Effect of topography on tree species composition and volume of coarse woody debris in an Oriental beech (Fagus orientalis Lipsky) old growth forests, northern Iran
iForest - Biogeosciences and Forestry, Volume 9, Issue 4, Pages 658-665 (2016)
doi: https://doi.org/10.3832/ifor1080-008
Published: Mar 17, 2016 - Copyright © 2016 SISEF
Research Articles
Abstract
There is an emerging interest in the relationships between forest structure and topographic aspects. Still, such patterns have been scarcely studied in undisturbed mixed beech forests in northern Iran. We investigated the influence of topographical factors including aspect, slope degree, and landform index (LI) on the distribution of dominant tree species and coarse woody debris (CWD). Tree density and basal area were not significantly correlated with any of the measured parameters, except a moderate relation between basal area and LI (r = - 0.376; P = 0.029). Redundancy analysis (RDA) of the tree layer revealed a significant relationship between the measured environmental variables and species distributions. CWD volume showed significant negative correlation with percent canopy coverage and was highly correlated with slope. Density of CWD in decay class IV was significantly correlated with aspect and percent of canopy cover. Analyses of CWD distributions in relation to both living vegetation and topographic gradients showed a highly complex interplay of factors driving the distribution of CWD across the forest stands.
Keywords
Stand Structure, Physical Geography, Landform, Caspian Forest, Fagus orientalis
Introduction
Environmental factors impact vegetation patterns and species distribution, and play an important role in structuring plant communities across the landscape ([21]). In mountain ecosystems, topographic conditions can influence resource and moisture availability, and daily insolation, which are important factors for species distribution, plant growth and mortality patterns. Therefore, sites with different topographical features support different forest types ([60], [24]).
Coarse woody debris (CWD) is generally considered as dead woody materials in various stages of decomposition, including fallen decaying stem and branches having a minimum diameter (or an equivalent cross-section) of 10 cm at the widest point ([19]). Several recent studies contribute to better understanding the extent and role of CWD in forest ecosystems ([11], [23], [45], [57]). Dead woody debris provides rooting substrate and nurse logs for regeneration of plants ([38], [15]), facilitates nutrient cycling and energy flows through forest ecosystems ([31], [30]), maintains hydrology and soil retention capacities ([27]), provides wildlife habitat, and increases biodiversity in forest ecosystems ([16], [26], [54]). CWD has been included in the list of improved European indicators for sustainable forest management by the Ministerial Conference on the protection of forests in European countries ([13]).
In the Oriental beech (Fagus orientalis Lipsky) old-growth forests along the Caspian sea, CWD volume varies according to successional stage, site conditions and management history. An important feature of natural forests of this region is their high amounts of dead wood in all stages of decay and the high proportions of old living trees with dead parts ([43], [59]), with fairly high amount of coarse woody debris (on average, 51 m3 ha-1 - [59]). Sefidi et al. ([56]) for the Hyrcanian beech-dominated forests, recorded volume of coarse and fine woody debris of 15 and 10 m3 ha-1, respectively. Amanzadeh et al. ([5]) in a study carried out in northern Iran reported standing and fallen dead wood volume ranging from 9.1-24.7 and 7.5-29 m3 ha-1, respectively. In contrast, oriental beech stands managed for timber production displayed a lower volume of coarse woody debris, with an average of 23 m3 ha-1 ([7]). These studies focused on the CWD amount in different successional stages and forest associations, but the influence of topographic gradient on the distribution of woody debris in forest stands had not been investigated. In northern forests, insufficient knowledge and scarce quantitative information about amount and quality of dead wood negatively influence forest management plans. In order to manage uneven-aged forest stands using the single selection method, it is of great importance to understand the factors that affect the dead wood pool dynamics and the distribution of CWD in topographically dissected area supporting specific forest stands, such as mountain beech forests in Western Asia ([12]), especially when woody debris is to be managed for maintenance of biodiversity and/or certain stand structural characteristics ([33]). In this stydy, we seek to quantify the effect of the topographic gradient on beech stand structure in the Caspian forest, although the relationship between woody vegetation and topographic gradients has been fairly well studied in beech stands of other northern Iranian regions ([20], [1]).
Oriental beech is a shade-tolerant tree species covering north-facing slopes of Elborz Mountain, in contrast to oak-dominated stands highly distributed across the south-facing slopes of northern Iran ([53]). The unique topographical position of beech stands in this area influence the formation and distribution of different forest vegetation types and coarse woody debris. Such stands, known as Caspian mixed forests, are characterized by minimal anthropogenic disturbances, the presence of old trees near their maximum longevity, large amounts of standing and lying deadwood, and a heterogeneous stand structure, both horizontally and vertically.
The main aim of this research was to quantify the influence of topographical factors on species composition, and quantity and quality of coarse woody debris in natural and undisturbed areas of the Caspian mixed forests.
Methods
Study area
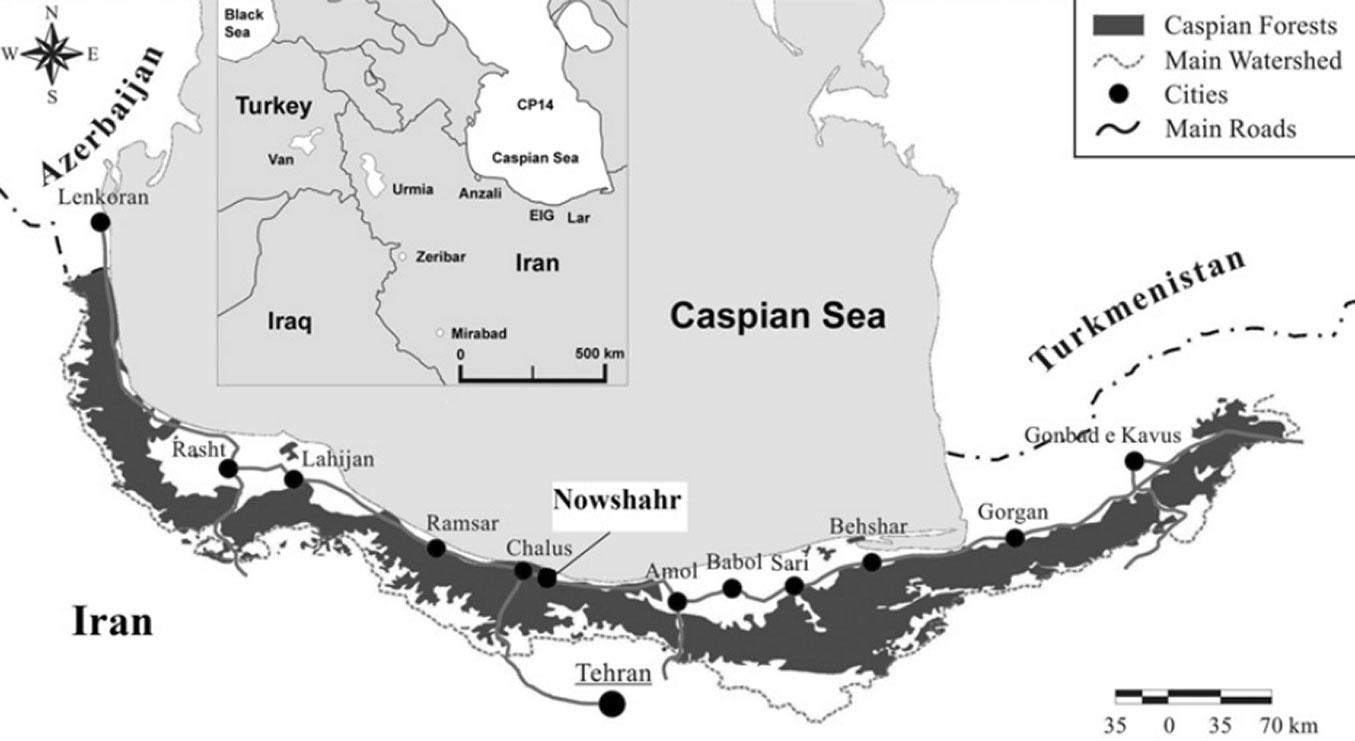
The study was conducted in the Gorazbon section of the Kheyrud Experimental Forest (northern Iran), which is owned and managed by the University of Tehran for education, research, and conservation purposes (Fig. 1). The forest covers a total area of almost 8000 ha and ranges from 36° 27′ N to 36° 40′ N of latitude and from 51° 32′ E to 51° 43′ E of longitude ([44]). The climate is sub-Mediterranean with a mean annual temperature of 9 °C and total annual precipitation of 1380 mm ([36]). Selected forest communities are established on plateaus or fairly inclined slopes which are dominated by moderately acidic to alkaline brown soils with deep, organic A-horizons, limestone bedrock, and a surface largely free of rocks ([18]). Most stands are uneven-aged where new seedling establishment occurs within canopy gaps ([36]). The undisturbed mature beech stands were classified as a climax forest and represent a regional example of old-growth forests with no historical cutting or harvesting of trees ([58]). The elevation of this area ranges between 1000-2000 m a.s.l.. Oriental beech and oriental hornbeam (Carpinus orientalis Miller) are the major species, with Persian maple (Acer velutinum Boiss), Cappadocian maple (Acer cappadocicum Gled.), largeleaf lime tree (Tilia platyphyllos Scop), Siberian elm (Zelkova carpinifolia (Pall.) Dipp), Caucasian Alder (Alnus subcordata C.A.M), Wych elm (Ulmus glabra Hudson), sweet cherry (Prunus avium L.), common yew (Taxus baccata L.), and wild service tree (Sorbus torminalis L.) as less common, but still important part of the forest composition ([53]).
Fig. 1 - The distribution of Caspian forests (dark grey) in the north of Iran (modified from [44]). The study area is indicated in the white box.
Field sampling
Data collection and field inventories took place during the summer of 2012. To characterize the coarse woody debris within stands, a 50×50 meter regular grid of sampling points was established within a natural beech stand, and 0.1-ha circular plots were set at 35 sampling points randomly selected using a systematic random sampling technique, with the aim of equally represent a wide variety of topographical factors ([37], [55]). Potential plot locations were identified using a topographic map ([6]). Each plot was established in order to have oriental beech as the dominant species in the canopy, and the CWD element closer to the intersection point of the grid was considered the plot center. CWD was defined as decaying dead trees or large branches with a diameter > 10 cm regardless to their length. To avoid any edge effects, plots were located at least 100 m away from the forest border and trails. Because of the high degree of complexity in the old growth beech stands, the plot size of 0.1 ha was chosen as larger plots often spread across more than one topographic feature ([51], [58]).
In each plot, we measured percent slope, aspect and land form. Instead of using descriptive categories (such as ridge, mid-slope, and valley), we quantified land form as a land form index (LI) by calculating the mean slope from the plot center to the topographic horizon every 45 degrees (8 total measurements) around the plot center using clinometer and compass ([41], [51]). The eight measurements were then averaged to obtain a single LI value for each plot. The use of the land form index (LI, dimensionless) instead of descriptive categories (ridge, mid-slope, valley) facilitated statistical analyses and avoided classification problems. In fact, using this index the effects of distance and height of landforms are compensated, e.g., a low, nearby ridge and ta high, distant ridge could have the same effect on LI, though obviously their ecological influence could differ ([41]).
The percent of canopy coverage was visually estimated in each plot and classified according to three canopy coverage classes: (I) canopy coverage > 75%; (II) coverage between 50 and 75%; and (III) canopy coverage < 50% ([37]).
In each plot, all woody stems greater than 7.5 cm DBH were identified, and their DBH recorded. For each piece of CWD, we recorded species, total length, type (log, snag, or stump), diameter at both ends, diameter at the midpoint (for stumps only the diameter at the midpoint was recorded), and decay class. For species not identified in the field, a small section of wood was excised and identified later in the lab based on its macro- and microscopic characteristics ([46]). Lengths and diameters were taken at the edge of the plot boundary if the log extended outside of the plot. The diameters of logs, snags and stumps were measured using a caliper; however, for taller snags, top diameters were estimated visually as suggested by Harmon & Sexton ([28]). Decay classes were defined according to Albrecht ([2]) as Class 1 (recently dead), Class 2 (bark loose with some decay in the sapwood), Class 3 (decay obvious throughout the secondary xylem) and Class 4 (woody debris mixing with soil, little structural integrity). Thus, according to these definitions a snag could never be identified as Class 4.
The Newton’s formula was used to calculate the log volume ([28] - eqn. 1):
where V is the volume in m3, L the length, and Ab, Am and At are the cross-sectional area at the base, middle, and top, respectively. The volume for snags and stumps was calculated as (eqn. 2):
where V is the volume in m3, Am is the cross-sectional area at the middle of the length, and L is the length.
Data analysis
Species basal area, density and relative importance values were calculated in each plot for both living and dead trees. Relative ecological importance value (RIV) was calculated for each species by averaging the relative density (number of stems), and the relative dominance (basal area) values. RIV ranks tree species within a site not only based upon the total number of individual trees but also based on the total amount of forest area occupied by the tree species. This index indicates how commonly a species occurs across the entire forest ([17]). Coarse woody debris RIV was calculated for each species in each plot by averaging the sum of each relative density and relative volume of CWD ([51]).
Pearson’s product-moment correlation analysis was used to investigate the relationships between basal area (volume for CWD), density, and RIV values for trees and CWD and plot parameters (LI, slope aspect, percent slope, and percent canopy cover).
We utilized a multivariate approach to investigate the relationships between live and dead trees distributions and plot parameters. A preliminary detrended correspondence analysis (DCA) was performed to assess the gradient length of the species data for each of the structural layers. DCA revealed the presence of short (linear) gradients, so we utilized redundancy analysis to assess the gradient length of the species data for each of the three structural layers ([34], [22], [61]).
Redundancy analysis (RDA) is a method of direct gradient analysis consisting in a constrained linear method of canonical ordination that allows species-environment relationships to be explored. RDA methods try to find values of a new variable that represents the best predictor for the values of all the species as a response variables.
RDA was performed separately for living trees and CWD using species RIV values. Three different RDA analyses were performed. In the first one, environmental and stand (living tree component) parameters were plotted, with no distinction between species. In the second one, environmental and stand parameters (explanatory variables) and RIV of species in the living tree component (response variables) were plotted. In the third one, environmental and stand parameters (explanatory variables) and RIV of species in the CWD component (response variables) were plotted. RDA with tree species data was performed using percent slope, transformed slope aspect, percent canopy cover and land form index. Explanatory variables included importance value of tree species, basal area, density of tree species, crown canopy percent, slope aspect and percentage and tree species in two RDA. Prior to bivariate and multivariate analyses, normality of species distributions and plot parameters were assessed using Kolmogorov-Smirnov normality tests, and appropriate transformations were performed to satisfy normality assumptions. Pearson correlation test was used to investigate the relationship among topographical variables and stand characteristics including stand density, basal area and CWD.
For slope aspect categories as nominal data set, the non-parametric Spearman’s rank correlation procedure ([68]) was used instead of the Pearson’s product-moment correlations. Multivariate analyses were performed using CANOCO version 4.5 ([34]). The significance level for correlation analysis was set at a significance level of α = 0.05.
Results
Plots
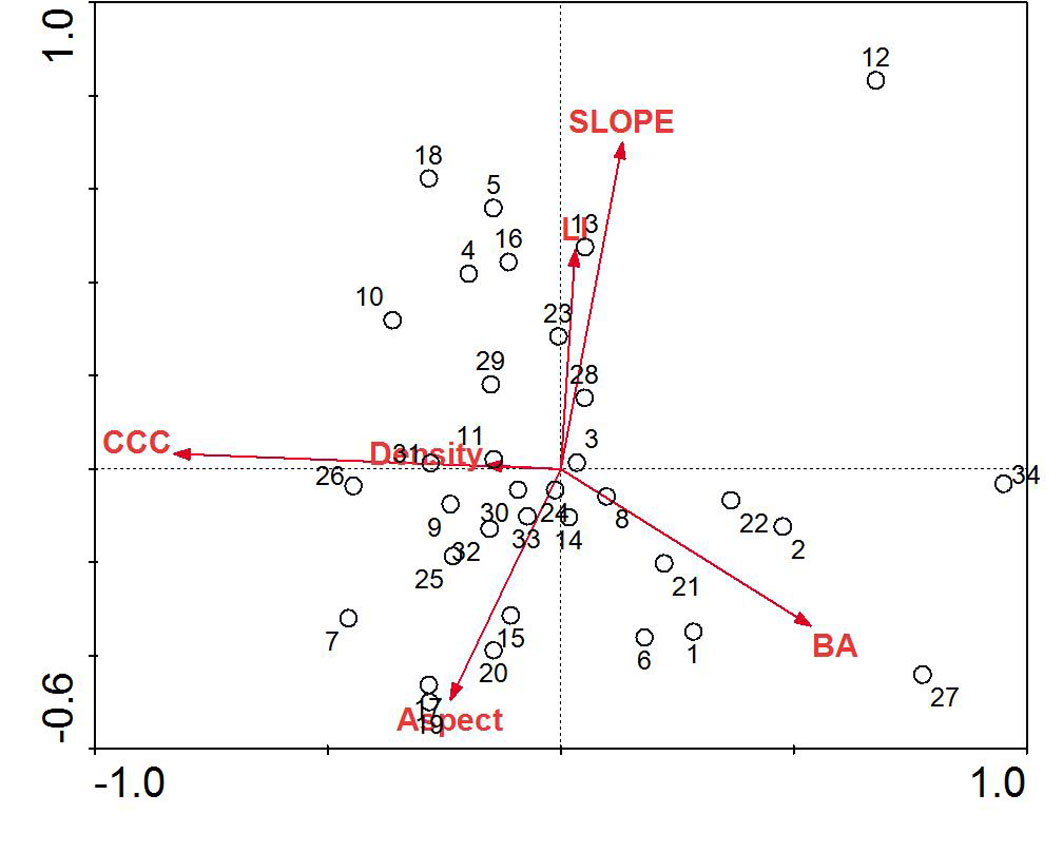
Individual plot parameters varied throughout sampling plots (Tab. 1). The mean DBH in sampling plots was 41.58 ± 1.2 cm, while mean CWD volume was 74.6 ± 18.1 m3 ha-1. Distribution patterns of sampling plots along the first two extracted canonical axes differed throughout the study site with respect to environmental parameters (Fig. 2). We performed a one-way ANOVA as an exploratory test to determine if differences in standard slope position categories (ridge, mid slope, valley) were detectable using LI. We found a significant difference between the three categories (F = 4.71, p = 0.016), and post-hoc analysis (Bonferroni’s multiple comparison test) revealed that all the three groups were significantly different ([51]).
Tab. 1 - Physiographical characteristics of the study site within old growth F. orientalis forests of northern Iran.
| Characteristic | Parameter | Value (%) |
|---|---|---|
| Percent slope |
Mean | 19.5 ± 1.8 |
| Min | 7 | |
| Max | 35 | |
| Land form index |
Mean | 11.8 ± 0.8 |
| Min | 5.3 | |
| Max | 28.3 | |
| Crown Canopy Coverage | Mean | 75.3 ± 3.1 |
| Min | 35 | |
| Max | 95 |
Fig. 2 - RDA biplot of sampling plots and environmental parameters for mixed beech stands, northern Iran. Arrows indicate environmental parameters used in the creation of the biplot: (BA): basal area of trees; (Density): density of trees; (Aspect): transformed slope aspect; (CCC): percent canopy cover; (LI): land form index; (Slope): percent slope.
Trees
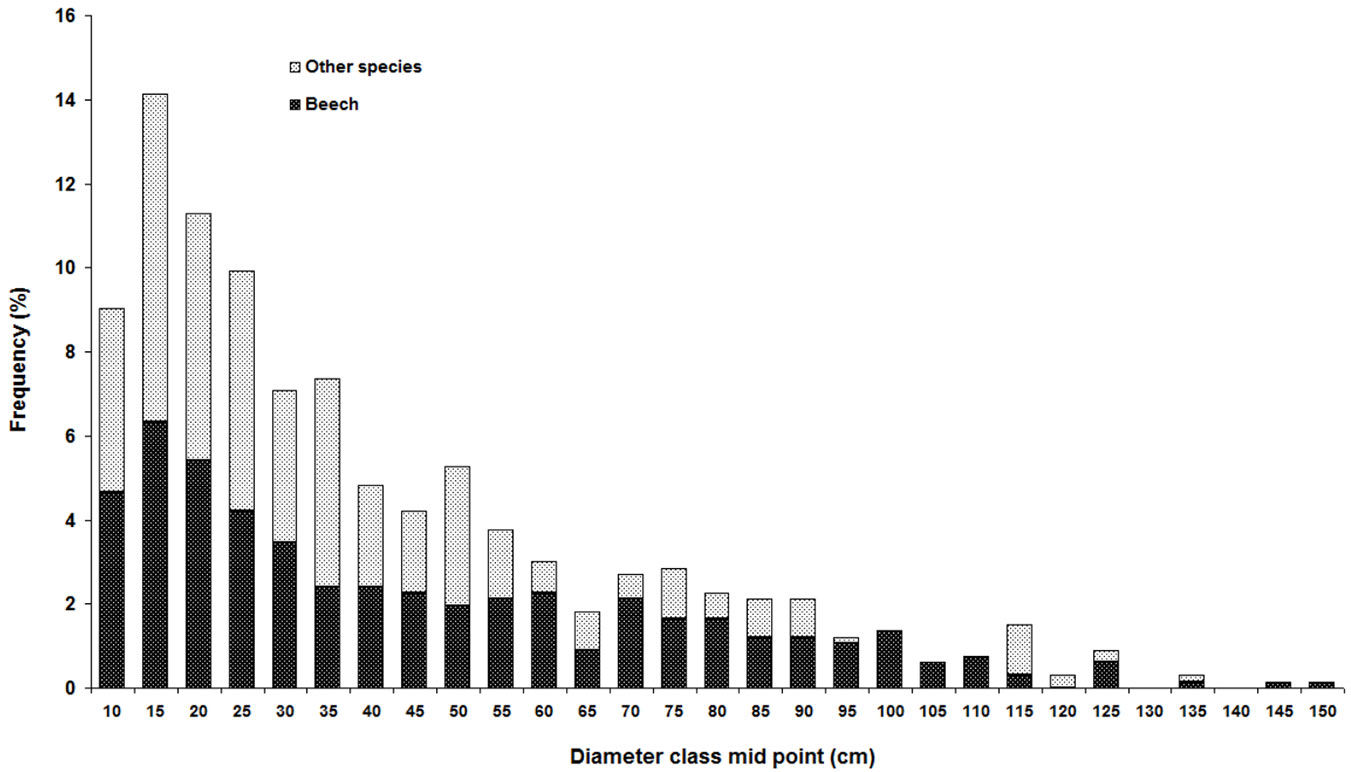
In the sampling plots, Fagus orientalis had the highest mean density, basal area, and RIV, followed by Carpinus betulus, Alnus subcordata, Acer velutinum and Tilia begonifolia (Tab. 2). Diameter distribution showed a reverse J-shape curve (Fig. 3). Tree density was not related to site parameters, but there was a significant correlation between basal area and land form index (Tab. 3).
Tab. 2 - Mean (± SE) density (stems ha-1), basal area (BA, m2 ha-1), relative density (RELDEN), relative basal area (RELBA), and RIV of trees (dbh > 7.5 cm) in the oriental beech forests of northern Iran.
| Species | Density | BA | RELDEN | RELBA | RIV |
|---|---|---|---|---|---|
| Fagus orientalis | 98.8 ± 10.5 | 23.9 ± 2.2 | 54.9 ± 5.6 | 61.3 ± 5.1 | 58.1 ± 5.1 |
| Carpinus betulus | 70.8 ± 15.5 | 5.9 ± 1.1 | 30.3 ± 5.5 | 17.1 ± 3.3 | 23.6 ± 4.3 |
| Acer velutinum | 13.2 ± 3.1 | 3.7 ± 0.8 | 7.4 ± 1.8 | 9.2 ± 2.4 | 8.3 ± 1.7 |
| Alnus subcordata | 11.5 ± 2.5 | 5.4 ± 1.4 | 6.1 ± 1.1 | 11.4 ± 2.8 | 8.7 ± 1.9 |
| Quercus castaneifolia | 0.6 ± 0.2 | 0.3 ± 0.3 | 0.3 ± 0.2 | 0.6 ± 0.5 | 0.4 ± 0.3 |
| Other species | 0.3 ± 0.2 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.3 ± 0.1 |
| Total, all plots | 195.2 ± 10.4 | 39.6 ± 2.3 | - | - | - |
Fig. 3 - Diameter distribution of mixed beech stands in northern Iran. Tree species are distinguished into two groups: F. orientalis (black) and all other tree species (grey).
Tab. 3 - Correlation test results for stand density, basal area and CWD in relation to the topographical variables.
| Variables | Density (N ha-1) |
Basal area (m2 ha-1) |
CWD (m3 ha-1) |
|||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Percent slope | 0.025 | 0.886 | -0.284 | 0.128 | 0.922 | <0.001 |
| Slope aspect | 0.012 | 0.945 | - 0.306 | 0.079 | 0.253 | 0.128 |
| Land form index | 0.055 | 0.757 | - 0.376 | 0.029 | 0.307 | 0.091 |
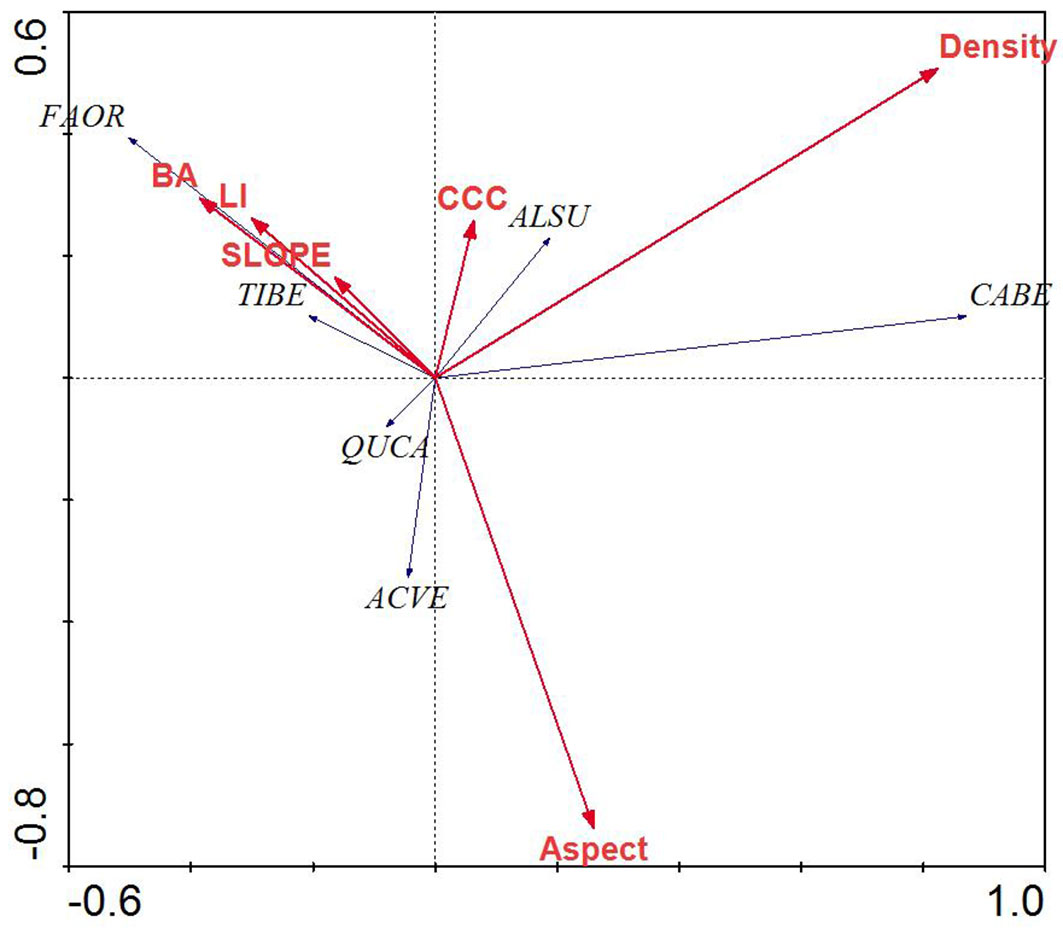
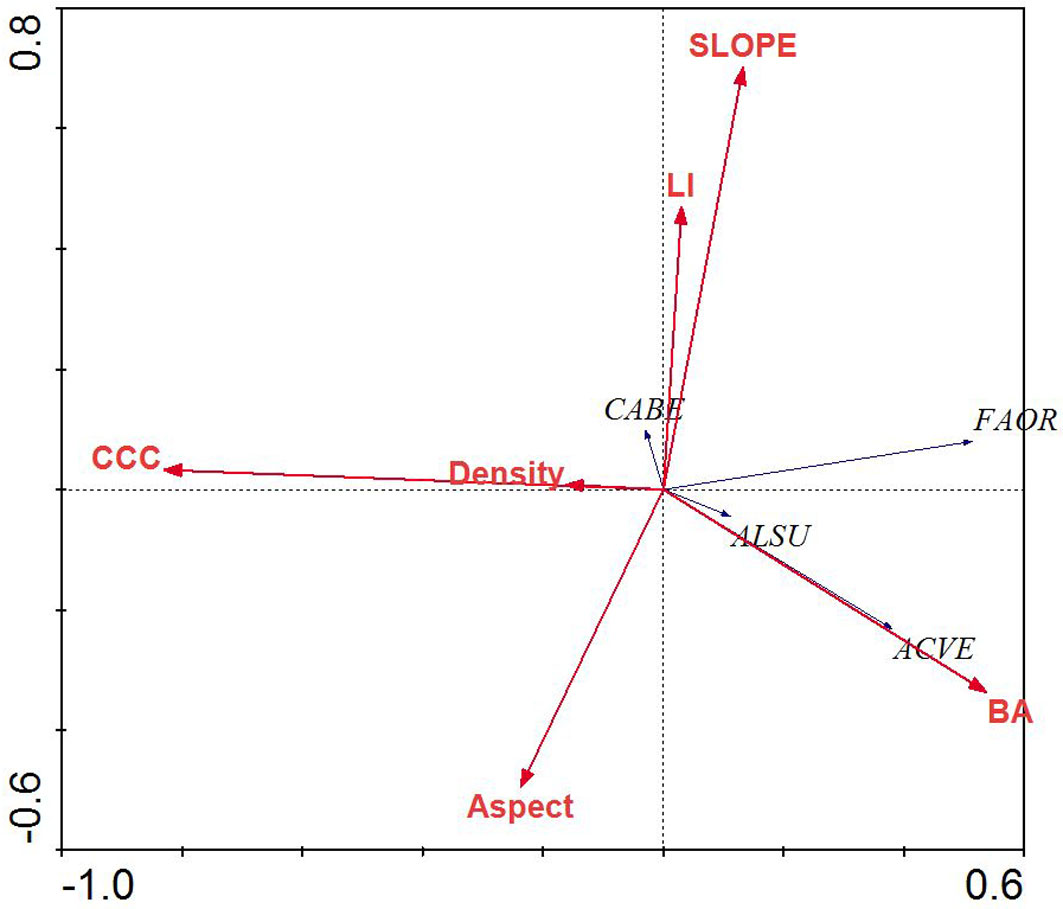
RDA of the tree layer revealed a significant relationship between the environmental variables and species distributions on the first canonical axis (F = 36.72, p < 0.001) and all four canonical axes (F = 8.17, p < 0.001). The environmental variables explained 63.3% of the variation in species distributions on the first two axes. Examination of the inter-set (correlation between species axes and environmental variables) and intra-set (correlation between environmental axes and environmental variables) correlation values revealed that both species axis 1 and environmental axis 1 were correlated with LI (r = 0.25 and 0.30, respectively), although the strength of the correlation was small. In contrast, species axis 2 and environmental axis 2 were highly correlated with aspect (r = 0.49 and 0.73, respectively).
Species distributions in the multi-dimensional space were consistent with changes in individual species presence and habitat affinities (Fig. 2). For example, mesophytic species such as F. orientalis and T. begonifolia ([53]) were found on the left side of the RDA biplot (northerly aspects and high LI scores, valleys). Additionally, C. betulus was found on the right side of the biplot; this is consistent with its distributions reaching maxima on more dry and exposed slope aspects and slope position (southerly and ridge - Fig. 4).
Fig. 4 - Redundancy analysis biplot of tree species and environmental parameters for mixed beech stands, northern Iran. Species name abbreviations are: (FAOR): Fagus orientalis; (TIBE): Tilia begonifolia; (ALSU): Alnus subcordata; (CABE): Carpinus betulus; (QUCA): Quercus castaneifolia; (ACVE): Acer velutinum. Arrows indicate the environmental parameters used in the creation of the biplot. (BA): basal area of trees; (Density): density of trees (ASPECT): transformed slope aspect; (CCC): percent canopy cover; (LI): land form index; (SLOPE): percent slope.
Coarse woody debris
Four major trees species encompass the highest amount of CWD in the sampling plots (Tab. 4). The most frequently CWD species were oriental beech, which was found in 65% of sampling plots, followed by Carpinus betulus (29%). F. orientalis had the highest and C. betulus had the second highest mean density, volume, relative density, relative volume, and RIV of all CWD (Tab. 4).
Tab. 4 - Mean (± SE) density (stems ha-1), volume (m3 ha-1), and relative density (RELDEN), relative volume (RELVOL) of CWD (dbh > 10 cm) in the oriental beech forests, Northern Iran
| Species | Density | Volume | RELDEN | RELVOL | RIV |
|---|---|---|---|---|---|
| Fagus orientalis | 6.7 ± 1 | 44.6 ± 15.7 | 51.9 ± 7.6 | 56.3 ± 8.1 | 54.1 ± 7.8 |
| Carpinus betulus | 2.9 ± 0.7 | 4.3 ± 2.8 | 20.3 ± 6.5 | 18.9 ± 6.3 | 19.8 ± 6.2 |
| Acer velutinum | 2.3 ± 0.7 | 17.2 ± 8.8 | 16.4 ± 5.8 | 15.2 ± 5.4 | 16.1 ± 5.7 |
| Alnus subcordata | 11.5 ± 2.5 | 8.4 ± 5.4 | 10.1 ± 5.1 | 9.4 ± 4.8 | 9.9 ± 5.1 |
| Other species | 0 | 0 | 0 | 0 | 0 |
| Total, all plots | 13.2 ± 1.2 | 74.6 ± 18.1 | - | - | - |
Correlation analysis revealed no significant relationships between CWD volume and LI and slope aspect. CWD volume was significantly negatively correlated with percent canopy cover (r = 0.423, p = 0.012) and highly correlated with slope (r = 0.922, p < 0.001). The CWD volume (m3 ha-1) was significantly correlated with tree basal area (r = 0.504, p = 0.002).
Tree density (N ha-1) and CWD volume (m3 ha-1) in each decay classes was not correlated with plot parameters, except class IV CWD density that was significantly correlated with aspect (r = 0.296, p = 0.044) and the percentage of crown canopy coverage (r = 0.335, p = 0.026).
The RDA of CWD data revealed a significant relationship between the CWD distributions and the environmental variables for the first axis (F = 6.97, p = 0.044) but not for all of the four extracted axes (F = 1.432, p = 0.142). The environmental variables explained 22.5% of the variation in the species data on the first two axes (Fig. 5).
Fig. 5 - RDA biplot of coarse woody debris species and environmental parameters for mixed beech stands, northern Iran. Species name abbreviations are: (FAOR): Fagus orientalis; (TIBE): Tilia begonifolia: (ALSU): Alnus subcordata; (CABE): Carpinus betulus; (QUCA): Quercus castaneifolia; (ACVE): Acer velutinum. Arrows indicate environmental parameters used in the creation of the biplot. (BA): basal area of trees; (Density): density of trees; (Aspect): transformed slope aspect; (CCC): percent canopy cover; (LI): land form index; (SLOPE): percent slope.
Species axis 1 and environmental axis 1 were most highly negatively correlated with canopy cover percent (r = 0.511 and 0.829, respectively), while species axis 2 correlated with parameters, but environmental axis 2 was most highly correlated with slope and aspect (r = 0.701 and 0.469, respectively).
Discussion
Based on our results, the distribution of woody species and CWD in the old growth oriental beech-dominated forest stands are influenced by topographic gradients. Natural stands in the mountain beech forests of northern Iran have a high stocking volume and basal area ([43]). The presence of over-mature beech trees with diameter > 200 cm increased the mean basal area (39.6 m2 ha-1). Sefidi ([58]) reported a mean basal area ranging 18-46 m2 ha-1 in a 75 ha beech forest in the north of Iran.
Different results were reported for primeval beech forests in the eastern Europe. Popescu-Zeletin & Dissescu ([49]) reported a mean basal area of 17-50 m2 ha-1 for a Carpathian beech forest, Urban et al. ([62]) a mean value of 33.8-48.2 m2 ha-1 in a mixed beech stands in the east of the Czech Republic, Hobi et al. ([29]) a value of 36.3 ± 0.8 m2 ha-1 in the southern slopes of the Carpathians in southwest Ukraine, and Petritan et al. ([48]) an average basal area of 47.3 m2 ha-1 in the Runcu-Grosi Natural Reserve in western Romania. The main reason for the aforementioned differences in basal area between Iranian and European mixed beech forests is that F. sylvatica is commonly mixed with different species such as silver fir (Popescu-Zeletin & Dissescu ([49]) and sessile oak ([47]), that contribute to a high proportion of forest stand volume. In contrast, oriental beech in the Iranian forests is mainly mixed with European hornbeam in the understory, which does not represent a high percentage of the total standing volume ([53], [55]). Moreover, the productivity and site-specific environmental factors can also influence stand basal area, thus accounting for the above differences.
Tree species distribution differed throughout the study site with respect to density and basal area. Mesophytic and shade tolerant species such as oriental beech were found with greater frequency on northerly aspects and lower-slope positions, while more xerophytic species such as hornbeam showed a higher frequency on drier, more exposed positions. F. orientalis is a shade tolerant and major canopy layer species, with greater mean basal area, relative basal area, and RIV than all other tree species (Tab. 1).
Oriental beech also showed a high volume and density of living and dead trees. In this study we found a mean CWD volume of 74.6 ± 18.1 m3 ha-1 with a range of 1.2-136.1 m3 ha-1, similar to several other studies carried out in the Iranian beech forests ([56], [5], [59]). Contrastingly, the total volume of dead timber at the Milesice Forest Reserve in the Czech Republic reached 324 m3 ha-1 ([64]), that was higher than our findings. Moreover, Hobi et al. ([29]) reported 135.9 ± 7.5 m3 ha-1 of lying coarse woody debris in the Uholka-Shyrokyi Luh primeval forest in the southern slopes of the Carpathians. Differences in CWD amount between European and Oriental beech forest are likely determined by differences in the stand composition and structure. The coarse woody debris pool reflects the past stand conditions, specifically an earlier successional period when different species dominated the overstory - a condition that is particularly common in old-growth stands ([40]). Moreover, in the European forests beech is mixed with softwood trees such as fir that are missing in the Iranian beech stands. Differences in decomposition rate among species can account for variation in the overstory and dead wood composition ([50]).
The distribution pattern of CWD along the first two extracted canonical axes were similar to patterns of living tree distribution, and were most likely influenced by physiological characteristics of the individual species. For example, oriental beech and maple CWD was mainly found on mesic sites such as valleys or plots with northerly aspects. In contrast, European hornbeam was found on xeric southerly aspects and ridges.
The significant correlation between volume and density of CWD and percent slope of plots revealed that high slope areas have high volume and density of CWD. Accumulation of CWD in the lower slopes (i.e., negative correlation between dead wood volume and the percent of slope) was expected because of the mechanical stability of trees growing on steep slopes and gravity-driven process. Nevertheless, our results revealed a high accumulation of CWD on high slopes that can be explained by the relatively homogeneity of topographic conditions of the study site. The median value of slope across the study site was 18 %; this means that 50% of sampling plots were located in relatively lower slope positions. On steep slopes, CWD tend to drop to lower-slope positions, therefore a negative correlation between the percent of slope and accumulation of CWD in lower slopes was observed. The negative correlation between CWD accumulation and slope was also reported by Rubino & McCarthy ([51]). The same authors also showed that different ecological conditions can affect decomposition rates and dynamics of woody debris as well as accumulation of CWD. Furthermore, Alidadi ([4]) reported that the decomposition of oriental beech CWD largely differs from that reported for European beech, as tree dimensions affect the dead wood pool within stands. Indeed, long-lived oriental beech trees can reach more than 2 meters in diameter at productive sites, and show a high amount of forest dead wood in the studied sites ([57], [43]).
The influence of topography on forest stand composition has been demonstrated in North America ([39], [51]), and similar evidence was also reported in Europe ([24]) and in Iran ([42], [63]). In the Caspian region, the most productive sites are located on the northern slopes. Valipour et al. ([63]) reported that physiographic characteristics (including steepness of slope and aspect) significantly affect the structure of the oak-dominated forests of western Iran in terms of density and diameter class distribution. The same authors found a greater CWD volume on more fertile north-facing slopes than on south-facing slopes. Moreover, in the north of Iran Alidadi et al. ([3]) showed dead wood decomposition rates of F. orientalis and Carpinus betulus significantly different between north facing and south facing slopes ([4]). In this study, slope aspect was not significantly correlated with CWD volume or density, despite an accumulation of CWD in north-facing slopes was expected. Indeed, warmer microclimatic conditions in southern aspects can enhance the CWD decay as a consequence of more intense insolation, higher air and soil temperatures ([9]). Temperature affects decomposition rate, as microbial and invertebrate activity is higher at higher temperatures ([66], [35]). It was demonstrated that an increase of 2 °C in temperature increases stem woody debris decay (expressed in density loss) by 9-55% ([67]).
The successional status of stands ([25], [52], [59], [58]), as well as the susceptibility to storm damage ([10]) or to pathogens ([65], [40]) are all factors affecting the accumulation of CWD. Banas et al. ([8]) stated that ecological and social functions of forests have a slight influence on the amount of CWD. Conservation of biological diversity is one of the most important goals of forest management and dead trees as micro habitats have a key role in maintaining biodiversity in forest ecosystems ([32]). The analysis of the influence of topographical gradients on the dead wood pool is crucial for the development of conservation strategies and for better monitoring the biological diversity in the forest ecosystems.
Physiographic factors can significantly affect tree characteristics in the oak dominated forests in western Iran. In this area, the greatest tree diameter, total height and crown cover were found on the lower slopes, eastern and northern aspects and high altitudes ([63]). Rubino & McCarthy ([51]) showed distribution of trees, saplings, and CWD varies across the fragmented landscape in oak dominated stands in southern Ohio forests. Similar results were reported for other forest types ([40], [25], [51]). In montane mixed forests in Italy, Castagneri et al. ([14]) indicated elevation, basal area of living trees and human impact as the most important factors influencing dead wood pool.
Knowledge on the spatial distribution of CWD in topographically dissected forest stands of the Caspian region is still limited. The evaluation of environmental factors influencing CWD distribution is necessary for a better management of coarse woody debris in beech mixed Caspian forests.
Acknowledgements
We would like to thank Prof. Dr. Namiranian, The president of the Kheyrud Experimental Forest in northern Iran which is owned and managed by the University of Tehran for education, research, and conservation. We are also grateful for the very detailed and constructive comments by two anonymous referees.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Faculty of Agriculture Technology and Natural resources, University of Mohaghegh Ardabili, Ardabil (Iran)
Department of Physical Geography, University of Mohaghegh Ardabili, Ardabil (Iran)
College of Agriculture & Natural Resources, University of Tehran,Tehran (Iran)
Corresponding author
Paper Info
Citation
Sefidi K, Esfandiary Darabad F, Azaryan M (2016). Effect of topography on tree species composition and volume of coarse woody debris in an Oriental beech (Fagus orientalis Lipsky) old growth forests, northern Iran. iForest 9: 658-665. - doi: 10.3832/ifor1080-008
Academic Editor
Emanuele Lingua
Paper history
Received: Jul 19, 2013
Accepted: Nov 11, 2015
First online: Mar 17, 2016
Publication Date: Aug 09, 2016
Publication Time: 4.23 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2016
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 50976
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 41177
Abstract Page Views: 4014
PDF Downloads: 4314
Citation/Reference Downloads: 71
XML Downloads: 1400
Web Metrics
Days since publication: 3637
Overall contacts: 50976
Avg. contacts per week: 98.11
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2016): 22
Average cites per year: 2.20
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Technical Reports
Population genetic structure of Platanus orientalis L. in Bulgaria
vol. 4, pp. 186-189 (online: 11 August 2011)
Research Articles
Long-term effects of single-tree selection cutting management on coarse woody debris in natural mixed beech stands in the Caspian forest (Iran)
vol. 10, pp. 652-658 (online: 20 June 2017)
Research Articles
Suitability of Fagus orientalis Lipsky at marginal Fagus sylvatica L. forest sites in Southern Germany
vol. 15, pp. 417-423 (online: 19 October 2022)
Research Articles
Influences of forest gaps on soil physico-chemical and biological properties in an oriental beech (Fagus orientalis L.) stand of Hyrcanian forest, north of Iran
vol. 13, pp. 124-129 (online: 07 April 2020)
Research Articles
Long-term dynamics of stand structure and regeneration in high-stocked selection fir-beech forest stand: Croatian Dinarides case study
vol. 14, pp. 383-392 (online: 24 August 2021)
Research Articles
Consistency among forest structure and biodiversity potential index (IBP): an assessment of stand structural complexity for floodplain poplar woodlands
vol. 18, pp. 335-343 (online: 04 November 2025)
Research Articles
Stand structure and regeneration of Cedrus libani (A. Rich) in Tannourine Cedar Forest Reserve (Lebanon) affected by cedar web-spinning sawfly (Cephalcia tannourinensis, Hymenoptera: Pamphiliidae).
vol. 11, pp. 300-307 (online: 13 April 2018)
Research Articles
Relationship between tree growth and physical dimensions of Fagus sylvatica crowns assessed from terrestrial laser scanning
vol. 8, pp. 735-742 (online: 11 June 2015)
Research Articles
Short-term effects in canopy gap area on the recovery of compacted soil caused by forest harvesting in old-growth Oriental beech (Fagus orientalis Lipsky) stands
vol. 14, pp. 370-377 (online: 10 August 2021)
Research Articles
Effect of soil-applied lead on mineral contents and biomass in Acer cappadocicum, Fraxinus excelsior and Platycladus orientalis seedlings
vol. 10, pp. 722-728 (online: 27 July 2017)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword