Effect of Funneliformis mosseae on growth, mineral nutrition, biochemical indexes and chlorophyll content of Ziziphus spina-christi seedlings at different salinities
iForest - Biogeosciences and Forestry, Volume 9, Issue 3, Pages 503-508 (2015)
doi: https://doi.org/10.3832/ifor1643-008
Published: Dec 08, 2015 - Copyright © 2015 SISEF
Research Articles
Abstract
Vast area of the land around the world is saline. Knowledge of plant behavior and their interaction with mychorrizal fungi in saline areas may help seedling establishment in such environments. This study aimed to determine the effects of the inoculation of the fungus Funneliformis mosseae (FM) on Ziziphus spina-christi (Rhamnaceae) plants grown under salt stress. Mycorrhizal and non-mycorrhizal seedlings were exposed to different levels of NaCl in the soil (0, 50, 100, and 150 mM). The following parameters were measured in both inoculated and non-inoculated plants: root colonization rate, seedling height, root diameter, root and shoot dry weights, chlorophyll a and b, total nitrogen (N), phosphorus (P), potassium (K) and sodium (Na+) content, proline accumulation in roots and leaves, superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) activities. The results showed that soil salinity hampered the root colonization by the fungus, and decreased basal diameter, seedling height, root and shoot dry weights, as well as some nutrients and chlorophyll a concentration, while increased leaves and roots Na+, SOD and POD activity, proline accumulation, as well as CAT activity in the roots. Contrastingly, no significant effect of soil salinity were detected on K and CAT of leaves, root N, and chlorophyll b. Inoculated plants had higher basal diameter, leaves and roots P, root and shoot dry weights, chlorophyll a and lower SOD content, proline accumulation in leaves and Na+, as compared with non-inoculated plants. Seedling height, root N, CAT and POD content, and chlorophyll b were not affected by inoculation with FM. These results demonstrated that FM inoculation is a promising method for improving the growth of Z. spina-christi seedlings under salt stress.
Keywords
Salinity, Peroxidase, Chlorophyll, Arbuscular Mycorrhiza, Ziziphus spina-christi
Introduction
Soil salinity is a chronic problem increasing worldwide, especially in arid and semi-arid areas ([4]). At least 6% of the global landmass is affected by salinity ([14]). Three types of physiological stress affect plant growth in saline soils: (i) toxic effects of specific ions, such as sodium and chloride, on plant cells ([23]); (ii) physiological drought in soil with low osmotic potential, due to the plant efforts to maintain a lower internal osmotic potential, thus preventing water egression from roots into the soil; and (iii) imbalances of the nutrient content caused by the decreased nutrient uptake and/or transport to the leaves ([3]).
To mitigate the effects of soil salt on plant growth, many strategies have been developed, including the use of seedlings with roots colonized by arbuscular mycorrhizal fungi (AMF - [41], [42]). AMF have symbiotic relationship with the roots of over 80% of the terrestrial plant species, including halophytes, hydrophytes and xerophytes ([19]). Indeed, It has been demonstrated that AMF colonization increases the tolerance of some plants to salt ([37]). AMF are mutually symbiotic and provide a direct physical link between the soil and plant roots ([15]).
AMF promotes salinity tolerance by increasing nutrient uptake ([12], [8]), improving rhizospheric and soil conditions ([6]), increasing photosynthesis and water use efficiency ([18]), the accumulation of compatible solutes ([12]) and enzymatic antioxidants such as superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) as a defense system to protect the plant cell from oxidative stress ([41], [25]).
Funneliformis mosseae (FM) is an arbuscular mycorrhiza used to alleviate salt stress in Arachis hypogaea ([5]), Capsicum annuum ([1]), Poncirus trifoliate ([41]) and Olea europaea ([33]) seedlings. Symbiotic relationships between Funneliformis mosseae and several species of the genus Ziziphus (Rhamnaceae) have been formerly reported ([9], [34]).
The aim of this study was to determine the effects of the mychorrizal fungus Funneliformis mosseae (FM) on the growth of Ziziphus spina-christi seedlings exposed to different soil salinity levels. Our starting hypothesis was that the FM inoculum could alleviate the effect of salinity stress in Z. spina-christi seedlings.
Material and methods
Plant material and AM inoculum
Experimental plants (Ziziphus spina-christi) were initially produced from seeds. The mycorrhizal inoculum of Funneliformis mosseae (FM, formerly known as Glomus mosseae) were originally purchased from the Tarbiat Modares University, Tehran, (Iran), and then propagated by trap culture technique in the rhizosphere of maize (Zea mays) roots for 5 months.
Growth conditions and methodology
The experiment was carried out in a forest nursery located in Mehran (western Iran) between February and August 2013 (6 months). The mean temperature was 19.5 °C and precipitation was 245.5 mm. Seeds of Z. spina-christi were scarified to overcome hard seed coat dormancy by removing a small portion of the coat at the cotyledon end with nail clippers. The seeds were germinated in a mixture of clay, silt and perlite (2:1:1 v/v - Tab. 1). About 10% (w/w) inoculum of FM was placed in the pots at sowing time. The FM inoculum consisted of soil, spores (50 spores g-1 inoculum), hyphae and root fragments. To ensure uniform soil conditions, sterilized inoculum was also added to the control pots (non-mycorrhiza).
Tab. 1 - Physico-chemical characteristics of soil used in this experiment. (EC): electrical conductivity; (OC): organic carbon; (Ca): Calcium; (N): Nitrogen; (P): Phosphor; (K): Potassium; (Mg): Magnesium; (Na+): Sodium.
| Parameter | Value |
|---|---|
| pH | 7.32 |
| EC (mmho cm-1) | 0.52 |
| OC (%) | 1.5 |
| Ca (%) | 5.4 |
| N (g kg-1) | 0.12 |
| P (ppm) | 19.6 |
| K (ppm) | 601 |
| Mg (ppm) | 0.6 |
| Na+ (g kg-1) | 1.1 |
| Texture | Loamy-Clay |
Seedling were grown for 5 weeks before being treated with one of four levels of NaCl (0, 50, 100 and 200 mM). The salt was added to the soil with the irrigation water. The soil was salinized step-wise to avoid subjecting the plants to osmotic shock. The NaCl concentration was gradually increased by 25 mM on alternative day to reach the required salinity. The pots were daily weighed to measure water loss, which was replaced with deionized water to avoid percolation and maintain the soil water potential at field capacity.
Determination of growth parameters and colonization
The plants were harvested 6 months after planting and the height and basal diameter of the seedlings were measured. The shoots (leaves and stems) and roots were then oven-dried at 70 °C for 72 h and their dry weight (DW) was calculated at 0.01 g precision ([26]).
Determination of the percentage of roots colonization was carried out according to the method suggested by Phillips & Hayman ([32]). Ten thin fragments of roots each with length of about 1 cm were collected from several seedlings for each treatment. The percentage of colonization (AM%) was determined by the following formula (eqn. 1):
where RLi and RLo were the infected and the overall root length, respectively.
Leaf and root nutrient analysis
Physiologically mature leaves and roots were randomly collected from selected seedlings in each treatment. Leaves were pooled, ground finely and sieved through a 40 µm mesh screen. Total nitrogen (N) was measured using the semi-micro Kjeldahl method ([29]). Potassium (K), phosphor (P) and sodium (Na) contents were determined by atomic absorption spectrophotometry (UV/VIS 9000).
Enzyme assays
Fresh matured leaves were detached from seedlings for enzyme measurement ([22]). Some 0.5 g of frozen leaves were ground in liquid nitrogen until a fine powder was obtained. The same method was applied for fine root samples. The powder was extracted using an ice-cold 50 nM phosphate buffer at pH 7.0. The extracts were centrifuged (Rotina 380; Hettich) at 4° C for 20 min at 13000 rpm, and the supernatant was collected for antioxidant enzyme analyses ([27]). The superoxide dismutase (SOD) activity was determined using potassium phosphate (pH = 7.5), Na2co3 (pH = 10.2) according to the method described by Giannopolitis & Ries ([16]). The peroxidase (POD) activity was determined using the Guaiacol oxidation method ([24]). The catalase (CAT) was measured using potassium phosphate (pH = 7.0) and H2O2 ([10]). Proline accumulation was determined using ninhydrin and sulfosalicylic acid ([7]).
Leaf chlorophyll content
Semi-mature leaflets (n = 32) were collected from seedlings to measure their chlorophyll content (a, b and total), which was extracted using 80% acetone ([17]). The supernatant was quantified with a spectrophotometer at 645 and 663 nm and compared to a blank 80% acetone standard. Chlorophyll content was expressed as mg g-1 fresh weight ([5]).
Experimental design and statistical analysis
The experiment was performed using a random design with 4 replications on 4 seedlings per treatment. All parameters were analyzed using the analysis of variance (ANOVA). Treatment means were compared using the post-hoc Duncan’ test at the significance level of 0.05.
Results
Root colonization
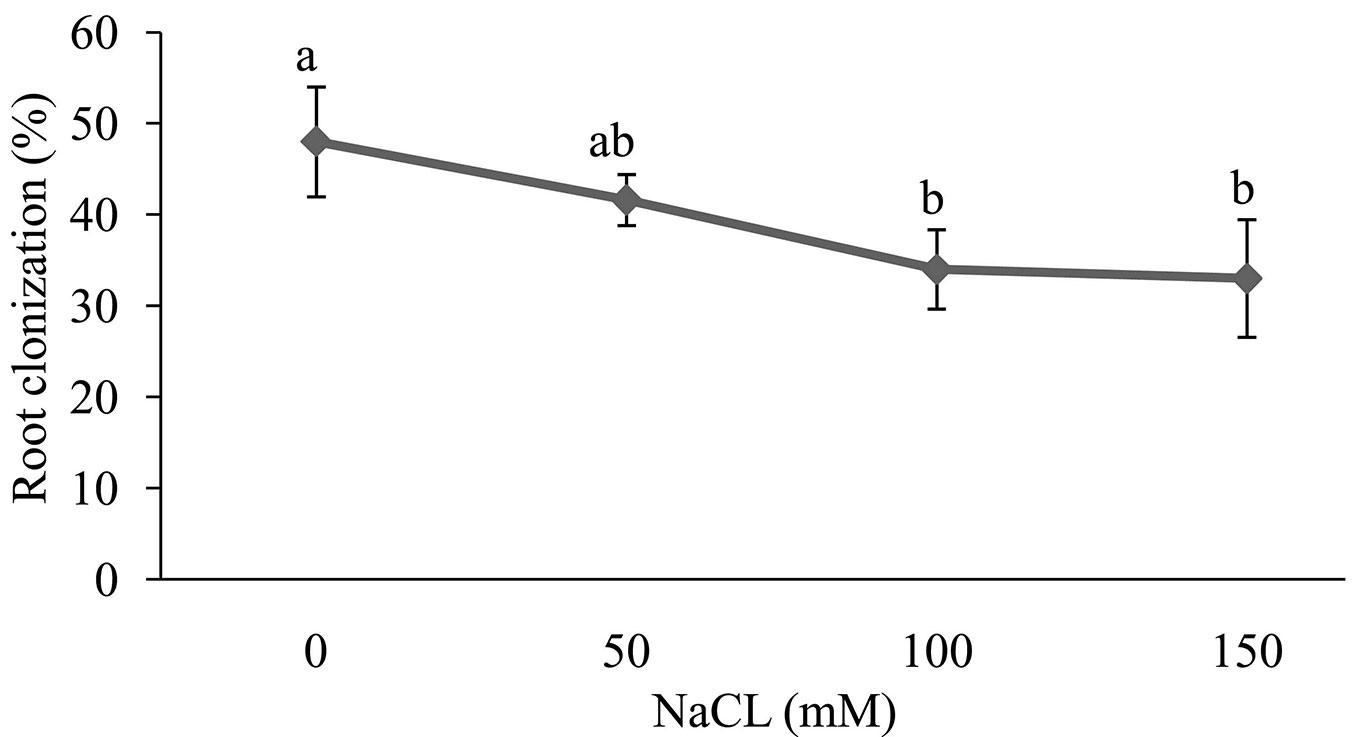
Overall, salt stress had a significant effect on colonization of Z. spina-christi seedling roots by Funneliformis mosseae. By increasing the soil salinity, FM colonization was decreased by 48% to 33%. However, no significant difference was observed between salt concentration in the soil of 100 and 150 mM. The highest level of FM colonization was in the control, while the lowest was in 100 and 150 mM treatments (Fig. 1).
Fig. 1 - Effect of salt stress on root colonization of Z. spina-christi seedlings. Different letters indicate significant differences between treatments (p < 0.05).
Growth indes
Tab. 2 shows the effects of FM on Z. spina-christi tolerance to salt stress as inferred from the change in growth indexes. Under salt stress seedlings’ basal diameters were significantly decreased, with the lowest values shown by seedlings grown at 150 mM soil salinity. At all levels of soil salinity, the basal diameter of FM-inoculated plants was significantly higher than that of non-inoculated seedlings. Likewise, salinity significantly decreased seedlings’ height. Contrastingly, no significant difference was detected between the height of inoculated and non-inoculated seedlings at low levels of salinity (0 and 50 mM). The root dry weight (RDW), a proxy of total dry matter, was also decreased under salt stress, while FM-inoculation increased RDW at all levels of salt stress (Tab. 2).
Tab. 2 - Effects of salt stress on diameter, height and RDW of mycorrhizal and non-mycorrhizal seedlings of Z. spina-christi. Means ± standard errors are reported. The signs (+, -) indicate the presence/absence of Funneliformis mosseae. Means in the same column followed by the same letter are not significantly different (p > 0.05) after the Duncan’s post-hoc test. (ns): not significant.
| Treatment (nM NaCl) |
AM inoculation |
Basal diameter (mm) |
Height (cm) |
RDW (g) |
|---|---|---|---|---|
| 0 | + FM | 3.93 ± 0.25 a | 28.8 ± 4.5 a | 4.12 ± 0.38 a |
| - FM | 1.33 ± 0.25 d | 28.8 ± 4.8 a | 1.52 ± 0.38 cd | |
| 50 | + FM | 3.17 ± 0.22 ab | 14.4 ± 1.9 b | 2.76 ± 0.27 b |
| - FM | 0.63 ± 0.17 de | 15.4 ± 1.9 b | 0.62 ± 0.24 ef | |
| 100 | + FM | 3.05 ± 0.40 b | 12.0 ± 1.4 b | 2.10 ± 0.20 bc |
| - FM | 0.61 ± 0.31 de | 7.8 ± 2.9 b | 0.30 ± 0.15 f | |
| 150 | + FM | 2.20 ± 0.22 c | 8.8 ± 0.7 b | 1.10 ± 0.22 de |
| - FM | 0.20 ± 0.17 e | 7.4 ± 2.0 b | 0.04 ± 0.02 f | |
| Main effects | NaCl | p<0.001 | p<0.001 | p<0.001 |
| AM | p<0.001 | ns | p<0.001 | |
| Interaction effects | NaCl × AM | ns | ns | ns |
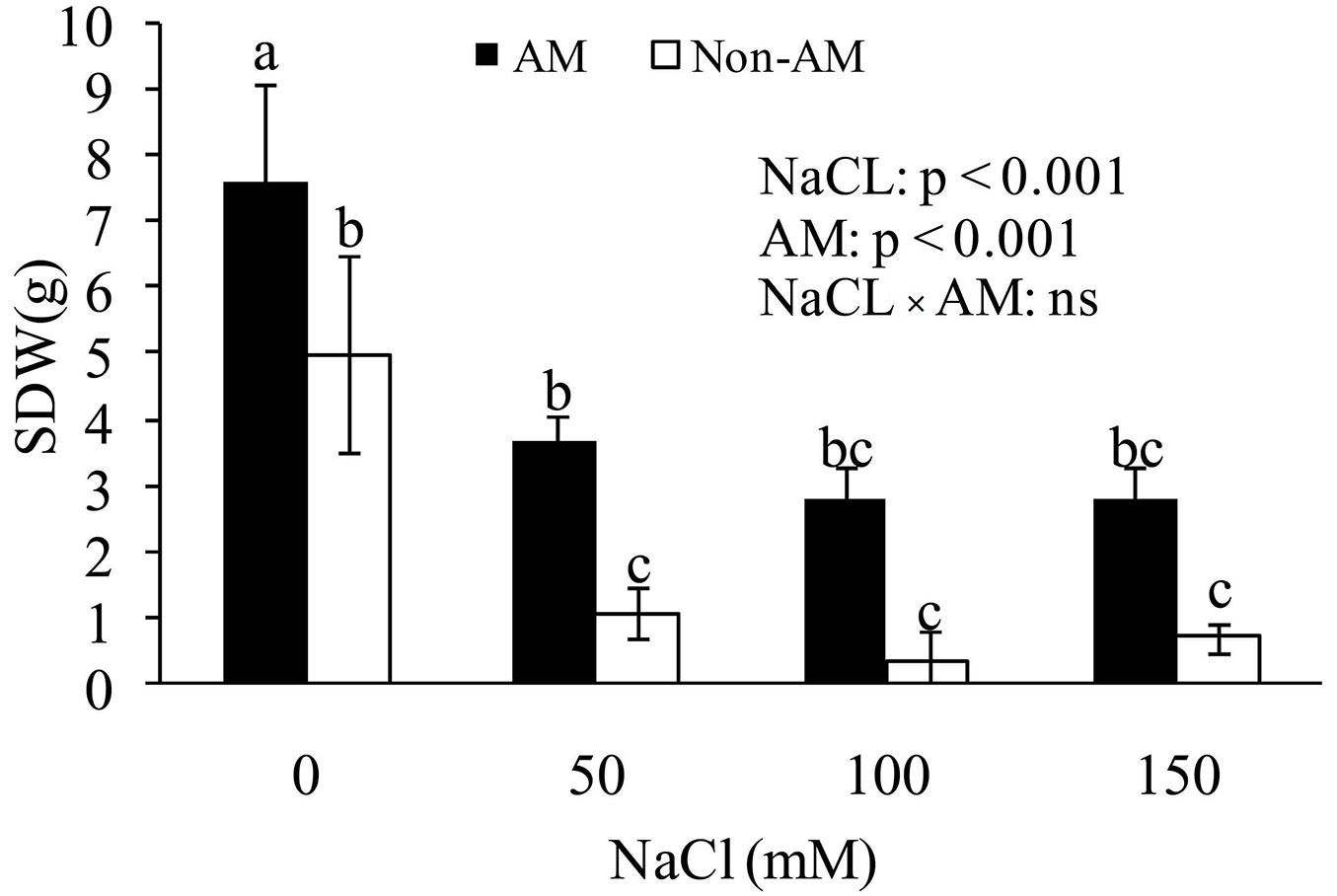
Shoot dry weight (SDW) under salinity was highly decreased, as the highest and lowest levels were in control and 150 mM of salinity, respectively. Moreover, it was observed that SDW of FM-inoculated seedlings was increased as compared with non-mycorrhizal plans. Height values showed that salt-stressed mycorrhizal Z. spina-christi had significantly greater SDW than salt-stressed non-mycorrhizal plants. Furthermore, there was an increase in SDW of mycorrhizal plants at all levels of salinity (0, 50, 100 and 150 mM); however, at high levels of salt stress (50 and 100 mM) a severe decrease in SDW in both mycorrhizal and non-mycorrhizal plants was evident. This may be related to the adverse effects of salinity on photosynthesis (Fig. 2).
Fig. 2 - Effect of salt stress on shoot dry weight (SDW) of mycorrhizal and non-mycorrhizal seedlings of Z. spina-christi. Different letters indicate significant differences between treatments (p < 0.05). (ns): not significant.
N, P, K, and Na+ concentrations
Leaf N significantly decreased by increasing NaCl concentration in the soil for both mycorrhizal and non-mycorrhizal plants. The FM inoculation increased the N absorption in leaves of seedlings grown at the lowest salinity by 44.1%, while such increase was 37.5% for seedlings grown at the highest salinity treatment. Contrastingly, no significant differences were observed in N absorption in the roots in seedlings grown under any soil salinity and FM treatments (Tab. 3).
Tab. 3 - Effect of salt stress on N, P and K absorption of mycorrhizal and non-mycorrhizal seedlings of Z. spina-christi. Means ± standard errors are reported. The signs (+, -) indicate the presence/absence of Funneliformis mosseae. Means in the same column followed by the same letter are not significantly different (p > 0.05) after the Duncan’s post-hoc test. (ns): not significant.
| Treatment (mM NaCl) |
AM inoculation |
N (gr kg-1) | P (ppm) | K (ppm) | |||
|---|---|---|---|---|---|---|---|
| Leaf | Root | Leaf | Root | Leaf | Root | ||
| 0 | + FM | 3.92 ± 0.29 a | 2.82 ± 0.53 a | 3.85 ± 0.47 a | 4.11 ± 0.24 a | 3.09 ± 1.02 a | 2.98 ± 0.83 a |
| - FM | 2.72 ± 0.27 b | 2.02 ± 0.36 a | 3.05 ± 0.47 ab | 3.31 ± 0.24 ab | 2.31 ± 1.01 a | 2.18 ± 0.83 ab | |
| 50 | + FM | 2.94 ± 0.28 b | 1.48 ± 0.10 a | 3.52 ± 0.30 a | 3.05 ± 0.12 abc | 2.76 ± 0.68 a | 2.59 ± 0.40 a |
| - FM | 2.74 ± 0.28 b | 1.28 ± 0.10 a | 2.72 ± 0.30 b | 2.25 ± 0.12 bcd | 1.96 ± 0.68 a | 1.80 ± 0.40 ab | |
| 100 | + FM | 2.42 ± 0.17 b | 1.50 ± 0.23 a | 2.12 ± 0.38 b | 1.93 ± 0.27 cd | 2.50 ± 1.42 a | 1.60 ± 0.33 ab |
| - FM | 2.32 ± 0.20 bc | 1.62 ± 0.24 a | 1.32 ± 0.38 bc | 1.13 ± 0.27 d | 1.74 ± 0.41 a | 0.80 ± 0.32 b | |
| 150 | + FM | 2.20 ± 0.27 bc | 1.78 ± 0.39 a | 0.98 ± 0.17 c | 1.85 ± 0.71 d | 1.19 ± 0.28 a | 1.44 ± 0.09 ab |
| - FM | 1.60 ± 0.23 c | 1.58 ± 0.39 a | 0.28 ± 0.12 c | 1.26 ± 0.60 d | 0.45 ± 0.25 a | 0.64 ± 0.09 b | |
| Main effects | NaCl | p < 0.001 | ns | p < 0.001 | p < 0.001 | ns | p < 0.001 |
| AM | p < 0.001 | ns | p < 0.001 | p < 0.001 | ns | p < 0.05 | |
| Interaction effects | NaCl × AM | ns | ns | ns | ns | ns | ns |
In general, a decreased in the P content of leaves and roots of seedlings grown under salinity stress was observed. However, the P content in leaves and roots increased respectively by 26.2% and 24.2% in FM-inoculated seedlings grown under the lowest salinity treatment, as compared to non-mychorrizal plants, while at the highest salinity treatment such increase was 28.0% in leaves and 20.8% in roots.
Concerning K content, salt stress and FM inoculation had no significant effects on seedling leaves. On the contrary, soil salinity decreased the K absorption in the roots. However, as compared with non-inoculated plants, mychorrizal seedlings showed an increase of K in the roots by 36.7% at the lowest and by 125.0% at the highest salinity treatments (Tab. 3).
Tab. 4 shows that the Na+ content increased in the leaves and roots of both mycorrhizal and non-mycorrhizal plants as the NaCl concentration in the soil increased. At all levels of soil salinity, the Na+ content in the leaves and roots of FM-inoculated seedlings was lower than that observed for non-inoculated seedlings.
Tab. 4 - Effect of salt stress on Na+ absorption and chlorophyll (a and b) on mycorrhizal and non-mycorrhizal seedlings of Z. spina-christi. Means ± standard errors are reported. The signs (+, -) indicate the presence/absence of Funneliformis mosseae. Means in the same column followed by the same letter are not significantly different (p > 0.05) after the Duncan’s post-hoc test. (ns): not significant.
| Treatment (mM NaCl) |
AM inoculation |
Na+ (g kg-1) | Chlorophyll (Mg g-1 fresh weight) | ||
|---|---|---|---|---|---|
| Leaf | Root | a | b | ||
| 0 | + FM | 22.90 ± 4.19 c | 16.56 ± 3.08 c | 3.02 ± 0.39 a | 0.43 ± 0.10 a |
| - FM | 22.90 ± 4.19 c | 22.80 ± 3.08 c | 1.62 ± 0.19 bc | 0.15 ± 0.08 a | |
| 50 | + FM | 37.26 ± 2.58 bc | 34.40 ± 2.40 b | 1.68 ± 0.18 bc | 0.27 ± 0.06 a |
| - FM | 43.50 ± 2.58 ab | 39.74 ± 2.40 ab | 1.14 ± 0.09 c | 0.19 ± 0.07 a | |
| 100 | + FM | 36.10 ± 3.58 bc | 32.80 ± 3.33 b | 1.38 ± 0.18 c | 0.25 ± 0.14 a |
| - FM | 42.40 ± 3.58 ab | 39.10 ± 3.33 ab | 1.42 ± 0.24 c | 0.17 ± 0.14 a | |
| 150 | + FM | 45.60 ± 3.41 ab | 40.50 ± 4.26 ab | 2.38 ± 0.60 ab | 0.11 ± 0.02 a |
| - FM | 51.90 ± 3.41 a | 46.82 ± 4.26 a | 0.98 ± 0.25 c | 0.02 ± 0.02 a | |
| Main effects | NaCL | p < 0.001 | p < 0.001 | p < 0.05 | ns |
| AM | p < 0.05 | p < 0.05 | p < 0.001 | ns | |
| Interaction effects | NaCl × AM | ns | ns | ns | ns |
Chlorophyll content
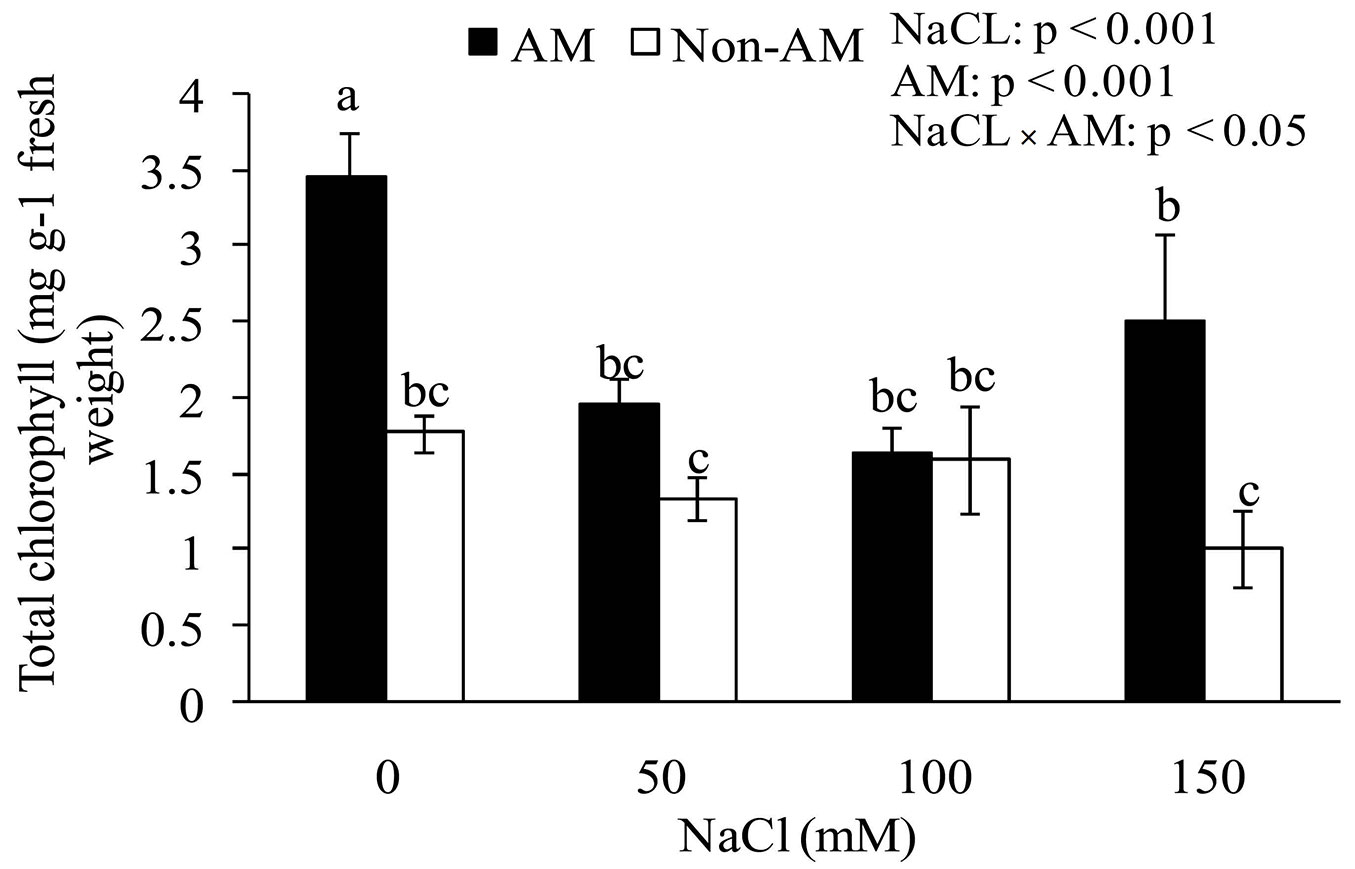
Total chlorophyll and chlorophyll a in mycorrhizal plants were significantly higher than in non-mycorrhizal plants at 0, 50, and 150 mM NaCl (Tab. 4, Fig. 3). In addition, soil salinity levels and the interaction between salinity and the fungus had significant effect on the total chlorophyll content. In other words, by increasing the salinity in the soil the total chlorophyll content of seedling leaves decreased. There were no significant differences between FM-inoculated and non-inoculated plants as for chlorophyll b. Similarly, the salt stress showed no significant effect on chlorophyll b content (Tab. 4).
Fig. 3 - Effects of salt stress on total chlorophyll of mycorrhizal and non-mycorrhizal seedlings of Z. spina-christi. Different letters indicate significant differences between treatments (p < 0.05).
Enzyme activity assessment
The levels of POD, SOD and CAT antioxidant enzymes in inoculated plants exposed to salt stress were lower than for non-inoculated plants. Salinity of the soil was observed to increase the SOD, POD and CAT activity (Tab. 5). In comparison to non-mycorrhizal plants, the activity of SOD enzymes decreased in mycorrhizal plants. In addition, FM inoculation did not significantly affect the activity of CAT and POD enzymes in both roots and leaves of Z. spina-christi seedlings.
Tab. 5 - Effect of salt stress on catalase (CAT), peroxidase (POD) and superoxide dismutase (SOD) of mycorrhizal and non-mycorrhizal seedlings of Z. spina-christi. Means ± standard errors are reported. (+, -): presence/absence of Funneliformis mosseae. Means in the same column followed by the same letter are not significantly different (p > 0.05) after the Duncan’s post-hoc test. (ns): not significant.
| Treatment (mM NaCl) |
FM inoculation |
CAT (U mg-1 protein) | POD (U mg-1 protein) | SOD (U mg-1 protein) | |||
|---|---|---|---|---|---|---|---|
| Leaf | Root | Leaf | Root | Leaf | Root | ||
| 0 | + FM | 5.31 ± 1.70 a | 9.95 ± 2.73 c | 6.19 ± 1.50 b | 2.75 ± 1.73 c | 0.98 ± 0.23 d | 1.57 ± 0.54 c |
| - FM | 7.21 ± 1.70 a | 11.85 ± 2.73 c | 8.09 ± 1.50 b | 2.75 ± 3.60 c | 2.88 ± 0.23 bcd | 3.47 ± 0.54 bc | |
| 50 | + FM | 7.56 ± 2.90 a | 14.60 ± 3.70 bc | 6.61 ± 2.10 b | 6.16 ± 1.82 bc | 2.11 ± 0.41 cd | 2.34 ± 0.73 bc |
| - FM | 11.46 ± 2.40 a | 18.90 ± 3.46 bc | 8.51 ± 2.10 b | 6.16 ± 3.70 bc | 4.41 ± 0.51abc | 4.24 ± 0.73 ab | |
| 100 | + FM | 15.36 ± 7.20 a | 11.02 ± 2.25 c | 15.20 ± 4.90 ab | 2.84 ± 0.46 c | 3.28 ± 1.08 bcd | 3.05 ± 0.66 bc |
| - FM | 19.06 ± 7.70 a | 14.90 ± 2.37 bc | 17.10 ± 4.90 ab | 2.84 ± 0.65 c | 5.18 ± 1.08 ab | 4.95 ± 0.66 ab | |
| 150 | + FM | 12.57 ± 2.60 a | 27.10 ± 5.70 b | 15.98 ± 3.90 ab | 10.93 ± 5.80 ab | 3.07 ± 0.76 bcd | 4.63 ± 1.25 ab |
| - FM | 22.47 ± 8.90 a | 40.90 ± 7.11 a | 21.80 ± 5.20 a | 10.93 ± 7.70 a | 6.17 ± 0.85 a | 6.78 ± 1.19 a | |
| Main effects | NaCl | ns | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| AM | ns | ns | ns | ns | p < 0.001 | p < 0.001 | |
| Interaction effects | NaCl × AM | ns | ns | ns | ns | ns | ns |
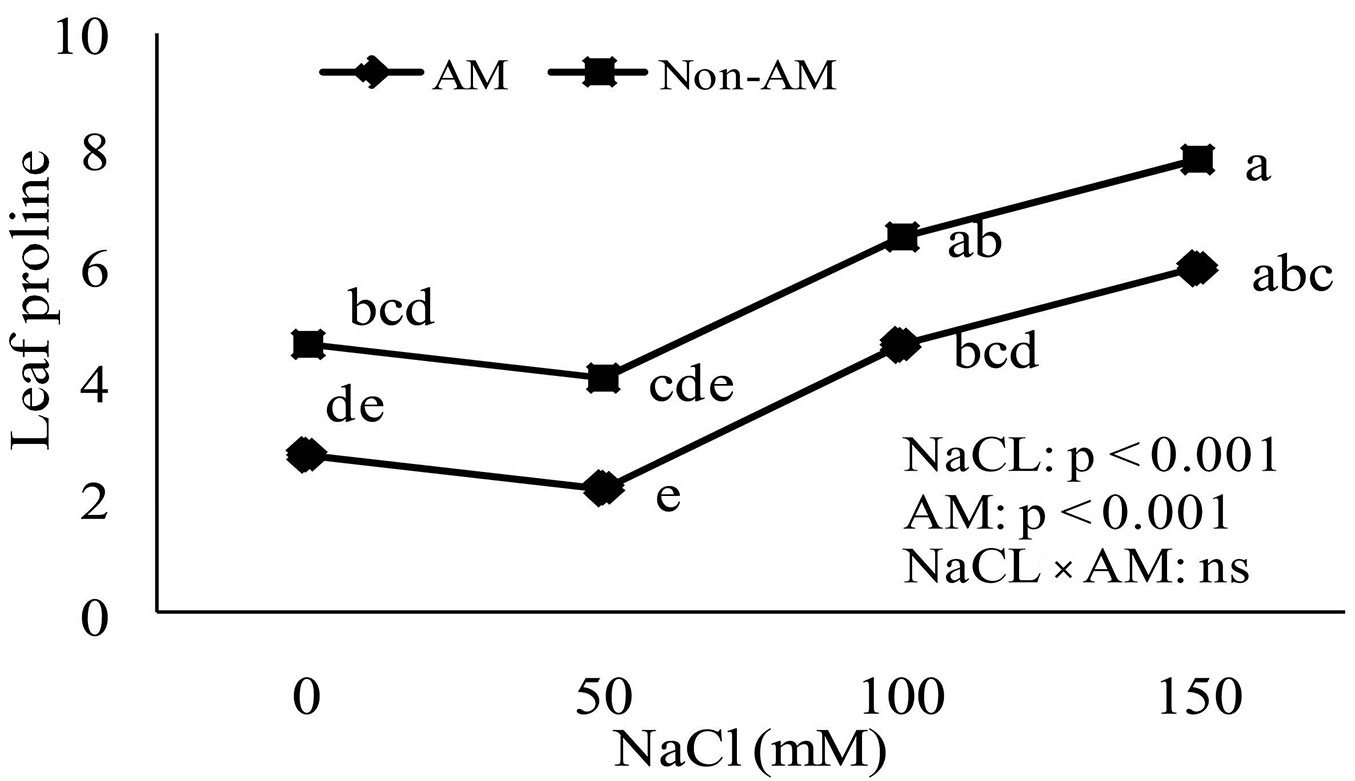
Proline accumulation
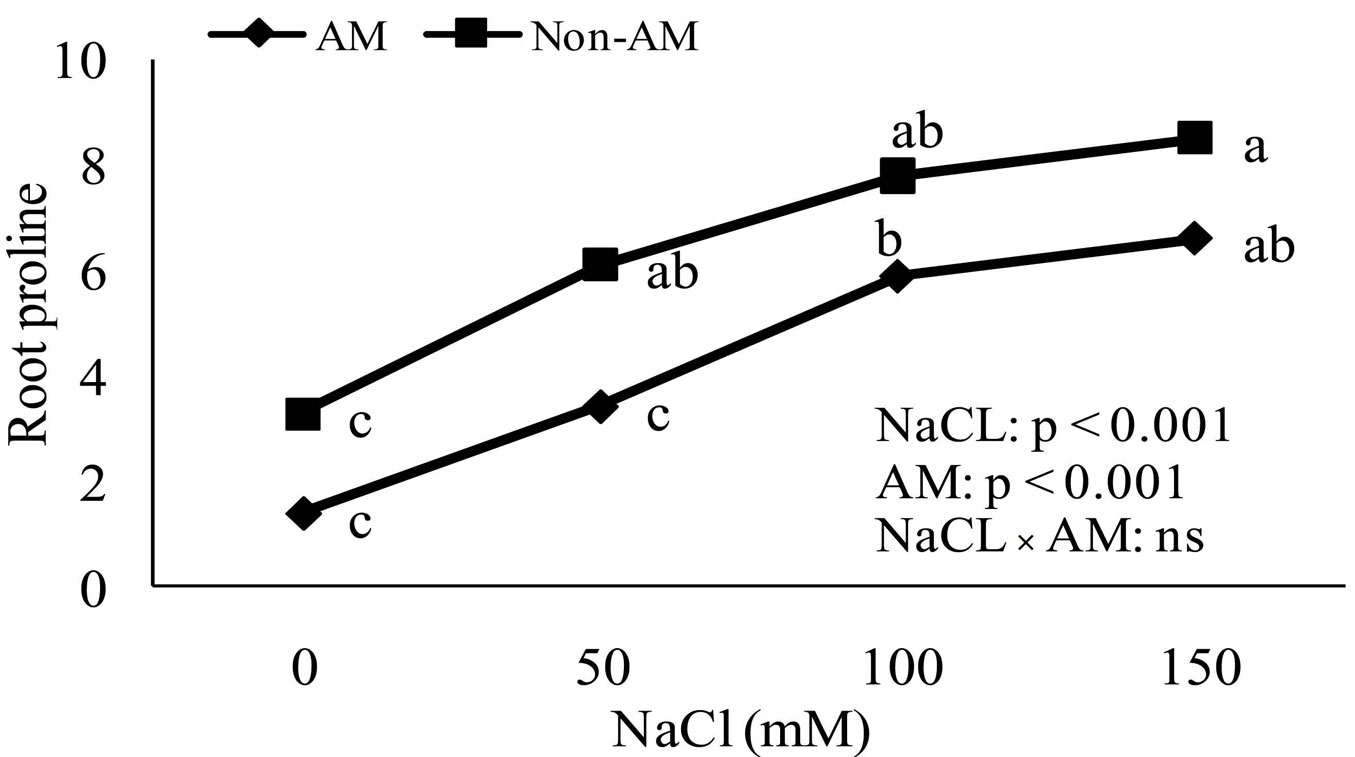
Accumulation of proline in non-mycorrhizal and mycorrhizal plants increased as soil salinity increased. The proline accumulation in leaves and roots of non-mycorrhizal plants increased significantly compared to mycorrhizal plants at all levels of soil salinity (Fig. 4, Fig. 5).
Fig. 4 - Effect of salt stress on proline accumulation in roots of mycorrhizal and non-mycorrhizal seedlings of Z. Spina-christi. Different letters indicate significant differences between treatments (p < 0.05). (ns): not significant.
Fig. 5 - Effect of salt stress on proline accumulation in leaves of mycorrhizal and non-mycorrhizal seedlings of Z. spina-christi. Different letters indicate significant differences between treatments (p < 0.05). (ns): not significant.
Discussion
The results of this study indicate that FM inoculation markedly improved the growth characteristics of Z. spina-christi seedlings under salt stress. Tian et al. ([37]) demonstrated that inoculation with FM fungi could improve growth of cotton plants under a variety of salt stress conditions. In the present investigation, the tolerance of FM-inoculated plants to salt stress increased compared to non-mychorrizal seedlings, as demonstrated by the increase of fresh weight and other parameters, including the level of colonization, basal diameter and RDW. Indeed, the abundance of fungus hyphae around the host roots may help absorbing poorly mobile nutrients such as P in the depletion zone of roots. These nutrients are transported into the host plants, resulting in the improvement of seedling growth ([30]).
According to Beltrano et al. ([8]), we found that the FM colonization of Z. spina-christi roots was inversely correlated to NaCl concentration in the soil (Fig. 1). This decrease in the fungus colonization under salt stress may be due to a reduced germination of fungal spores ([39], [5]).
The beneficial effects of mycorrhiza on growth under saline conditions have been studied in various plant species and families ([5], [13]). In the present study, when the plants were exposed to high concentrations of NaCl in the soil, seedling SDW substantially decreased regardless the presence or absence of mycorrhizal fungi (Fig. 2). The main reasons for the detrimental effects of salinity may be related to the negative osmotic pressure created by salt in the root zone ([21]) or to growth inhibition caused by cell injury in transpiring leaves ([38]).
In general, FM helped to partially alleviate NaCl stress; this was evident in the growth of inoculated plants compared to non-inoculated plants. The beneficial effect of FM symbiosis on plant growth has been largely attributed to the higher uptake of phosphorus ([28]). In the present study, plants inoculated with FM showed higher P contents at all salinity levels, primarily in the roots (Tab. 3). This suggests that the effect of FM on P uptake constitutes a major mechanism for increasing plant tolerance to salinity.
In this study, the Na+ concentrations in mycorrhizal seedlings were significantly smaller than in non-mycorrhizal plants. Low Na+ concentration in leaves and roots of mycorrhizal plants may be due to positive effect of FM fungus on water absorption. Previous studies have also indicated that FM fungi increase plant growth by reducing Na+ uptake ([37], [4]) and increasing the uptake of other nutrients such as P, K, and N ([4], [11]).
The results shown in Fig. 3 indicate that the total chlorophyll and chlorophyll a contents increased in the leaves of FM-inoculated plants as compared with non-mychorrizal plants. However, at 100 mM salinity the chlorophyll contents were at very close range and showed no statistically significant difference (p > 0.05). The higher chlorophyll content of FM-inoculated seedlings may reflect the higher photosynthetic rate necessary to support the carbon cost of association with the fungus ([40]). The increased photosynthesis in FM plants may be mediated by the increased P nutrition, as evidenced by increased plant growth. At higher NaCl concentrations in the soil, the total chlorophyll content decreased (Fig. 3). It has previously been reported that salinity decreased chlorophyll content ([36]); therefore, high levels of NaCl can decrease the chlorophyll content of leaves.
On the other hand, salt stress also enhanced the SOD and POD activity in roots and leaves and CAT activity just in roots of Z. spina-christi seedlings. It is well known that these enzymes represent an effective mechanism for preventing the negative effects of reactive oxygen species (ROS) under salinity stress ([27]). In addition, if the stress lasts for a long time, these enzymes will negatively influence the plant ([2]). FM inoculation acts as a preventive mechanism by decreasing SOD in leaves and roots, thus favoring the avoidance of oxidative damage induced by salt stress ([20]). Finally, this leads to survive the plant under salt stress ([31]).
Proline accumulation is a symptom of stress in less salt-tolerant plants. Proline plays multiple roles in stress tolerance as a mediator of osmotic adjustment ([43]). It also protects macromolecules during dehydration ([35]). In the present study, both salt-stressed mycorrhizal and non-mycorrhizal Z. spina-christi accumulated free proline (Fig. 4, Fig. 5). The increase in free proline in salt-stressed non-mycorrhizal plants was significantly higher than in inoculated plants at all levels of salinity. This suggests that FM inoculation may favor osmotic adjustments in seedlings by promoting the synthesis of solutes such as proline.
Conclusion
This study focused on the effects of the mychorrizal fungus Funneliformis mossae (FM) on the growth of Ziziphus spina-christi seedlings under different levels of soil salinity. The results showed that FM inoculation improved the tolerance of plants to salt stress, alleviated the detrimental effects of salinity on growth and improved the nutrition uptake, as evidenced by the higher K, P, N and lower Na+ concentrations in leaf tissues. The use of FM-inoculated seedlings is a sustainable and environmentally safe treatment to improve tolerance to salinity in Ziziphus spina-christi seedings. Therefore, root inoculation and colonization by FM can be recommended as an effective strategy to alleviate the deleterious effects of salt stress.
References
Online | Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Corresponding author
Paper Info
Citation
Mirzaei J, Mirzaei Y, Naji HR (2015). Effect of Funneliformis mosseae on growth, mineral nutrition, biochemical indexes and chlorophyll content of Ziziphus spina-christi seedlings at different salinities. iForest 9: 503-508. - doi: 10.3832/ifor1643-008
Academic Editor
Silvano Fares
Paper history
Received: Mar 11, 2015
Accepted: Jul 21, 2015
First online: Dec 08, 2015
Publication Date: Jun 01, 2016
Publication Time: 4.67 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 49862
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 41386
Abstract Page Views: 3084
PDF Downloads: 4151
Citation/Reference Downloads: 32
XML Downloads: 1209
Web Metrics
Days since publication: 3692
Overall contacts: 49862
Avg. contacts per week: 94.54
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2016): 4
Average cites per year: 0.40
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Salinity strongly drives the survival, growth, leaf demography, and nutrient partitioning in seedlings of Xylocarpus granatum J. König
vol. 10, pp. 851-856 (online: 26 October 2017)
Research Articles
Two Populus deltoides W.Bartram ex Marshall clones cope differentially with sodium salinity stress
vol. 18, pp. 259-266 (online: 10 October 2025)
Research Articles
Calibration of a multi-species model for chlorophyll estimation in seedlings of Neotropical tree species using hand-held leaf absorbance meters and spectral reflectance
vol. 9, pp. 829-834 (online: 17 May 2016)
Research Articles
Shifts in the arbuscular mycorrhizal fungal community composition of Betula alnoides along young, middle-aged plantation and adjacent natural forest
vol. 13, pp. 447-455 (online: 07 October 2020)
Research Articles
Arbuscular mycorrhizal colonization in black poplar roots after defoliation by a non-native and a native insect
vol. 9, pp. 868-874 (online: 29 August 2016)
Review Papers
Arbuscular mycorrhizal fungi as a tool to ameliorate the phytoremediation potential of poplar: biochemical and molecular aspects
vol. 7, pp. 333-341 (online: 17 April 2014)
Research Articles
Short- and long-term natural regeneration after windthrow disturbances in Norway spruce forests in Bulgaria
vol. 11, pp. 675-684 (online: 23 October 2018)
Research Articles
Arbuscular mycorrhizal fungal symbiosis with Sorbus torminalis does not vary with soil nutrients and enzyme activities across different sites
vol. 8, pp. 308-313 (online: 03 September 2014)
Research Articles
Effects of arbuscular mycorrhizal fungi on microbial activity and nutrient release are sensitive to acid deposition during litter decomposition in a subtropical Cinnamomum camphora forest
vol. 16, pp. 314-324 (online: 13 November 2023)
Research Articles
Post-fire effects and short-term regeneration dynamics following high-severity crown fires in a Mediterranean forest
vol. 5, pp. 93-100 (online: 30 May 2012)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword