The combined effects of Pseudomonas fluorescens CECT 844 and the black truffle co-inoculation on Pinus nigra seedlings
iForest - Biogeosciences and Forestry, Volume 8, Issue 5, Pages 624-630 (2015)
doi: https://doi.org/10.3832/ifor1334-007
Published: Jan 08, 2015 - Copyright © 2015 SISEF
Research Articles
Abstract
The inoculation of forest seedlings with mycorrhizal fungi and rhizobacteria can improve the morphology and physiology of the seedlings and benefit the reforestation of Mediterranean areas and the reintroduction of mycorrhizal fungal inocula into these areas. Pinus nigra subsp. salzmannii,a forest component of the Mediterranean natural ecosystems, is currently used in the reforestation of Mediterranean regions. Its roots are able to form an ectomycorrhizal symbiosis with the Ascomycetes fungus Tuber melanosporum Vitt., the black truffle. The ecological, economic and social values of this ectomycorrhizal fungus is well known. Previously, we demonstrated that the inoculation of Pinus halepensis seedlings with Pseudomonas fluorescens CECT 844 rhizobacteria and the black truffle T. melanosporum improved the plant growth and N absorption of the seedlings. Furthermore, the addition of P. fluorescens CECT 844 doubled the rate of mycorrhization of T. melanosporum. In the present work, P. nigra seedlings were produced in a nursery under well-watered conditions. We studied the morphophysiological response of these seedlings to a combined T. melanosporum and/or a rhizobacteria P. fluorescens CECT 844 inoculation. Five months after inoculation, the growth parameters (seedling height, basal diameter, and shoot and root dry weight), mycorrhizal colonization, water parameters (osmotic potential at both full and zero turgor and modulus of elasticity), and the total contents and concentrations of N, P, and K in the seedlings roots and shoots were measured. The root growth potentials were subsequently estimated. The addition of P. fluorescens CECT 844 did not significantly improve the mycorrhizal colonization by T. melanosporum on P. nigra seedlings. Additionally, the P. fluorescens inoculation caused few significant improvements in the growth and water parameters. Moreover, apparently opposing effects were observed between the two inoculations regarding the seedlings P absorption. We discuss whether P. fluorescens CECT 844 could act as a Mycorrhizal Helper Bacterium (MHB) through different mechanisms depending on the environmental conditions.
Keywords
Rhizobacteria, Black Truffle, Mycorrhiza, Mycorrhiza Helper Bacteria
Introduction
Water and nutrient availability are the main constraints on plant productivity in semi-arid Mediterranean ecosystems. The preservation of mycoflora diversity depends on the status of plant roots ([27]). Forest species in these areas often develop specific strategies to improve their water usage in response to drought ([26]).
Several studies have been conducted to improve the quality of seedlings produced in nurseries ([7]). Several authors reported that soil amendment with ectomycorrhizal fungi and plant-growth-promoting rhizobacteria (PGPR) increased plant survival and seedling quality, especially in soils with low microbial activity ([8], [34]).
Pseudomonas fluorescens generally shows several characteristics of an effective PGPR. It is easily cultivated in vitro, and it colonizes a wide range of ecological niches, including plant rhizospheres ([4]). Additionally, P. fluorescens genomes are highly diverse, which most likely increases the P. fluorescens survival ([44]). The ecological flexibility of such bacteria allows them to exploit a wide variety of nutrients to adapt to environmental changes for survival. P. fluorescens also improves plant growth by producing phytohormones such as auxins (e.g., IAA - [25]). It also has a high capacity for phosphorus solubilization and can produce siderophores ([28]).
Despite the very well-known positive effects of P. fluorescens on plant survival, only a few studies have been conducted to study its influence on the growth of forest species ([38], [31]). We recently demonstrated that the inoculation of Aleppo pine (Pinus halepensis Mill.) with P. fluorescens CECT 844 improved the vegetative growth and N absorption of the P. halepensis seedlings ([16]).
The use of environmental-friendly natural microbial inocula, such as PGPR or mycorrhizal fungi, is presented in this study as a potential alternative fertilizers. These microorganisms are also beneficial for the maintenance of pre-existing soil microflora, thus contributing to the conservation of soil biodiversity. The amended soil in the nursey increases the vegetative vigor and morphophysiological quality of forest species growth for reforestation purposes ([8]).
Information regarding the productivity of ectomycorrhizal fungi, their ecological functions and their contributions to the productivity and recovery of altered agroecosystems is increasingly valuable in agroforestry. In Spain, the black truffle (Tuber melanosporum Vitt.) is of substantial economic and social value in rural areas of the Mediterranean ([35]), although studies on the contributions of T. melanosporum to the growth and physiology of forest plants are scarce ([12]). Moreover, the ecological value of such symbiosis in the recovery of Mediterranean ecosystems has not been well characterized.
The inoculation of black truffle-producing species (including Quercus ilex, Quercus faginea and Corylus avellana) with T. melanosporum is an important practice, supporting truffle silviculture in natural areas ([35]). However, preliminary experiments with other non-ascocarp-producing species are only now being initiated. These non-black truffle ascocarp-producing species (including Pinus nigra and Pinus halepensis) are components of mixed stands of natural ecosystems in which the black truffle is found ([13], [21]). The above pines are also able to form an ectomycorrhizal symbiosis with the black truffle ([33]).
The association of T. melanosporum with other indigenous microorganisms may improve plant growth and increase plant nutrient concentrations, thereby protecting the host plant from drought, which is common in the Mediterranean region. The adhesion of and colonization by mycorrhizal helper bacteria (MHB), such as P. fluorescens, on the surfaces of several ectomycorrhizae can improve the symbiotic relationship and the pre-symbiotic stages, and can benefit the host plant ([18], [11]). In some cases, the co-inoculation of mycorrhizal fungi and P. fluorescens may also increase the root colonization by Pseudomonas ([31], [16]), and the mycorrhizal fungus. In other cases, synergistic effects on the plant growth were observed ([37]), although co-inoculation did not affect the fungal colonization.
Several authors suggested the presence of P. fluorescens to be linked to different stages of ascocarp maturation in the genus Tuber ([10], [2]), especially in T. melanosporum ([40]).
We previously demonstrated that the inoculation of P. halepensis seedlings with P. fluorescens CECT 844 rhizobacteria and the black truffle T. melanosporum improved plant growth and the N absorption of seedlings, and that the addition of P. fluorescens CECT 844 doubled the mycorrhization rate of T. melanosporum ([16]). In the present study, inoculations (both combined and single) were performed using T. melanosporum and P. fluorescens CECT 844 in P. nigra seedlings. Seedling growth, water relations and nutrient uptake were studied. Mycorrhizal colonization was analyzed, and the effect of inoculation on the root growth potential of seedlings was also investigated.
Our starting hypothesis was that the combined inoculation of both microorganisms - P. fluorescens CECT 844 and T. melanosporum - could have synergistic effects and positively influence the P. nigra seedling physiology, thereby improving the plant quality at early developmental stages. We also hypothesized that this rhizobacterial strain could behave as a mycorrhizal-helper bacteria on several forest species forming mycorrhizae of black truffle.
Methods
Plant material
Seeds of P. nigra subsp. salzmannii were collected from Maestrazgo, Castellon, Spain and kept in closed polyethylene bags at 4 °C until sowing. New containers of Forest Pot 300 ® (Nuevos Sistemas de Cultivo S.L., Girona, Spain), each composed of 50 alveoli, were used. Culture substrates were prepared in mid-May 2011 with vermiculite and Sphagnum light and dark peat mixtures, pH 6, with a ratio of 3:1 peat:vermiculite, plus 3.7 % (w/v) CaCO3 and 1.8 % (w/v) KOH. The peat was previously sterilized in an autoclave at 120 °C for 2 hours. At sowing, the substrate pH was close to 8. The seeds were selected by flotation and immersed in water 24 hours before planting. Prior to sowing, seeds were immersed in 30 % H2O2 for 15 minutes for disinfection and then washed several times in distilled water. The assays were performed at the E.T.S.I. Mountains, Madrid, Spain. A total of 24 bins (1200 alveoli) were sown. In each alveolus, 3-8 seeds were placed and allowed to germinate, with each socket containing a single pine seedling. Sowing was performed in a greenhouse in May 2011. Seedlings were irrigated daily until soil saturation at a culture temperature between 20 and 30 °C until inoculation.
Fungal inoculum
The black truffle inoculum was prepared from ascocarps collected in February 2011 in Molina de Aragón (Guadalajara, Spain). T. melanosporum fruiting bodies were selected, superficially cleaned and flame-sterilized. The samples were then stored in closed polyethylene bags at 4 °C until the liquid spore inoculum was prepared several days before inoculation. The fruiting bodies were ground, diluted in distilled water and stored at 4 °C until inoculation. The fungal inoculum was estimated to contain approximately 3.4 × 104 spores ml-1.
Bacterial cultures and inoculation assays
The lyophilized inoculum of P. fluorescens CECT 844 was obtained from the CECT (Spanish Type Culture Collection) at the University of Valencia and stored at 10-15 °C until use. The lyophilized bacteria were pre-incubated for recovery in a standard liquid nutrient medium suitable for the growth of P. fluorescens (1 g meat extract, 2 g yeast extract, 5 g peptone, 5 g NaCl and 1 L distilled water, pH 7.2) and maintained at 28 °C for 12 h at 200 rpm. A second culture was grown at 30 °C for 12 h before inoculation. The inoculum was prepared at the final concentration of 3 × 107 CFU ml-1 as estimated by plate counting.
We used a 4-level univariate design (inoculation of P. fluorescens CECT 844 [Ps], T. melanosporum [T], T. melanosporum x P. fluorescens CECT 844 [T × Ps] and control) distributed into 6 randomized blocks (1 × 4 × 6) with 200 plants per block (4 containers with 50 plants/container).
The inoculum of P. fluorescens CECT 844 was applied in two steps separated by 7 days (July 22 and 29, 2011). Half of the seedlings were inoculated with 10 ml plant-1 (5 + 5 ml, 3 × 108 CFU plant-1). The inoculum of T. melanosporum was applied on July 27, 2011. Half of the seedlings were inoculated with 5 g fresh carpophore/20 ml distilled water/plant (6.8 × 105 spores plant-1). After the second inoculation with P. fluorescens CECT 844, the seedlings were removed from the greenhouse and watered daily until saturation. The HOBO® data logger was used to measure the temperature and relative humidity. The plants were then maintained in an outdoor nursery at an average temperature of 2-30 °C and relative humidity 40-80%.
Pressure-volume curves and water parameter analysis
In November 2011, pressure-volume (PV) curves were built as described by Tyree & Hammel ([51]) and Robichaux ([41]) using the stem water potential measured by a Scholander pressure chamber ([43]). From each pressure-volume curve, the following three parameters were calculated: the osmotic potential at saturation (Ψπfull), the osmotic potential at the turgor loss point (Ψπ0) and the modulus of elasticity (Emax - [9], [24], [52], [5]). Twelve randomly chosen plants per treatment were analyzed (1 PV curve per plant; two plants per block). The seedlings inoculated with T. melanosporum (treatments [T] and [T x Ps]) were later analyzed to confirm the presence of the mycorrhizal fungus.
Plant vegetative growth and mycorrhizal colonization measures
Eighteen plants per treatment (three plants per block) were randomly selected in late November 2011. The height and diameter of each plant was measured. We analyzed the colonization by the mycorrhizal fungi by characterizing and identifying the mycorrhizae ([1]). Subsequently, the mycorrhizal counts of T. melanosporum and other naturally occurring mycorrhizal fungi were determined on the total roots as follows. The root ball of each plant was submerged in water to clean the roots by removing most of the substrate. Roots were then chopped into 1- to 2-cm pieces that were cleaned, rinsed in distilled water and placed into a Petri dish with water for analysis. The percentage and number of root tips in both the inoculated and non-inoculated plants were calculated. Then, the dry weight of shoots and roots was measured for each sample after oven-drying at 65-70 °C for 48 h.
Plant nutrition attributes
In November 2011, concentration and content of key nutrients (nitrogen, phosphorus, and potassium) assimilated in the shoots and roots of seedlings were analyzed. Random samples of 36 plants per treatment (6 plants per block) were divided into three groups. The aerial portion and the roots of each seedling were regrouped, cleaned and dried in an oven at 65-70 °C for at least 48 hours. The tissue was finely ground and homogenized by a pestle. The N, P and K analyses were performed using a Continuous Flow Analyzer SLAMAS SAO++ (Skalar Analytical B.V., Breda, The Netherlands) after Kjeldahl digestions in H2SO4 following the manufacturer’s instructions.
Root growth potential
The root growth potential, i.e., the ability of the plant roots to initiate and extend further in a given time period under optimal growth conditions ([39]), was determined by random sampling 18 plants per treatment (3 plants per block) on March 16, 2012. The height and basal diameter of each plant were measured. Subsequently, each plant with its root ball was carefully transplanted to a 3-L prismatic pot filled with inert white perlite. The pots were placed randomly in the greenhouse in E.T.S.I. Mountains and allowed to grow under optimal growth conditions for 30 days ([46]). The plants were irrigated by maintaining the substrate at saturation. The environmental conditions were monitored in the greenhouse, and the temperature was set to 22 °C. The HOBO® data logger was used to measure the temperature and relative humidity. During the analysis, the average air temperature was maintained between 13 and 28 °C, with a relative humidity between 25-85%. After one month, each plant was carefully removed, and the new roots (distinguishable by their distinct color and greater thickness) greater than 1 cm were counted, and the total length of new roots measured for each plant.
Data analysis
All of the statistical analyses were performed using the software package Statgraphics Plus® (StatPoint Technologies Inc., Warrenton, Virginia, USA). Analysis of variance (ANOVA) for the proposed parameters and the Duncan’s mean comparison test were performed using a confidence level of 5 %. In the case of non-homogeneous variances, the non-parametric Kruskal-Wallis test was applied. For the analysis of the root growth potential, the height and the diameter were selected as covariates. Similar results were obtained for each covariate, therefore only results obtained using the height covariate are presented.
Results and discussion
Seedling growth and mycorrhizal colonization
Several authors argued that the microflora associated with Tuber sp. are stable and selectively represented by the genus Pseudomonas and the aerobic spore-forming bacteria actinomycetes and rhizobacteria ([10], [2]). Rivera et al. ([40]) found that the predominant species in the ascocarps of T. melanosporum was P. fluorescens.
The adhesion of and colonization by MHB (which are metabolically active) at the mycorrhizal surface can affect and improve the symbiotic relationship and the pre-symbiotic stages ([18], [11]). The stimulatory effects of P. fluorescens and ectomycorrhizal fungi when grown together have been alreay reported ([36], [11]). Additionally, MHB may be beneficial to certain fungi but may adversely affect others ([50]). However, MHB seem to be fungus-specific and not plant-specific ([20]).
In this study, CO3Ca and KOH were added to the substrate where P. nigra seedlings were grown. Under such conditions, no significant improvement was found in the colonization by black truffle of the roots of P. nigra seedlings by P. fluorescens CECT 844 inoculation (Tab. 1). However, we have demonstrated that P. fluorescens CECT 844 can facilitate the formation and establishment of T. melanosporum ectomycorrhizae in P. halepensis seedlings under non-optimal soil pH conditions, when calcium carbonate is not added to the growing peat substrate ([16]). According to Garbaye & Duponnois ([20]), MHB are not plant-specific, but they are clearly selective in their interactions with various fungal species, being therefore fungus-specific. Because P. fluorescens CECT 844 may be a MHB not specific to Pinus sp., we hypothesize that P. fluorescens CECT 844 as MHB could significantly increase the colonization by T. melanosporum only when the environment (soil) is unsuitable for fungal growth, as suggested by Brule et al. ([6]) for the fungus Laccaria bicolor. Under unfavorable conditions, it may be also hypothesized that the fungus is not able to prepare a suitable environment to promote mycorrhization, e.g., potentially increasing the mycorrhizal root tips or creating nutritional stress to promote the fungus-plant symbiosis.
Tab. 1 - Water relation parameters, growth parameters, mycorrhizal colonizations, and root growth potential of Pinus nigra seedlings. (C): control; (Ps): Pseudomonas fluorescens CECT 844; (T): Tuber melanosporum. (Ψπfull): osmotic potential at full turgor; (Ψπ0): osmotic potential at zero turgor; (Emax): modulus of elasticity near full turgor. (Total): number of total root tips/plant; Total number and length of new roots/plant; covariate using the height parameter. Means and standard errors are reported. N = 12 (water relations); N =18 (growth, mycorrhizal colonization and root growth potential parameters). Values in the same row and labeled with different letters differ significantly (p<0.05) according to the Duncan’s test.
| Group | Treatment | C | Ps | T | T × Ps |
|---|---|---|---|---|---|
| Water relation parameters |
Ψπfull (MPa) | 0.70 ± 0.09 a | 0.76 ± 0.06 a | 0.82 ± 0.09 a | 0.70 ± 0.10 a |
| Ψπ0 (MPa) | 0.99 ± 0.13 a | 1.18 ± 0.13 a | 1.28 ± 0.15 a | 1.11 ± 0.13 a | |
| Emax (MPa) | 6.92 ± 1.38 a | 7.33 ± 1.13 a | 4.56 ± 0.96 a | 7.54 ± 1.39 a | |
| Growth | Height (cm) | 6.64 ± 0.32 a | 6.83 ± 0.14 a | 7.08 ± 0.28 a | 6.50 ± 0.43 a |
| Basal diameter (mm) | 1.36 ± 0.07 a | 1.38 ± 0.07 a | 1.44 ± 0.05 a | 1.43 ± 0.05 a | |
| Shoot (g) | 0.13 ± 0.01 b | 0.14 ± 0.01 ab | 0.16 ± 0.02 ab | 0.16 ± 0.01 a | |
| Root (g) | 0.13 ± 0.01 a | 0.14 ± 0.01 a | 0.14 ± 0.01 a | 0.12 ± 0.01 a | |
| Mycorrhizal colonization |
T (%) | 0 b | 0 b | 24 ± 3 a | 27 ± 3 a |
| Total (n/plant) | 99 ± 4 b | 102 ± 4 b | 166 ± 13 a | 165 ± 13 a | |
| Root growth potential |
New roots (n/plant) | 7.34 ± 0.75 a | 7.79 ± 0.75 a | 7.49 ± 0.75 a | 6.81 ± 0.75 a |
| New roots (cm/plant) | 7.49 ± 0.84 a | 6.55 ± 0.84 a | 7.18 ± 0.84 a | 6.15 ± 0.84 a |
In this study, the effect of P. fluorescens on the growth of P. nigra seedlings was only significant for the shoot dry weight when P. fluorescens was co-inoculated with T. melanosporum (Tab. 1). P. fluorescens is a plant growth stimulator that efficiently promotes seed germination, accelerates growth in the early stages, induces root initiation, enhances the formation of roots and root hairs, facilitates root regeneration and helps control pathogens in some forest species ([23]). These effects have been observed specifically in P. halepensis inoculated with P. fluorescens Aur6 ([38]). However, the isolated effects of P. fluorescens CECT 844 on the root initiation (Root Growth Potential test) were not apparent in this or previous studies (Tab. 1 - [16]), though the inoculation of this strain have been observed to significantly improve other growth parameters.
In the present study, the inoculation of T. melanosporum significantly increased the total number of root tips in both the simple inoculation [T] and the co-inoculation [T × Ps] treatments. However, the fungus did not significantly increase the root dry weight, indicating that the mycorrhizal fungus T. melanosporum increased the root branching in P. nigra seedlings without affecting the root biomass production. Splivallo et al. ([49]) suggested that this could be facilitated by the production of phytohormones by the fungus. In a similar study on P. halepensis grown with no CaCO3 or other pH correctors in the substrate Domínguez et al. ([15], [16]) observed that the inoculation of T. melanosporum did not significantly increase the total production of root tips. Such results suggest that the fungus could increase the total production of root tips (both mycorrhizal and non-mycorrhizal) only in soils with available CaCO3 or with a high pH. However, it seems that inoculation with P. fluorescens (in combination with T. melanosporum or alone) does not significantly affect the total production of root tips (Tab. 1).
These inoculations did not cause the regeneration of new roots the following spring (root growth potential), in contrast to observations made in previous studies ([25], [23]); this may suggest different effects of different strains of Pseudomonas fluorescens on root regeneration. Furthermore, we did not observe any positive synergistic effects of the fungus-MHB partnership on root growth or the regeneration of new roots. However, increasing the number of plants sampled and/or the duration of the radical regeneration trial could enhance the appearance of treatment effects in the seedlings.
Nutrient uptake
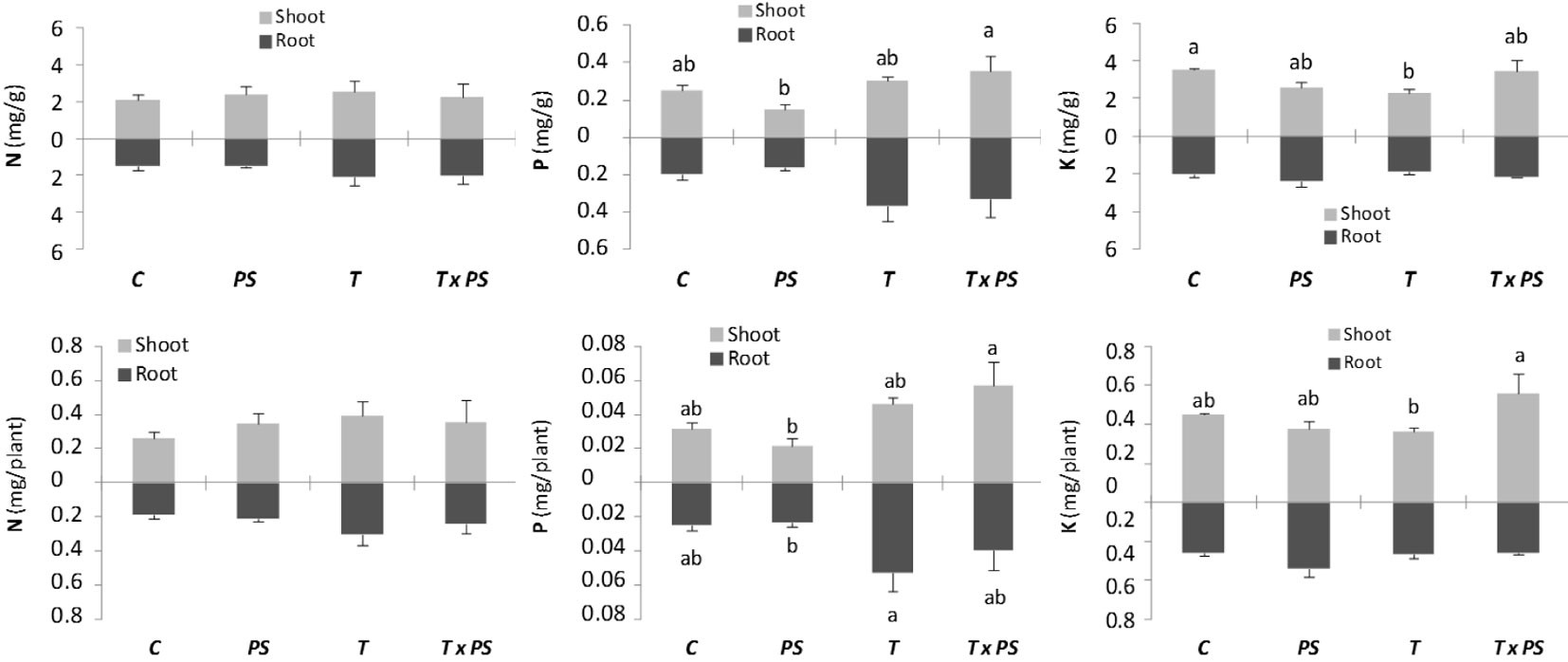
In our study, the co-inoculation treatment [T × Ps] significantly increased the concentration and total P content in the shoot as compared with the [Ps] inoculation treatment. Additionally, the [T × Ps] inoculation significantly increased the K content in the shoot compared with that of the [T] inoculation. Moreover, the T. melanosporum [T] inoculation significantly reduced the K concentration in the shoot compared with that of the control, and significantly increased the P content compared with that of the [Ps] inoculation (Fig. 1).
Fig. 1 - Nutrients concentrations and contents of Pinus nigra seedlings. (C): control; (Ps): Pseudomonas fluorescens CECT 844; (T): Tuber melanosporum. Bars represent the standard error (N = 3). Columns with same color and different letters differ significantly (p<0.05) according to the Duncan’s test.
Improvement of host plant vigor by mycorrhizae is often observed under limited nutrient supply ([47]). Rincón et al. ([37]) observed different effects of ectomycorrhizal fungi on the nutrient uptake in seedlings of P. halepensis, as a likely consequence of nutritional demands that were dependent on the fungal species ([30]). Also, in our previous studies on T. melanosporum, inoculation was shown to improve growth and nutrition, particularly in P. halepensis seedlings ([15], [16]).
In Mediterranean calcareous soils, P is a limiting nutrient for early growth of P. halepensis ([42]), and the Ca availability in the substrate can hinder the P and K uptake from the soil. On the other hand, P. fluorescens has a high capacity for phosphorus solubilization. However, in this study such beneficial effect was not observed, despite seedlings were produced under limited nutrient availability (no added fertilizer) in the nursery. Moreover, the two inoculations apparently caused opposite effects on the P and K absorption by seedlings. Dominguez et al. ([16]) observed that P. fluorescens CECT 844 inoculation may decrease the P and K uptake in P. halepensis seedlings co-inoculated with T. melanosporum and growing on a substrate with no CaCO3. However, in the present study, P uptake of P. nigra seedlings grown on substrate with CaCO3 and co-inoculated with T. melanosporum was not significantly affected by P. fluorescens inoculation. Contrastingly, K content of the mycorrhizal seedlings co-inoculated with T. melanosporum was significantly improved by P. fluorescens inoculation. All the above result suggests that T. melanosporum and P. fluorescens inoculations can cause different effects in P and K uptake, depending on the absence or presence of CaCO3 in the peat substrate (lowered availability of nutrients). P. fluorescens can either block or allow the P uptake to the host plant, depending on whether the level of nutritional stress was sufficient to promote mycorrhization. Frey-Klett et al. ([18]) demonstrated the potential of MHB to affect the N nutrition of host seedlings.
Although P. nigra var. salzmannii and P. halepensis Mill. are two calcicolous species, they show different strategies to cope with water and nutritional stress ([19]). Based on our results, it could be hypothesized that P. halepensis is more efficient than P. nigra in the use of their rhizospheric microorganisms.
Alterations of microbial populations in the rhizosphere may lead to quantitative and qualitative changes in the absorption of nutrients by associated plants. Rincón et al. ([38]) found that the [Ps] inoculation may change the concentration of soil nutrient available for P. halepensis seedlings in the presence of different bacterial populations in the mycorrhizosphere. Additionally, ectomycorrhizal symbiosis may affect the bacterial community in the mycorrhizosphere ([17]), which in turn can alter the nutrient availability ([45]), in particular N and P ([32], [22]).
Water relations
The regulation of the osmotic potential and the increase of the cell wall elasticity allow plants to maintain the cell turgor, thus tolerating negative water potentials in the soil and withstanding water stress ([53]). In the present study, the osmotic potentials were not affected by any of the inoculations carried out (Tab. 1). Moreover, T. melanosporum inoculation has caused a slight (though not significant) increase in the elasticity of cell walls (4.56 MPa [T] vs. 6.92 to 7.54 MPa in the other treatments), as already noted by Domínguez et al. ([15]).
Domínguez et al. ([14]) reported that T. melanosporum can reduce the water deficit of the host plant during drought. Furthermore, Domínguez et al. ([15]) observed that mycorrhization with black truffles under conditions of abundant water availability can cause an elastic adjustment of cell walls in P. halepensis seedlings, which may be a mechanism of resistance to water stress. Hormonal effects may also be involved in the water stress tolerance of plants inoculated with PGPR, since some bacteria can produce abscisic acid (ABA), a plant hormone produced in response to drought ([3]). Rincón et al. ([38]) found that P. fluorescens Aur6 can enhance the water efficiency of associated forest species, but only when plants are subjected to a period of water stress.
In this study, the increased stiffness of cell walls may be related to the plant response to the flagellin produced by the bacteria. The presence of flagellin has been shown to induce stomatal closure ([29]) and the modification of the protein content of cell walls ([54], [48]). However, [Ps] inoculation did not cause significant decreases in cell elasticity in our study.
P. nigra does not usually produce T. melanosporum ascocarps, but only ectomycorrhizae. Some authors ([21], [16]) suggested the use of black truffle mycorrhized seedlings as “carriers” of inocula of wild truffles for application in forestry truffle culture. In the present study, we observed the effects of co-inoculation [T. melanosporum x P. fluorescens] in P. nigra seedlings growing on substrate with CaCO3. Based on our results and those from similar study ([16]), we suggest that both inoculations can cause different effects in nutrients uptake and mycorrhizal colonization of seedlings, depending on the absence or presence of CaCO3. Mycorrhizal P. nigra seedlings do not seem particularly sensitive to addition of P. fluorescens. However, further studies are needed to better understand the effects of co-inoculation [T x Ps] in seedlings of species symbiontic with the prized black truffle and to improve the environmental stress tolerance of forest plants through the co-inoculation of soil microorganisms.
Acknowledgments
This work was part of the project A/030 952/10 “Application of mycorrhizae in sustainable management of silvopastoral systems in the Mediterranean”, PCI-AECID (Program of cooperation between universities and scientific research between Spain and Mediterranean countries, Spanish International Cooperation Agency for Development).
References
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Marcelina Medina
Marta Berrocal-Lobo
ETSI Mountains, Polytechnic University of Madrid, Av. Ciudad Universitaria s/n, E-28040 Madrid (Spain)
Ada Albanesi
Faculty of Agronomy and Agroindustries, Nacional University of Santiago de Estero, Av. Belgrano (S) 1912, Santiago del Estero (Argentina)
Corresponding author
Paper Info
Citation
Dominguez-Nuñez JA, Medina M, Berrocal-Lobo M, Anriquez A, Albanesi A (2015). The combined effects of Pseudomonas fluorescens CECT 844 and the black truffle co-inoculation on Pinus nigra seedlings. iForest 8: 624-630. - doi: 10.3832/ifor1334-007
Academic Editor
Gianfranco Minotta
Paper history
Received: Apr 30, 2014
Accepted: Sep 11, 2014
First online: Jan 08, 2015
Publication Date: Oct 01, 2015
Publication Time: 3.97 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 60626
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 47857
Abstract Page Views: 7324
PDF Downloads: 4137
Citation/Reference Downloads: 24
XML Downloads: 1284
Web Metrics
Days since publication: 4058
Overall contacts: 60626
Avg. contacts per week: 104.58
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 6
Average cites per year: 0.55
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Conservation of Betula oycoviensis, an endangered rare taxon, using vegetative propagation methods
vol. 13, pp. 107-113 (online: 23 March 2020)
Research Articles
Controlled-release fertilizers combined with Pseudomonas fluorescens rhizobacteria inoculum improve growth in Pinus halepensis seedlings
vol. 8, pp. 12-18 (online: 12 May 2014)
Research Articles
Use of overburden waste for London plane (Platanus × acerifolia) growth: the role of plant growth promoting microbial consortia
vol. 10, pp. 692-699 (online: 17 July 2017)
Research Articles
Fertilisation of Quercus seedlings inoculated with Tuber melanosporum: effects on growth and mycorrhization of two host species and two inoculation methods
vol. 10, pp. 267-272 (online: 13 December 2016)
Research Articles
Size and age: intrinsic confounding factors affecting the responses to a water deficit in black spruce seedlings
vol. 8, pp. 401-409 (online: 09 December 2014)
Technical Reports
Effects of different mechanical treatments on Quercus variabilis, Q. wutaishanica and Q. robur acorn germination
vol. 8, pp. 728-734 (online: 05 May 2015)
Technical Reports
De novo adventitious root formations in mini-cuttings of Azadirachta indica in response to different rooting media and auxin treatments
vol. 8, pp. 558-564 (online: 09 December 2014)
Short Communications
Effect of four levels of shade on survival, morphology and chlorophyll fluorescence of Nothofagus alessandrii container-grown seedlings
vol. 8, pp. 638-641 (online: 08 January 2015)
Research Articles
Effects of different nut pretreatments and substrates on germination and seedlings growth of Neocarya macrophylla Sabine in Basse Casamance, Senegal
vol. 17, pp. 346-352 (online: 03 November 2024)
Research Articles
Seedling quality and short-term field performance of three Amazonian forest species as affected by site conditions
vol. 17, pp. 80-89 (online: 21 March 2024)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword