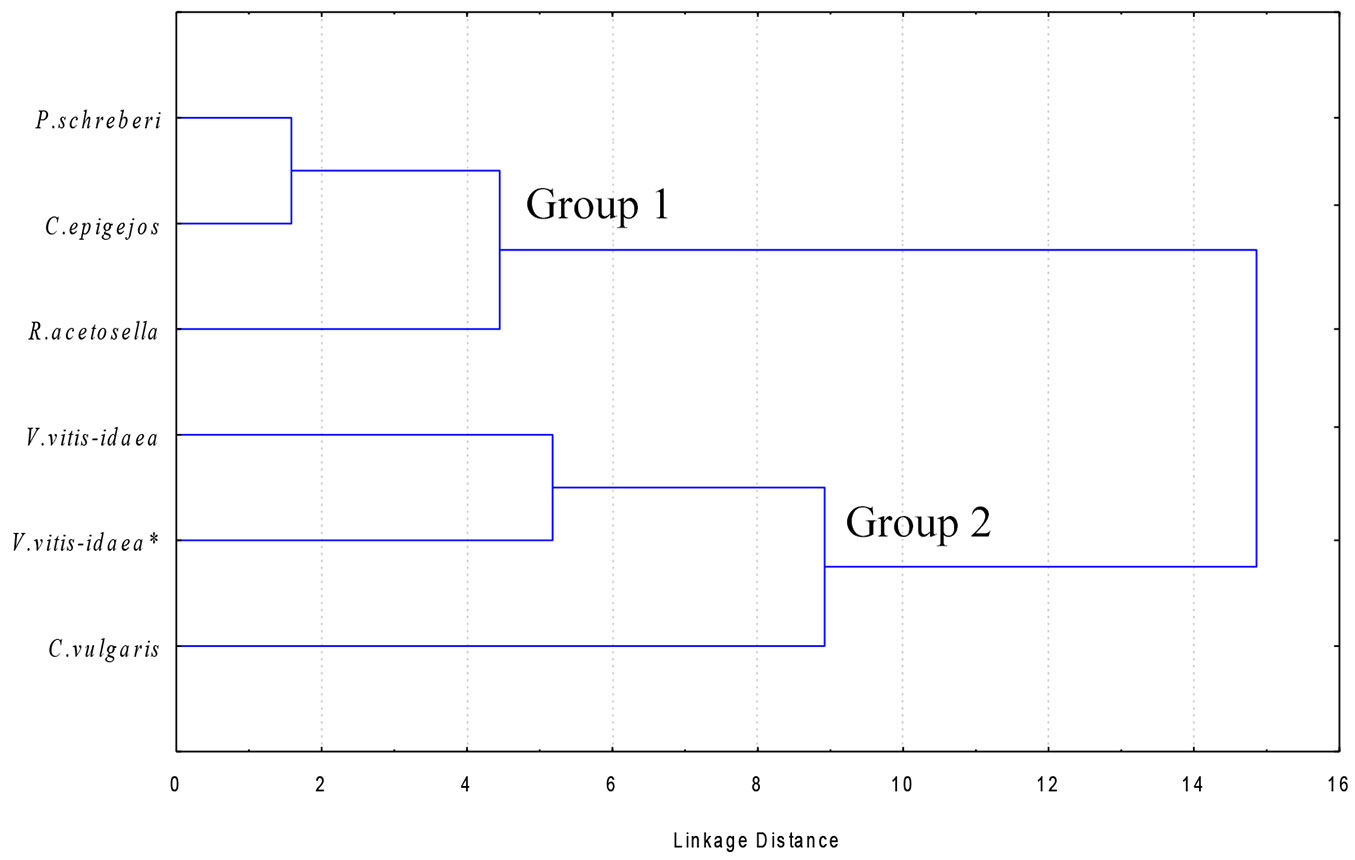
Quantitative and qualitative differences in allelochemicals may explain the different extent of suppressive allelopathic effects among dominant species in the understory. In order to identify the underlying patterns of species allelopathic effects, the dominant species were clustered on the basis of their chemical properties. As the lowest content of IPC was found in the roots, cluster analysis was applied on data from the shoots of the dominant species. Two main groups of dominant species were identified based on their shoot IPC content. According to the dendrogram reported in Fig. 1, V. vitis-idaea and C. vulgaris clustered together in the group showing the strongest inhibition effect (Group 2), whereas P. schreberi, C. epigejos and R. acetosella grouped to a different group (Group 1) characterized by weaker suppression allelochemical activity on Scots pine seedlings.
Fig. 1 - Cluster analysis of the dominant species in the understory of clear-cuts in Scots pine stands, based on the identified phenolic compounds in their shoot extracts.
All the phenolic compounds identified in this study have been considered as potential allelopathic agents in previous studies ([21], [20]). According to Boudet ([5]), the lower variety in allelochemicals identified in plant roots might be related to the lower content of phenolic compounds in shoots, as opposed to that in roots. Our results confirmed the higher IPC in shoot than in root in all the dominant species examined (Tab. 3, Tab. 4). The highest content in IPC was observed in V. vitis-idaea L. shoot from 1-yr-old and the 2-yr-old cuts. The highest root IPC was determined in R. acetosella from the 2-yr-old clear-cuts (1.19 ± 0.16 mg g-1) and in V. vitis-idaea and C. vulgaris extracts from the 1-yr-old clear-cuts.
In general, we found a significant correlation between the identified phenolics in extracts of dominant species and their inhibitory effect on seed germination in Scots pine (Tab. 5), though such effect varied across different phenolic compounds. Consistently with previous reports ([8], [18]), a significant negative correlation (r = -0.39, p<0.05) was detected between trans-ρ-coumaric acid and seed germination, as well as between the above compound and seedling parameters, namely radicle length (r = -0.43, p<0.05) and hypocotyl length (r = -0.45, p<0.05). Therefore, our results confirmed that the tested dominant species may influences Scots pine recruitment in clear-cuts through their phenolic compounds, which inhibit seed germination and seedling growth.
Various phenolic compounds have been experimentally proven to have allelopathic activity, such as ferulic acid ([7]), caffeic acid ([20]), p-hydroxybenzoic and cinnamic acids ([1], [13]). In this study, a significant negative correlation of the trans-sinapic acid content was observed with seed germination (r = -0.47, p<0.05), and seedling radicle growth (r = -0.34, p<0.05). Noteworthy, a similar allelochemical effect of trans-sinapic acid was previously reported by Chung et al. ([7]). Furthermore, a significant negative correlation was observed between the length of Scots pine seedling hypocotyls and ferulic acid (r = 0.45, p<0.05), caffeic acid (r = 0.35, p<0.05) and hydroxycinnamic acid (r = 0.33, p<0.05). The highest contents of ferulic, caffeic and hydroxycinnamic acids were detected in V. vitis-idaea extracts from both the 1-yr-old and 2-yr-old clear-cuts. The highest content of trans-sinapic acid was measured in R. acetosella extracts from the 2-yr-old clear-cuts (4.09 ± 0.20 mg g-1) and in C. vulgaris aqueous extracts from the 1-yr-old clear-cuts (2.00 ± 0.13 mg g-1). The highest content of trans-ρ-coumaric acid was found in R. acetosella and V. vitis-idaea extracts in the 2-yr-old clear-cuts, as well as in V. vitis-idaea and C. vulgaris extracts from the 1-yr-old clear-cuts. Our findings are in line with those of Mallik ([22]), who demonstrated that the accumulated litter of ericaceous plants (Vaccinium, Calluna spp.) contains an array of phenolic compounds that is inhibitory to conifer seed germination, primary root growth, and ectomycorrhizal growth. Moreover, many of these phenolic compounds might provoke soil nutrient imbalance through the reduction of available nitrogen (by forming protein-phenol complexes), thus leading to long-term site degradation ([16]).
Based on the results of this investigation, the accumulation of high amounts of allelochemicals by species such as C. vulgaris and V. vitis-idaea could be hypothesized to be one of the primary reasons for conifer regeneration failure after forest disturbance in Lithuania. The negative effect of dominant understory species on Scots pine early growth might have practical significance in the forest management, e.g., reforestation might be preferred to natural regeneration in presence of such negative impact.
Conclusions
The inhibitory allelopathic effect of aqueous extracts depends on the dominant species in clear-cuts, the plant part, the quantity and composition of phenolic compounds, and cut’s age. The aqueous extracts of C. vulgaris of 1-yr-old clear-cuts and R. acetosella of 2-yr-old clear-cuts exhibited the strongest phytotoxicity, inhibiting Scots pine seed germination due to their highest phenolics accumulation. Our results showed that shoot extracts has higher content and variety of phenolic compounds compared to root extracts. The highest content of phenolic compounds was obtained in extracts of V. vitis-idaea (both from 1-yr-old and 2-yr-old clear-cuts), R. acetosella (2-yr-old clear-cuts) and C. vulgaris (1-yr-old clear-cuts). Moreover, strong negative correlations between the content in phenolic compounds of extracts and both seed germination and seedling growth were observed.
Species such as V. vitis-idaea, C. vulgaris and R. acetosella can negatively affect the process of natural reforestation due to high contents and variety of phenolic compounds in their biomass or in plant debris. Furthermore, the knowledge of allelopathic activity of dominant species in the understory of clear-cuts might help choosing the best strategies to mitigate the negative phytotoxic impact of herbaceous species on early pine growth.
Acknowledgments
We thank the researchers and PhD students of the Department of Biology, Vytautas Magnus University in Lithuania for the lab support.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Online | Gscholar
Authors’ Info
Authors’ Affiliation
Department of Ecology, Forest Research Institute, Lithuanian Research Centre for Agriculture and Forestry (Lithuania)
Institute of Environment and Ecology, Aleksandras Stulginskis University (Lithuania)
Department of Biology, Vytautas Magnus University (Lithuania)
Corresponding author
Paper Info
Citation
Šežiene V, Baležentiene L, Maruška A (2017). Identification and allelochemical activity of phenolic compounds in extracts from the dominant plant species established in clear-cuts of Scots pine stands. iForest 10: 309-314. - doi: 10.3832/ifor1791-009
Academic Editor
Giorgio Matteucci
Paper history
Received: Aug 05, 2015
Accepted: Sep 12, 2016
First online: Feb 23, 2017
Publication Date: Feb 28, 2017
Publication Time: 5.47 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2017
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 50597
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 42091
Abstract Page Views: 2971
PDF Downloads: 4431
Citation/Reference Downloads: 21
XML Downloads: 1083
Web Metrics
Days since publication: 3285
Overall contacts: 50597
Avg. contacts per week: 107.82
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2017): 11
Average cites per year: 1.22
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Allelopathic effects of dominant ground vegetation species on initial growth of Pinus sylvestris L. seedlings in response to different temperature scenarios
vol. 12, pp. 132-140 (online: 27 February 2019)
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Research Articles
Optimum light transmittance for seed germination and early seedling recruitment of Pinus koraiensis: implications for natural regeneration
vol. 8, pp. 853-859 (online: 22 May 2015)
Research Articles
Use of brassinosteroids to overcome unfavourable climatic effects on seed germination in Pinus nigra J. F. Arnold
vol. 17, pp. 1-9 (online: 02 February 2024)
Research Articles
Effects of brassinosteroid application on seed germination of Norway spruce, Scots pine, Douglas fir and English oak
vol. 10, pp. 121-127 (online: 02 October 2016)
Research Articles
Are Mediterranean forest ecosystems under the threat of invasive species Solanum elaeagnifolium?
vol. 14, pp. 236-241 (online: 10 May 2021)
Technical Reports
Effects of different mechanical treatments on Quercus variabilis, Q. wutaishanica and Q. robur acorn germination
vol. 8, pp. 728-734 (online: 05 May 2015)
Research Articles
Influence of mother plant and scarification agents on seed germination rate and vigor in Retama sphaerocarpa L. (Boissier)
vol. 7, pp. 306-312 (online: 08 April 2014)
Research Articles
Seed germination traits of Pinus heldreichii in two Greek populations and implications for conservation
vol. 15, pp. 331-338 (online: 24 August 2022)
Research Articles
Role of serotiny on Pinus pinaster Aiton germination and its relation to mother plant age and fire severity
vol. 12, pp. 491-497 (online: 02 November 2019)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword