Adaptability of Indocalamus decorus to climate change based on physiological and biochemical responses to elevated carbon dioxide and ozone
iForest - Biogeosciences and Forestry, Volume 9, Issue 2, Pages 311-317 (2015)
doi: https://doi.org/10.3832/ifor1571-008
Published: Oct 22, 2015 - Copyright © 2015 SISEF
Research Articles
Abstract
Carbon dioxide (CO2) and ozone (O3) are important greenhouse gases that contribute to global climate change. The effects of elevated CO2 and/or O3 on plants remain unclear. Plant responses to mixtures of the two gases at high concentrations are likely to be complex. Previous studies have shown that the ability to tolerate elevated levels of the two gases varies among plant species; physiological adaptability in the face of changing atmospheric composition also differs among taxa. However, the effects of mixtures of the two greenhouse gases on the growth and physiology of bamboo are largely unexplored, even though bamboos are important vegetation elements throughout tropical and subtropical regions of the planet. In this study, we used open-topped chambers (OTCs) to double the concentrations of atmospheric CO2 and O3, and examined changes in membrane lipid peroxidation, photosynthetic physiology, and antioxidase activities in Indocalamus decorus leaves. After 103 days of treatment, elevated O3 depressed net photosynthetic rate (Pn) without changing stomatal function, but caused no significant oxidative damage in the leaves. High levels of antioxidase activities were maintained in the leaves, indicating that this species had a strong tolerance to elevated O3. Decreases in reactive oxygen content and antioxidase activity in the leaves highlighted the significant positive effects of elevated CO2 on photosynthesis in I. decorus. When a mixture of both gases was supplied at high concentrations, we detected no oxidative damage, although photosynthetic capacity was reduced. Negative effects of O3 were very marked during the early part of the treatment period, but the effects of CO2 were positive. CO2 mitigated the oxidative damage caused by O3 and promoted the growth of I. decorus. Thus, I. decorus tolerated the two greenhouse gases, and was able to adapt to elevated CO2 and O3 levels. These findings contribute to the current knowledge base on the response of bamboo to global climate change.
Keywords
Antioxidant Enzyme, Carbon Dioxide, Indocalamus decorus, Membrane Lipid Peroxidation, Ozone, Photosynthetic Physiology
Introduction
The impacts of global climate change on terrestrial ecosystems are intrinsically complex. In spite of the complexity, they are issues of broad public concern. Carbon dioxide (CO2) and ozone (O3) are important greenhouse gases that drive global climate change. A wide range of human activities, such as the rapid development of industry and transportation, heavy use of nitrogen fertilizers in agriculture, significant emissions of nitrogen oxides (NOx) and oxygen-containing volatile organic compounds (VOCs), have increased atmospheric CO2 and O3 concentrations to levels that are significantly higher than those prior to the industrial revolution. The rate of increase has been relatively stable over time. By 2050, the atmospheric CO2 concentration is expected to reach concentrations that will be double those of preindustrial levels; O3 is increasing by up to 2.5% annually ([15]). Significant increases in CO2 and O3 concentrations will lead to increase radiation levels and contribute to global warming, and they will also directly affect the microscopic structures, physiological and biochemical functions, and the growth and development of plants and ecosystems. Thus, the impacts of increased surface layer CO2 and O3 concentrations on plants have been a focus of attention worldwide ([10], [25]).
O3 is a strong oxidant that enters plant tissues via the stomatal apertures. This gas may cause visible leaf injuries ([11]), inhibit plant growth, and reduce plant height, leaf area ([2]) and biomass ([27]). O3 also increases leaf malonyldialdehyde (MDA) and reactive oxygen species contents, membrane permeability, and membrane lipid peroxidation ([31]). It reduces chlorophyll content, changes chloroplast structure, reduces the number and activity of photosynthetic enzymes and the photosynthetic rate, and changes the distribution of photosynthetic products ([22], [24]).
CO2 is a photosynthetic substrate; changes in its atmospheric concentration are likely to affect physiological and biochemical reactions in plants. Many previous studies have demonstrated that elevated CO2 levels may promote plant growth ([24], [26]) and improve plant photosynthesis performance ([6], [33]). Plants subjected to high CO2 levels also reduce their rates of reactive oxygen species generation, thereby balancing metabolic function and maintaining cell stability ([32]). Thus, elevated O3 levels may damage plants, but elevated CO2 concentrations may promote photosynthesis and accelerate metabolism. Is the damage caused by increases in O3 offset by the benefits conferred by increased CO2 levels? The results of investigations into plant responses to combinations of elevated CO2 and O3 are inconsistent. Some studies indicate that elevated CO2 may reduce stomatal conductance, thereby limiting the volume of O3 entering leaves, or raise antioxidant enzyme activity to alleviate the damage inflicted by O3 ([9], [5]). However, other studies indicate that elevated CO2 does not mitigate the negative effects of O3 on plants ([28]); long-term elevation of CO2 may even exacerbate the damage caused by O3 ([34]).
Bamboo is an unusual evergreen plant. It grows rapidly, can be sustainably managed after reafforestation, and provides a variety of useful products and services for human use. It has important roles in regional water and soil conservation, carbon fixation, oxygen emission, and climate regulation, making it an important element of terrestrial ecosystems. Indocalamus decorus, a member of the subfamily Bambusoideae (family Poaceae), has sympodial rhizomes and large green leaves. The species is very adaptable across a wide range of environmental conditions, including low temperatures, drought and barren soil. Accordingly, it is widely used in soil stabilization and landscaping (hedging, stratified planting, etc.). In the present study, we aimed to identify the mechanisms underlying the physiological and biochemical responses of bamboo plants to elevated atmospheric CO2 and O3, and provide reference data for the adaptive management of bamboo plants during changes in global climate. Thus, we investigated the shifts in membrane lipid peroxidation, photosynthetic physiology, and antioxidant enzymes in I. decorus leaves exposed to doubled CO2 and O3 concentrations in open-topped chambers (OTCs).
Methods
Experimental site
The experimental site was located in the Hangzhou Lin’an Taihuyuan Ornamental Bamboo Planting Garden, Taihuyuan Township, Lin’an City, Zhejiang Province, China (29° 56′ - 30° 23′ N, 118° 51′ - 119° 72′ E). The region has a typical central Chinese subtropical climate with an annual precipitation of 1250-1600 mm. The average annual temperature was 15.4 °C; the maximum and minimum temperatures were 40.2 °C and -13.3 °C, respectively. The average annual frost-free period was 233 days (d).
Experimental materials
I. decorus specimens were planted with soil-free roots into 120 pots during March 2011. Each black plastic pot held 2051.5 cm3 of soil. The potting medium was a uniform mixture of red soil and silver sand (3:1 vol/vol) with a pH value of 5.8, a hydrolyzable nitrogen level of 198.47 mg kg-1, an available phosphorus level of 67.25 mg kg-1, and an available potassium level of 74.16 mg kg-1. We transplanted 15 annual specimens with normal rhizomes in clumps and dark green leaves into each pot. The mean diameter and height of the plants were 4.54 ± 0.08 mm and 37.28 ± 0.15 cm, respectively. We manually watered at regular intervals, and managed bamboo shoots and weeds regularly. The experimental treatments with elevated CO2 and O3 began in July 2011.
Experimental design and methods
OTCs were constructed from stainless steel tubes and colorless clear glass. The eight-prism chamber had an octagonal base, a 4-m high aboveground section and a 0.8-m belowground section; the upper walls leaned inward at an angle of 45°. The aboveground section was wrapped with impermeable clear glass, leaving the top totally open. The bottom was an octagon with sides of 1.5 m and a height of 4 m. Carbon dioxide and ozone were obtained from steel cylinders of pure CO2 and from a CFG-20 O3 generator (Sankang Environmental Technology Co., Ltd., Jinan, China), respectively. Ambient atmospheric air was filtered through activated carbon. The supplementary gases were supplied to the chambers through pressure-relief valves. The volume of additional air added was calculated from the air chamber volume and the flow velocity through an axial flow ventilator; adjustments were made with a flow meter. Supplementary gases were supplied to the OTCs by 750 W ventilation pumps.
We applied four treatments: (i) controls (ambient air, with an O3 concentration of 40 ± 5 nmol ml-1, and a CO2 concentration of 360 ± 20 μmol ml-1); (ii) EO treatment (O3 concentration of 100 ± 10 nmol ml-1, CO2 concentration of 360 ± 20 μmol ml-1); (iii) EC treatment (O3 concentration of 40 ± 5 nmol ml-1, CO2 concentration of 700 ± 35 μmol ml-1); and (iv) ECEO treatment (O3 concentration of 100 ± 10 nmol ml-1, CO2 concentration of 700 ± 35 μmol ml-1). Each treatment was replicated threefold. We placed ten pots containing experimental I. decorus seedlings at a similar growth stage in evenly spaced positions within each air chamber. The O3 and CO2 concentrations in the OTCs were monitored using a Model 205 double beam UV-O3 analyzer (Kangzhuo Automation Systems Engineering Service Co., Ltd., Shanghai, China) and a CO2 infrared sensor (Zhuoxing Environmental Instrument Co., Ltd., Shanghai, China), respectively. Concentrations were measured in the upper, middle, and lower regions of the air chambers at 3 d intervals.
The experiment began on 10 July 2011 and ended on 30 October 2011. We provided O3 on each experimental day from 07:00 to 17:00. CO2 was provided continually.
Determination of photosynthetic gas exchange parameters
During a one hour period (09:00-10:00) on two sunny days in the course of the treatment process (days 55 and 103), we selected three complete mature leaves of I. decorus in each OTC and measured their net photosynthetic rate (Pn), transpiration rate (E), stomatal conductance (Gs), and intercellular CO2 concentration (Ci) using a LiCor-6400 portable photosynthesis system analyzer (Licor, Lincoln, NE, USA) equipped with a standard assimilation chamber (2 × 3 cm) under standardized conditions (photon flux: 900 μmol m-2 s-1; relative humidity: 75 %; temperature: 29 °C).
Determination of physiological indicators
In the mornings (10:00-10:30) of treatment days 55 and 103, we randomly selected a mixed sample of 10-12 mature leaves of I. decorus from each air chamber (following completion of photosynthetic gas exchange parameter measurements) and determined their main physiological and biochemical indexes.
Chlorophyll content and electrolyte leakage rate
Chlorophyll content was estimated following the method described by Zhang & Chen ([36]), with slight modifications. We extracted 50 mg samples in a mixture of 2.5 ml acetone and 2.5 ml ethanol for 24 h in darkness at room temperature. After extraction, we measured absorbances spectrophotometrically at 663 and 645 nm to calculate the contents of chlorophyll a and b, and total chlorophyll.
Electrolyte leakage rate was estimated by measuring electric conductance. Twenty leaf discs with a diameter of 8 mm were rinsed with distilled water, submerged in 20 ml distilled water, vacuum infiltrated for 20 min and then shaken for 2 h to measure the initial electric conductance (S1). Samples were digested in water at 100 °C for 20 min to determine the final electric conductance (S2). The electrolyte leakage was calculated as: EL (%) = (S1/S2) × 100.
Superoxide anion radical (O2·-) and MDA contents
O2·- content was determined by the hydroxylamine oxidation method of Ke et al. ([17]), with slight modification. Fresh leaves (0.5 g) were ground in liquid nitrogen with 5 ml of 50 mM (pH 7.8) phosphate buffer using a mortar and pestle. The homogenate was filtered through a 45 μm nylon mesh and centrifuged at 10 500 × g for 20 min at 4 °C. We added 1 ml of hydroxylammonium chloride (1 mM) to 0.5 ml of the supernatant and incubated the combination for 10 min at 25 °C. We subsequently added 1 ml 4-aminobenzenesulfonic acid (17 mM) and 1 ml α-aphthylamine (7 mM) and held the mixture for 20 min at 25 °C to develop the color. Specific absorption was measured at 530 nm. Sodium nitrite was used as the standard solution to calculate the content of O2·-.
The MDA content was determined by the thiobarbituric acid (TBA) method. We transferred 1.5 ml of the centrifugation supernatant to a stoppered test tube containing 2.5 ml of 0.5 % TBA solution. The mixture was incubated in a boiling water bath for 20 min, cooled and centrifuged. We measured the absorbances of the supernatant at 532 nm, 600 nm and 450 nm, and calculated MDA concentration (μmol L-1) as: MDA = 6.45 × (OD532-OD600) - 0.56OD450 ([4]).
Enzyme extraction and assay
Fresh leaves (0.5 g) were ground in liquid nitrogen using a mortar and pestle; ground samples were individually homogenized in an ice bath in 10 ml of 50 mM phosphate buffer (pH 7.8). The homogenate was centrifuged at 10 500 × g for 15 min at 4 °C. The supernatant was used for the following enzyme assays.
Superoxide dismutase (SOD; EC 1.15.1.1) activity was assayed by monitoring the inhibition of the photochemical reduction of nitroblue tetrazolium (NBT). The 3 ml reaction mixture contained 50 mM phosphate buffer (pH 7.8), 13 mM methionine, 75 μM NBT, 2 μM riboflavin, 0.1 mM EDTA, and 0.05 ml of enzyme extract. Reaction mixtures were illuminated for 20 min at a photon flux of 72 μmol m-2 s-1. One unit of SOD activity was defined as the amount of enzyme required to cause a 50% inhibition of NBT (monitored at 560 nm - [4]).
Peroxidase (POD; EC 1.11.1.7) activity was measured using the guaiacol oxidation method. The assay mixture contained 1 ml of 0.3% H2O2, 0.95 ml of 0.2% guaiacol and 1 ml of 50 mM phosphate buffer (pH 7.0). We added 0.05 ml of enzyme solution to the reaction mixture to make up a total volume of 3.0 ml. To calculate POD activity, we started recording changes in the absorbance of guaiacol (at 470 nm) 30 s after the reaction mixture had been made up, and finished recordings 3.0 min later.
Catalase (CAT; EC 1.11.1.6) activity was determined by directly measuring the decomposition of H2O2 at 240 nm. The reaction mixture contained 1.0 ml of 0.3 % H2O2, 1.9 ml of H2O, and 0.1 ml of enzyme solution. We mixed 0.1 ml of enzyme solution with 2.9 ml of reaction mixture, then added 1.0 ml of 0.3 % H2O2 to begin the reaction. We recorded absorbances at 240 nm at 30 s intervals. CAT activity was followed by the decrease in absorbance in the period 0.5-3.0 min after the reaction had been initiated. A decrease of OD by 0.01 per minute was defined as an activity unit ([4]).
Ascorbate peroxidase (APX; EC 1.11.1.11) activity was determined by following the decrease in absorbance at 290 nm (extinction coefficient 2.8 mM-1 cm-1) in 3.0 ml of a reaction mixture containing 2.4 ml of 0.5 mM ascorbate, 0.3 ml of 2 mM H2O2, and 0.3 ml of enzyme extract ([19]).
Statistical analysis
Microsoft Excel® ver. 2007 was used for data sorting and constructing graphical plots. We used the SPSS® ver. 16.0 statistical software (SPSS Inc., Chicago, IL, USA) to perform one-way analyses of variance (ANOVA); the least significant difference (LSD) multiple comparisons test was used for pairwise comparisons (p < 0.05). All values presented are means ± SD.
Results
Effects of elevated CO2 and O3 on contents of O2·- and MDA, and electrolyte leakage rates
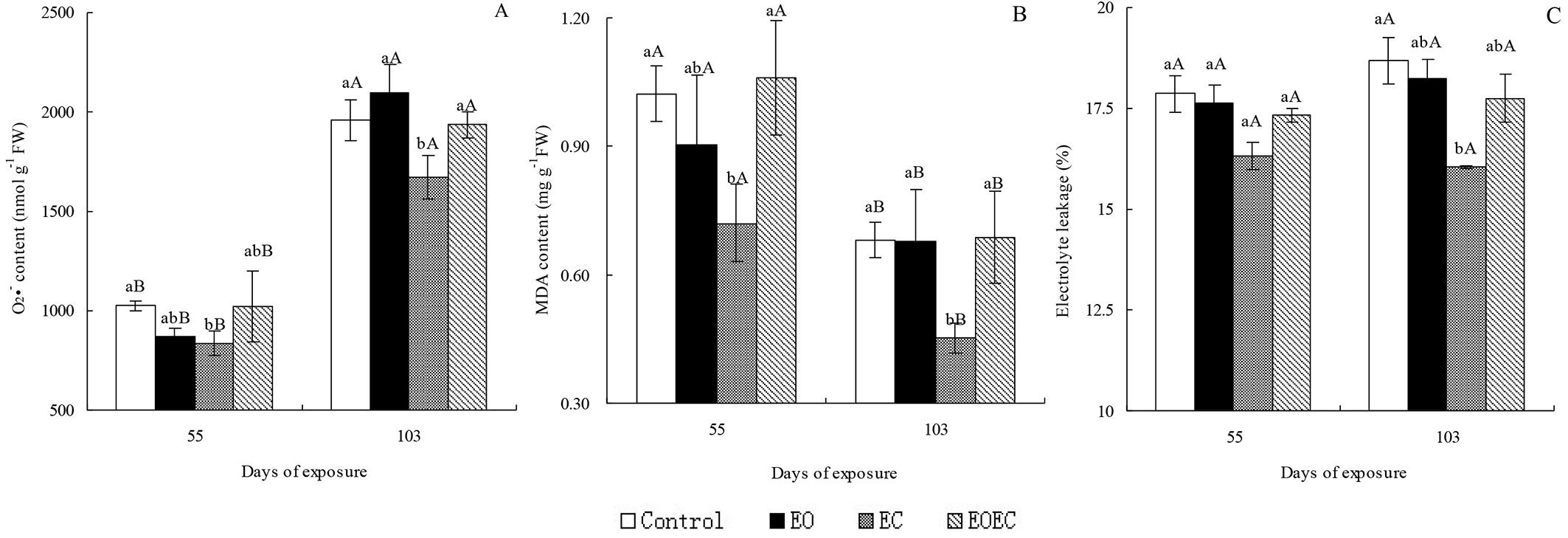
By day 55 of the experimental period, the contents of O2·- and MDA, and the electrolyte leakage rate of leaves in the EO treatment had decreased slightly in comparison with controls. In contrast, the contents of O2·- and MDA in the EC treatment had decreased markedly (p < 0.05 - Fig. 1); the electrolyte leakage rate had decreased slightly in comparison with the controls. By day 103, the O2·- content in the EO treatment had increased slightly, but the MDA content and electrolyte leakage rate had decreased slightly. Measured parameters in the EC treatment had decreased significantly (p < 0.05) compared with the controls. By days 55 and 103 of EOEC treatment, the contents of O2·- and MDA, and the electrolyte leakage rate had changed only slightly in comparison with leaves in the control and EO treatment. However, the contents of O2·- and MDA in the EOEC treatment were significantly higher than those in the EC treatment (p < 0.05). O2·- contents after 103 days of exposure to all treatments were significantly higher than those measured on day 55, but MDA contents on day 55 exceeded those on day 103. Electrolyte leakage rates were not significantly different (p > 0.05) between days 55 and 103 across all treatments.
Fig. 1 - Effects of elevated ozone and/or elevated carbon dioxide on (A) superoxide anion radical (O2·-) content, (B) malonyldialdehyde (MDA) content, and (C) electrolyte leakage in the leaves of Indocalamus decorus. Values are means ± standard deviation of the three independent open-topped chambers (OTCs). Different upper case letters indicate significant differences between days of exposure (p < 0.05); different lower case letters indicate significant differences among treatments (p < 0.05).
Effects of elevated CO2 and O3 on leaf photosynthetic physiology
Compared with the controls, Pn, Gs and Tr values of I. decorus leaves after 55 and 103 days of EO treatment had decreased significantly (p < 0.05), but Ci had increased significantly (p < 0.05). The total chlorophyll in leaves after 55 d of EO treatment had decreased significantly in comparison with the controls (p < 0.05); after 103 d under the same conditions, the chlorophyll content was only slightly different from the controls (Fig. 2). Compared to the controls, the EC treatment caused slight changes in Pn and chlorophyll contents, but Cs, Ci and Tr values in this treatment were significantly higher (p < 0.05) by day 55. In treatment EC, all photosynthetic parameters were significantly elevated (p < 0.05) by day 103. Pn and chlorophyll contents were not significantly different between the EOEC treatment and the controls (p > 0.05), but Ci and Tr values in the EOEC treatment were significantly higher than those in controls (p < 0.05) on days 55 and 103. Gs values in the EOEC treatment were significantly higher than those in the controls (p < 0.05) after 55 d of exposure, but the treatment effect was slight by day 103. Other than Ci, all of the photosynthetic parameters of I. decorus leaves subjected to EOEC treatment were significantly higher than those in the EO treatment (p < 0.05), and significantly lower than those in the EC treatment (p < 0.05) (Fig. 2). Pn and E values of I. decorus leaves after 55 d of exposure to all treatments were significantly higher than those after 103 d of exposure (p < 0.05), but this trend was reversed for Ci. After 55 d of exposure, Gs values in the EOEC treatment, and chlorophyll contents in the control and treatment EOEC were also significantly higher than those on day 103 (p < 0.05). Overall, the responses of Gs and chlorophyll contents to EO and EC treatments were slight on days 55 and 103.
Fig. 2 - Effects of elevated ozone and/or elevated carbon dioxide on (A) photosynthetic rate (Pn), (B) stomatal conductance (Gs), (C) intercellular CO2 concentration (Ci), (D) transpiration rate (E), and (E) chlorophyll content in the leaves of Indocalamus decorus. Values are means ± standard deviation of the three independent open-topped chambers (OTCs). Different upper case letters indicate significant differences between days of exposure (p < 0.05); different lower case letters indicate significant differences among treatments (p < 0.05).
Effects of elevated CO2 and O3 on antioxidant enzyme activities
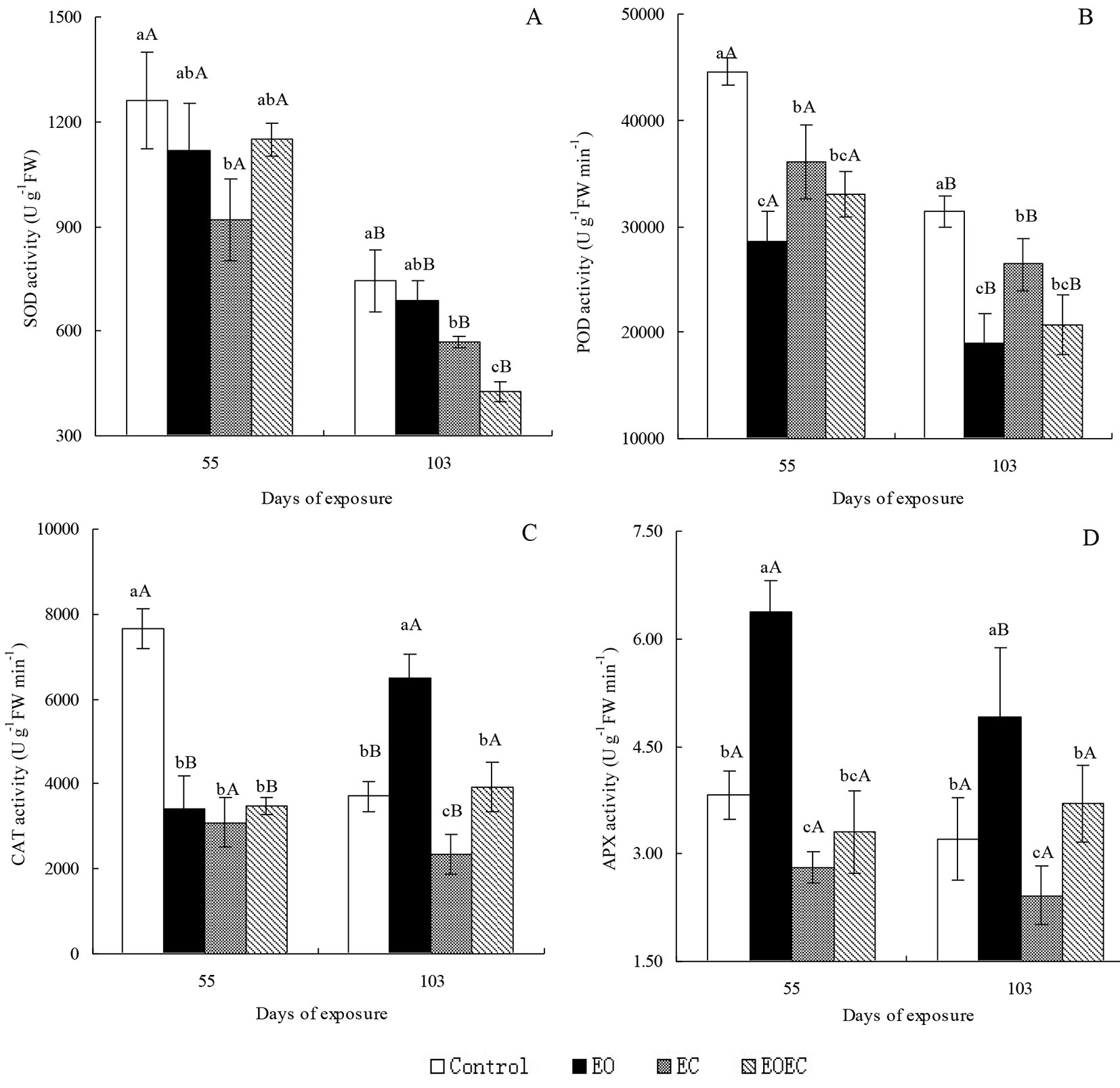
Compared with the controls, the APX activity of I. decorus leaves after 55 d of EO treatment was significantly elevated (p < 0.05), but the activities of SOD, POD and CAT were significantly reduced (p < 0.05) (Fig. 3). After 103 d of the same treatment, the activities of CAT, APX and POD were significantly elevated (p < 0.05), but SOD activity changed only slightly. After 55 and 103 d of exposure to the EC treatment, the activities of all enzymes had decreased significantly (p < 0.05). After 55 d of exposure to the EOEC treatment, the activities of SOD, POD and CAT were significantly reduced (p < 0.05), but APX activity was little affected. After 103 d of exposure to the same treatment, the activities of SOD and POD were significantly reduced (p < 0.05), but the activities of CAT and APX were little affected. The antioxidant enzyme activities of I. decorus leaves in the EOEC treatment were generally lower than those in the EO treatment, but higher than those in the EC treatment. On day 55, the activities of SOD and POD in all treatments were significantly higher than those on day 103 (p < 0.05). CAT activities in the control and EC treatments followed the trends in SOD and POD activities; however, CAT activities in the EO and EOEC treatments tracked a reverse trend. APX activity after 55 d of exposure to the EO treatment was significantly higher than the activity on day 103, but this difference in APX activity between experimental days was not apparent in other treatments.
Fig. 3 - Effects of elevated ozone and/or elevated carbon dioxide on the activity of (A) superoxide dismutase (SOD), (B) peroxidase (POD), (C) catalase (CAT), and (D) ascorbate peroxidase (APX) in the leaves of Indocalamus decorus. Values are means ± standard deviation of the three independent open-topped chambers (OTCs). Different upper case letters indicate significant differences between days of exposure (p < 0.05); different lower case letters indicate significant differences among treatments (p < 0.05).
Discussion
Elevated O3 tolerance
Yan et al. ([35]) reported severe oxidative damage and breakage of membrane structures in selected crops and deciduous trees subjected to O3 treatment. In contrast, we found that the contents of O2·- and MDA, and the electrolyte leakage rate in the leaves of I. decorus were not significantly affected by elevated O3 treatments (100 or 80 nmol ml-1). Thus, this bamboo species is strongly tolerant of elevated O3 levels. This adaptive trait has also been reported for subtropical evergreen tree species, such as Pinus elliottii and Ilex integra ([37]).
However, the photosynthetic physiology parameters of I. decorus leaves were sensitive to elevated O3. Pn decreased significantly after 55 d of exposure, which is a common effect among plants. Gs reduction may be an important protective mechanism that limits the entry of O3 into the leaves through the stomatal apertures when the gas is at elevated concentrations, as demonstrated in previous investigations on Populus ([12]), Ginkgo biloba ([14]), and Quercus mongolica ([35]). The reversed trends in Gs and Ci values in the leaves of I. decorus under elevated O3 conditions indicate that the Pn decline was not due to a Ci decrease caused by changes in stomatal conductance (Gs); the decline of photosynthesis was mainly due to a decline in the activity of photosynthetic organs. Based on the reverse trends of Gs and Ci in the leaves, Pn reduction was most likely caused by non-stomatal factors, in accordance with the theory of Farquhar & Sharkey ([8]). The chlorophyll content of I. decorus leaves decreased significantly in elevated O3 treatments, which also indicates that Pn reduction was caused mainly by the activity of the photosynthetic system (including light energy utilization) and not by stomatal limitation ([20]). At 103 d of exposure, Pn also decreased significantly; chlorophyll content had changed slightly by that time, indicating that the Pn decrease was caused mainly by inhibition of the the carboxylation process by elevated O3 levels. Thus, the carbon fixation rate was reduced ([23], [35]).
At 55 d of elevated O3 exposure, the activities of SOD, POD and CAT of I. decorus leaves decreased significantly compared with control leaves. The O2·- and MDA contents, and the electrolyte leakage rate also decreased to some extent. All of these indicators show that elevated O3 resulted in stomatal closure, thereby preventing the entry of this gas into the leaf mesophyll ([39]). Thereafter, the generation of O2·- and MDA, and the substrate for enzyme synthesis decreased over time. We found that APX was strongly sensitive to elevated O3 concentrations. After 103 d of exposure to elevated O3 levels, the activities of CAT and APX were significantly elevated, but the contents of O2·- and MDA, and the electrolyte leakage rate were little affected. Yan et al. ([35]) demonstrated that elevated O3 levels enhance the activities of antioxidant enzymes, thereby maintaining a balance between the generation and elimination of reactive oxygen species (ROS) and the integrity of the cell membrane structures in the leaves. POD with IAA oxidase properties is an important H2O2 eliminator that also catalyzes oxidation products of the electron donor to generate ROS that promote plant aging ([1]). We showed that the POD activity of I. decorus leaves decreased significantly when O3 concentrations were elevated, demonstrating that POD played a vital role in H2O2 elimination. The enhancement of antioxidant enzyme activities in leaves during the late treatment period increased O3 tolerance in I. decorus.
Physiological and biochemical responses to elevated CO2 levels
CO2 is the substrate of Rubisco. The concentration of this gas affects the activity and catalytic direction of Rubisco, and adjusts the electron transfer rate and the proportions of diverse metabolic pathways. Thus, elevated CO2 levels may impact the photosynthetic process, but may also adjust the states of reactive oxygen species ([21]). Elevated CO2 levels can inhibit the formation of reactive oxygen species ([16]) and reduce the degree of membrane lipid peroxidation ([9]) by increasing the CO2/O2 ratio. We showed that elevated CO2 levels reduced the generation of reactive oxygen species in the leaves of I. decorus, reduced the degree of membrane lipid peroxidation, maintained the integrity of the cell membrane, and enhanced acclimation capability through 103 d of treatment. The effects of CO2 elevation are probably expressed in the next shoot growth season. It is likely that increased CO2 benefits shoot sprouting, growth and development in young bamboo plants with a high carbohydrate demand.
Since CO2 is a substrate for plant photosynthesis, carboxylation and photosynthetic rates will be improved by enhancing CO2 competition for binding sites on the Rubisco molecule (which also catalyzes a reaction between the ribulose-1.5-bisphosphate and molecular oxygen), and by inhibiting photorespiration ([7], [13], [25]). However, elevated CO2 may promote photosynthetic acclimation and a reduction in Pn ([38]). We found that Pn increased significantly in I. decorus during the period of elevated CO2 treatment, suggesting that photosynthetic acclimation did not occur during the treatment; thus, we identified a significant positive effect of elevated CO2. In most cases, elevated CO2 reduces Gs in leaves, reduces Tr, and elevates Ci ([18], [3]). However, that was not the case in our study. After 103 d of CO2 exposure, Gs and Tr had increased significantly, and Ci had significantly decreased. We propose that these responses represent an adaptive adjustment that prevented the occurrence of photosynthetic acclimation. However, the mechanism underlying this adjustment remains unclear. Chlorophyll content increased significantly after 103 days of CO2 exposure. We propose that the extended elevated CO2 treatment promoted photosynthesis in I. decorus, enhanced leaf transpiration, and accelerated plant growth rate.
The antioxidant enzyme activities of I. decorus leaves decreased significantly in the elevated CO2 treatment, confirming an earlier proposal that elevated CO2 levels shift antioxidant enzymes into a “slack” state ([30]). Elevated CO2 levels increase intercellular CO2 concentrations and raise the CO2/O2 ratio at Rubisco binding sites to promote increased carboxylation efficiency of the enzyme, thereby enhancing the photosynthetic phosphorylation in I. decorus leaves. Increased carbohydrate contents in leaves improves NADP+ utilization by the PSI electron acceptor and limits electron flow to molecular oxygen, thereby inhibiting the Mehler reaction and reducing the generation rate of toxic O2 and reactive oxygen species. Antioxidant enzyme activity decreases in response ([29]).
Physiological and biochemical responses to combinations of elevated O3 and CO2
The combined effects of elevated O3 and CO2 on contents of O2·- and MDA, and electrolyte leakage rate were similar to those of the elevated O3 treatment, and were slightly different from those in the controls. Thus, we detected no significant interaction between elevated O3 and elevated CO2 on leaf membrane lipid peroxidation.
At 55 d of the combined O3 and CO2 treatment, the Gs and Ci values of I. decorus leaves had increased significantly, but those of Pn and chlorophyll content decreased slightly. However, Gs values in the combined gas treatment were significantly higher than those in elevated O3 treatment, and lower than those in the elevated CO2 treatment. Thus, we detected an interaction between the elevated levels of O3 and CO2, and suggest that the elevated CO2 increased stomatal conductance, thereby increasing the volumes O3 and CO2 entering the tissues simultaneously. We propose that the measured reduction in the photosynthetic rate was related to the elevated inflow of O3 and the metabolic dominance of this gas, as reported in a previous study of soybean (Glycine max - [3]). After 103 d of combined gas treatment, the Gs and Ci levels in the bamboo leaves decreased; these reductions contributed to the balancing of intracellular CO2 and O3 levels, improving photosynthesis and reducing transpiration, thereby maintaining normal physiological activities in the plants. The process was associated with significant increases in chlorophyll content, which promoted a significant increase in Pn and a reduction of Tr in leaves, indicating that CO2 may have been dominant.
At 55 d of the combined gas treatment, the activities of SOD, POD and CAT decreased significantly; APX activity declined slightly. This combination of effects may have been related to the dominance of O3 on day 55. The low levels of reactive oxygen species in the leaves indicate that that the antioxidant enzymes had played a role in the elimination of reactive oxygen species. The sensitivities of SOD, POD and CAT exceeded that of APX. After 103 d of the combined gas treatment, the activities of SOD and POD were significantly reduced, but the activities of CAT and APX had not changed significantly. This combination of effects may be related to CO2 dominance, which promoted an increase in the CO2/O2 ratio, reductions in the generation of reactive oxygen species, and decreases in membrane peroxidation.
Conclusions
Elevated O3 levels inhibited photosynthesis to a measurable extent in I. decorus. Pn limitation was related to non-stomatal factors, and was not associated with membrane lipid peroxidation. Thus, I. decorus was strongly tolerant of elevated O3.
Elevated CO2 levels significantly promoted photosynthesis in I. decorus, increased the CO2/O2 ratio, and reduced the reactive oxygen species content and antioxidant enzyme activities in leaves. Therefore, elevated CO2 had significant positive effects on I. decorus growth.
The combination of elevated CO2 and O3 concentrations did not reduce photosynthesis or cause oxidative damage in I. decorus; we detected an interactive effect between the gases. In the early treatment period (after 55 days), O3 may have been dominant, with consequently negative effects on I. decorus growth, but later in the treatment period (103 days), CO2 may have been dominant, with consequently positive effects on I. decorus growth.
Ackowledgements
The project was supported by the Natural Science Foundation of Zhejiang Province (No. LY13C160001) and by fundamental research funds for Central Non-Profit Research Institutes (No. RISF2014006).
References
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Yingchun Li
Shuanglin Chen
Qingping Yang
Research Institute of Subtropical Forestry, Chinese Academy of Forestry, Hangzhou Zhejiang, 311400 (P.R. China)
College of Environmental Sciences and Engineering, Peking University, Beijing 100871 (P.R. China)
Corresponding author
Paper Info
Citation
Guo Z, Zhuang M, Li Y, Chen S, Yang Q (2015). Adaptability of Indocalamus decorus to climate change based on physiological and biochemical responses to elevated carbon dioxide and ozone. iForest 9: 311-317. - doi: 10.3832/ifor1571-008
Academic Editor
Silvano Fares
Paper history
Received: Jan 23, 2015
Accepted: Aug 18, 2015
First online: Oct 22, 2015
Publication Date: Apr 26, 2016
Publication Time: 2.17 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 49501
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 41386
Abstract Page Views: 2926
PDF Downloads: 3793
Citation/Reference Downloads: 17
XML Downloads: 1379
Web Metrics
Days since publication: 3755
Overall contacts: 49501
Avg. contacts per week: 92.28
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2016): 3
Average cites per year: 0.30
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
A new approach to ozone plant fumigation: The Web-O3-Fumigation. Isoprene response to a gradient of ozone stress in leaves of Quercus pubescens
vol. 1, pp. 22-26 (online: 28 February 2008)
Research Articles
Soil drench of ethylenediurea (EDU) protects sensitive trees from ozone injury
vol. 4, pp. 66-68 (online: 05 April 2011)
Research Articles
Photosynthesis of three evergreen broad-leaved tree species, Castanopsis sieboldii, Quercus glauca, and Q. myrsinaefolia, under elevated ozone
vol. 11, pp. 360-366 (online: 04 May 2018)
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Research Articles
A comparison between stomatal ozone uptake and AOT40 of deciduous trees in Japan
vol. 4, pp. 128-135 (online: 01 June 2011)
Research Articles
Changes in the proteome of juvenile European beech following three years exposure to free-air elevated ozone
vol. 4, pp. 69-76 (online: 05 April 2011)
Research Articles
Species-specific morphological and physiological characteristics and progressive nitrogen limitation under elevated CO2 concentration
vol. 13, pp. 270-278 (online: 03 July 2020)
Research Articles
Tracing the acclimation of European beech (Fagus sylvatica L.) populations to climatic stress by analyzing the antioxidant system
vol. 14, pp. 95-103 (online: 01 March 2021)
Research Articles
Prediction of ozone effects on net ecosystem production of Norway spruce forest
vol. 11, pp. 743-750 (online: 15 November 2018)
Research Articles
Exploring machine learning modeling approaches for biomass and carbon dioxide weight estimation in Lebanon cedar trees
vol. 17, pp. 19-28 (online: 12 February 2024)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword