Woody species recruitment under monospecific plantations of pioneer trees - facilitation or inhibition?
iForest - Biogeosciences and Forestry, Volume 5, Issue 1, Pages 1-5 (2012)
doi: https://doi.org/10.3832/ifor0601-009
Published: Feb 06, 2012 - Copyright © 2012 SISEF
Research Articles
Abstract
The successional model of forest restoration is based on the facilitation process, in which the establishment of pioneer tree species favors the late successional tree species. We tested the hypothesis of facilitation through a case study, comparing tree species diversity in the understory of two Neotropical native tree species plantations, Trema micrantha (L.) Blüme (Cannabaceae) and Schinus molle L. (Anacardiaceae). Results obtained under the plantations were compared with an adjacent area under spontaneous secondary succession, analyzing the ligneous plant diversity, soil physic-chemistry and shading. Additionally, the seed rain under the two plantations was analyzed. The area of spontaneous secondary succession and the area with T. micrantha had the highest ligneous plant diversity. The understory of T. micrantha plantation had the lowest light intensity and higher late successional species abundance. In addition, T. micrantha had higher plant diversity than S. molle, which could be explained at least partially by higher diversity in the seed rain. Higher litterfall and mineral content of leaves and twigs of T. micrantha did not coincide with higher topsoil mineral content under that species. Instead, soil under S. molle had higher level of P than the soil under T. micrantha. Data suggest that a high diversity of ligneous plants could be thrived by the spontaneous succession. On the other hand, T. micrantha had promoted a higher richness and abundance of late successional species, which could be related to a more pronounced shade effect, in agreement with the facilitation conception. The lowest diversity and density of ligneous plant species under S. molle characterizes an inhibition effect. T. micrantha could be included in restoration plans assembled with other species or combined with areas without intervention, whenever the spontaneous regeneration is possible.
Keywords
Forest restoration, Secondary succession, Shading, Solanum mauriatianum, Schinus terebinthifolius
Introduction
In southern and southeastern Brazil, the expansion of monocultures in the last decades brought an increasing reduction and fragmentation of native forests. The Atlantic Forest was reduced to only 7.5% of its original cover ([24]). In the present time, its cover is highly reduced and fragmented ([31]). The Subtropical Atlantic Forest, also named Seasonal Forest, is an ecoregion of the Atlantic Forest. With an original cover of 1 000 000 km2 including Brazil, Argentine and Paraguay, nowadays it exhibits only 6% of that area ([3]).
The concern with ecosystem restoration in the Atlantic Forest has been recently growing among Brazilian communities and scientists. In such wise, different methods of forest restoration have been investigated ([9]). Strategies of forest restoration include imitating natural secondary succession, direct seed sowing, monospecific or mixed tree species plantation ([17], [22]), or nucleation with key species that could favor the succession ([30]). In several cases, the seedling plantings constitute a necessary step to obtain satisfactory regeneration since other strategies such as direct sowing or natural regeneration have not produced the expected results ([28], [6]). Additionally, tree establishment contributes to a fast recovery of ecological processes and parameters e.g., the incremental accumulation of litter, nutrient cycling, and the increase in faunal and floral diversity ([7], [34]).
In the secondary succession, the facilitation model considers that only pioneer species are able to colonize following a perturbation. The early-succession species could modify the environment, making it more suitable for later-succession species. Oppositely, in the inhibition model, the pioneer species prevent the establishment of other species, which could only colonize the site when a new perturbation occurs. Alternatively, the tolerance model predicts that the later-succession species are able to survive and grow under the presence of the early-succession species, despite the environmental modifications provoked by them ([11]). The balance between facilitation and competition (inhibition) could be circumstantial and dependent of abiotic factors such as soil nutrient status and moisture ([5]).
Models of restoration based on a heterogeneous pool of tree species of different successional status have been proposed in Brazil over the last two decades ([19], [21], [20]) and more recently in Asia ([35]). In these successional models, it is assumed that secondary and shade-tolerant tree species, which present poor (if any) establishment in open and degraded areas, could be favored by the pioneer species through facilitation. However, there is scarce evidence of facilitation in restoration or secondary succession in the Neotropics, and in certain cases the evidences are contrary ([20]). Besides, monospecific plantations, particularly some long-lived pioneer tree species, can hinder the return of later successional species ([22], [26]).
We carried out an assessment of woody plants diversity in the understory of plantations of two native tree species from southern Brazil, Trema micrantha (L.) Blume (Cannabaceae) and Schinus molle L. (Anacardiaceae), in comparison with the spontaneous regeneration observed in an adjacent area. These species are pioneer trees from Neotropics, with fast growth, good adaptation to open areas and also adequate to plantations in degraded sites ([8], [1]). As pioneer trees with fast initial growth, such species could hypothetically exert a facilitation role when colonizing open areas. Consequently, we expected higher diversity of woody plants under the plantations than in the abandoned area. We tested that hypothesis evaluating the spontaneous regeneration under two plantations of those species, which are established side by side. To elucidate the mechanisms under the differences observed, we had measured: light intensity, seed rain, litterfall, topsoil fertility. We discuss the results in the perspective of the successional model and its underlying hypothesis of facilitation.
Methods
The investigation was undertaken at the Instituto Regional de Desenvolvimento Rural/ UNIJUÍ, FIDENE, Augusto Pestana, Rio Grande do Sul State, Brazil (28° 26’ 32” S, 54° 00’ 12” W). The soil is a red Oxisoil and the predominant forest is a Subtropical Semi-deciduous Seasonal Forest. The plantations of T. micrantha and S. molle were side by side, 3.0 x 2.0 m spacing, both established in June 2001. The area was formerly a pasture occupied by dairy cattle. The terrain was subsoiled only in the planting lines. The plantations were established with seedlings (15-25 cm height) produced in individual conic containers (75 cm3) in the Viveiro Regional, IRDeR/UNIJUÍ, Augusto Pestana, Rio Grande do Sul State, Brazil. Each seedling received 100 g of granulated NPK fertilizer (04-14-08), which was mixed with the soil around the seedling at the moment of planting. The abandoned area without plantation was adjacent to the two plantations. Trees in plantations of T. micrantha showed an average diameter of 52 ± 12 cm at 20 cm above the ground and an average height of around 7.0 m. Trees in plantations of S. molle had 43 ± 11 cm of diameter at 20 cm above the ground and near 7.5 meters height on average.
The spontaneous regeneration of ligneous species in the understory of the two plantations and in the shrubby secondary vegetation was measured through a phytosociological survey carried out between April and September 2009, with plots of 2.0 x 1.3 m. Only ligneous plants with height > 30 cm were included in the sample. The ligneous plants included trees, shrubs and climbing species. The sampled area was the same in the three sites (104 m2 each). The sample sufficiency has been assessed with the species-point curve. The values of absolute and relative density, frequency and abundance were calculated through usual equations. The Shannon index H’ has been calculated from the values of relative abundance ([2]).
Species were classified in three successional groups, namely: pioneers, secondary and late successional, corresponding to the classification of Denslow ([12]). The light intensity (photosynthetically active radiation) was measured between September 2009 and June 2010, always between 11 and 12 a.m. at a 0.5 m above the soil, with a light meter device LD-209 Instrutherm®. On each date, ten random replications were obtained on each area.
Soil analysis was accomplished from four different samples on each area. Each sample was a mixture of five different subsamples, obtained between 0-10 cm depth. The physico-chemical profile was obtained under the recommendations of Tedesco et al. ([36]). Analyses were carried out at the Soil Laboratory of UNIJUÍ (Ijuí, Rio Grande do Sul State, Brazil).
The mineral content of leaves and twigs of the two planted species was measured on 100 g samples collected in January 2010 from ten plants of each species. Only twigs and leaves of the last two growth units (less than one year old) were included. The samples were dried in an air forced circulating oven at 40 °C. Chemical data were provided by the UFRGS Laboratory (Porto Alegre, Rio Grande do Sul State, Brazil) through methods recommended by Tedesco et al. ([36]).
Litterfall was estimated through ten traps of 0.5 x 0.5 m on each plantation area during the year 2004. The values were expressed in dry weight basis.
The seed rain was estimated with 30 circular traps with 0.25 m of diameter on each plantation, amounting 1.47 m2 on each area. The trapped seeds were gathered monthly between December 2009 and November 2010.
Results
Richness and abundance of ligneous plant species were higher under T. micrantha (TM) and in the spontaneous vegetation (SV) than under S. molle (SM). The proportion of secondary and late successional species was higher in TM (Tab. 1).
Tab. 1 - Abundance and diversity data of ligneous plant species from the understory of T. micrantha (TM), understory of S. molle (SM), and spontaneous secondary regeneration area (SV).
| Parameters | TM | SM | SV |
|---|---|---|---|
| Individuals | 105 | 17 | 119 |
| H’ (Shannon) | 1.40 | 1.06 | 1.67 |
| Species | 14 | 5 | 12 |
| Pioneer | 6 | 4 | 8 |
| Secondary | 4 | 1 | 3 |
| Late successional | 4 | - | 1 |
In all areas, twenty different species were recorded in the phytosociological inventory and four additional species were recorded out of the plots (Tab. 2). Among them, Hovenia dulcis and Morus nigra are exotic species.
Tab. 2 - Mechanism of dispersion (DM) and relative abundance (expressed as percentage) of ligneous plant species from the understory of T. micrantha (TM), understory of S. molle (SM), and spontaneous secondary regeneration area (SV), for each successional guild. (Z): zoochoric; (A): autochoric; (W): wind-dispersed; (?): unknown; (-): absent; (•): observed out of the plots.
| Species | DM | TM | SM | SV |
|---|---|---|---|---|
| Pioneer | ||||
| Schinus terebenthifolius Raddi | Z | 58.0 | 37.5 | 6.7 |
| Ocotea puberula (Rich.) Ness. | Z | 14.5 | - | - |
| Baccharis punctulata DC | W | 11.0 | - | 2.5 |
| Senna macranthera (DC ex Colland.) Irwin & Barneby | ? | 1.0 | - | - |
| Baccharis dracunculifolia DC | W | - | 50.0 | 18.5 |
| Aegiphila brachiata Vell. | Z | - | 6.3 | 1.7 |
| Solanum mauritianum Scop. | Z | - | - | 44.5 |
| Morus nigra L. | Z | - | - | 1.7 |
| Schinus molle L. | Z | - | - | 1.7 |
| Acacia recurva Bentham | A | • | - | 0.8 |
| Hovenia dulcis Thunberg | Z | • | - | - |
| Secondary | ||||
| Nectandra lanceolata Ness | Z | - | 6.3 | - |
| Zanthoxylum rhoifolium Lam. | Z | 3.0 | - | - |
| Celtis iguaneae (Jacq.). Sargent | Z | 1.0 | - | - |
| Matayba elaeagnoides Radlk. | Z | - | - | 17.7 |
| Cupania vernalis Camb. | Z | 1.0 | - | 2.5 |
| Gledtisia amorphoides (Griseb.) Taubert | ? | - | - | 0.8 |
| Prunus myrtifolia (L.) Urban | Z | • | - | - |
| Rollinia salicifolia Schltdl. | Z | - | • | - |
| Late successional | ||||
| Trichilia elegans A. Juss. | Z | 7.5 | - | - |
| Trichilia catigua A. Juss. | Z | 2.0 | - | - |
| Allophylus edulis (A. St.-Hil., Camb. & A. Juss.) | Z | 1.0 | - | - |
| Allophylus guaraniticus A. St.-Hil. | Z | - | - | 0.8 |
| Ilex paraguariensis A. St. Hil. | Z | • | - | - |
Similarity among the studied areas was very low. Only S. terebinthifolius was shared in between TM and SM. Between SM and SV areas there were three species in common, all of them were pioneers. TM and SV presented four species in common, three pioneers and one secondary (Tab. 2).
The zoochory predominates, corresponding to 19 species or 79% of the ligneous flora (Tab. 2).
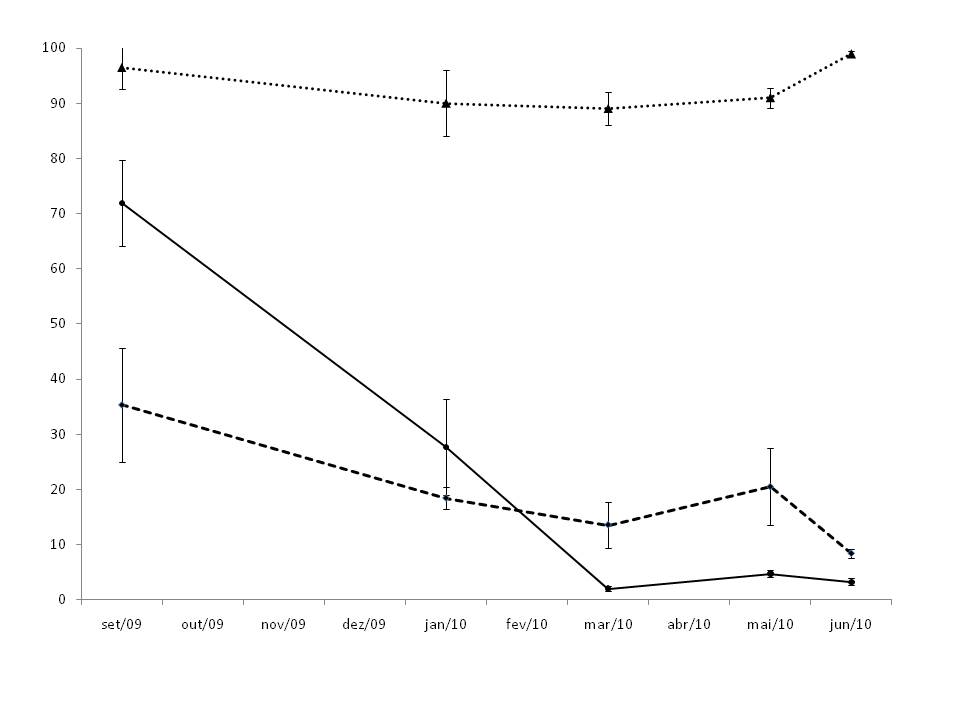
The light intensity at 0.5 m above the ground was higher in the SV. TM presented a higher light intensity than SM area in the end of winter and early spring (September), thereafter the effect of frosts between July and August. The frosts cause the total loss of leaves in T. micrantha. The leaf cover was restored during the summer, so that the light intensity in March onwards was lower in TM plantation (Fig. 1).
Fig. 1 - Light intensity (% of the full sun light) between September 2009 and June 2010, under T. micrantha (solid line) and S. molle (dashed line) plantations, and in the spontaneous secondary regeneration (triangles, dotted line). Vertical bars indicate standard error.
Litter production (dry weight) was higher under TM than under SM (677 g m-2 year-1 and 325 g m-2 year-1, respectively). Data on litterfall in the spontaneous vegetation were not available.
The mineral contents of the soil are higher in the SV area for S, Ca and Mg. SV also have larger values of CEC and base saturation (Tab. 3). The plantations of TM and SM had higher levels of Cu and P. The SM area showed elevated values of Zn (Tab. 3).
Tab. 3 - Physico-chemical soil profile in the top soil (0-10 cm) under T. micrantha and S. molle plantations, and in the spontaneous secondary regeneration. Different letters among columns indicate significant differences after Tukey’s test (P < 0.05).
| Mineral | Trema micrantha | Schinus molle | Spontaneous vegetation | |||
|---|---|---|---|---|---|---|
| P | 7.1 | b | 10.6 | a | 3.7 | c |
| K | 110.0 | a | 123.8 | a | 140.5 | a |
| S (mg/dm³) | 2.0 | a | 1.5 | a | 6.3 | b |
| Cu | 10.8 | b | 11.7 | b | 8.5 | a |
| Zn | 3.2 | ab | 4.3 | a | 2.5 | b |
| Mn | 77.8 | a | 64.6 | a | 38.6 | a |
| Al | 0.2 | a | 0.1 | a | 0.1 | a |
| Ca (cmolc/dm3) | 5.4 | a | 6.6 | ab | 8.1 | b |
| Mg | 2.1 | a | 2.2 | a | 4.3 | b |
| pH | 5.15 | a | 5.4 | a | 5.65 | a |
| CEC (pH = 7.0) | 13.9 | a | 14.0 | a | 18.5 | b |
| V % | 54.3 | a | 64.7 | ab | 69.0 | b |
| M % | 3.0 | a | 1.4 | a | 0.6 | a |
| O.M. (%) | 3.5 | a | 3.6 | a | 3.2 | a |
The mineral content of leaves and twigs of T. micrantha was higher than of S. molle as a general rule (Tab. 4).
Tab. 4 - Mineral contents of leaves and twigs of T. micrantha and S.molle. From N to S data as a percentage, from Cu to Al data as ppm. (*): significant differences (P<0.05) after Tukey’s test.
| Mineral | S. molle | T. micrantha | Prob. |
|---|---|---|---|
| N | 1.60 | 3.70 | * |
| P | 0.24 | 0.26 | n.s. |
| K | 1.48 | 2.16 | * |
| Ca | 1.21 | 1.82 | * |
| Mg | 0.25 | 0.57 | * |
| S | 0.14 | 0.16 | * |
| Cu | 8.60 | 7.00 | n.s. |
| Zn | 25.20 | 23.40 | n.s. |
| Fe | 91.20 | 165.80 | * |
| Mn | 161.40 | 383.00 | * |
| Al | 61.60 | 83.40 | n.s. |
The seed rain in the plantation of T. micrantha included 34 seeds of six different species, corresponding to 23.1 seeds m-2 year-1. Under the S. molle plantation, a total of five seeds of four different species were collected, which accounts for 3.4 seeds m-2 year-1 (Tab. 5).
Tab. 5 - Seed rain under the plantations of Trema micrantha and Schinus molle. The seeds of T. micrantha and S. molle were excluded.
| Species | T. micrantha | S. molle |
|---|---|---|
| Schinus terebinthifolius | 25 | 1 |
| Cupania vernalis | 5 | - |
| Sebastiana commersoniana | 1 | 1 |
| Ilex paraguariensis | 1 | - |
| Celtis iguanae | 1 | - |
| Trichilia cf. elegans | 1 | - |
| Zanthoxylum sp. | - | 2 |
| Unidentified | - | 1 |
| Total | 34 | 5 |
Sebastiana commersoniana (Baill.) Smith & Downs and Celtis iguanae (Jacq.) Sarg. were observed among collected seeds, but no individuals of these species were found among saplings. Data on seed rain in the spontaneous vegetation were not available.
Discussion
The spontaneous succession and the plantation of T. micrantha promoted highest ligneous species diversity. However, the floristic composition was clearly different among them. The proportion of secondary and late successional species was higher under T. micrantha, which probably is related to more shaded conditions. Regeneration under T. micrantha indicates facilitation to species such as Ocotea puberula and Trichilia elegans, while an inhibition effect should be considered for others, e.g., Matayba eleagnoides, Solanum mauritianum and Baccharis dracunculifolia. Our results are in contrast with those from Butler et al. ([4]), who reported a systematic lower diversity in the spontaneous regeneration compared to plantations. A possible explanation may lie in the former land use of the study areas. In our investigation, the area with natural regeneration was a cattle pasture with low animal densities, while in the study of Butler et al. the land activity was rice production, followed by cattle pastures. Past land use is decisive to the quality and rate of regeneration ([37]).
The presence of exotic species did not differentiate the areas, and could be considered low, as only two individuals (of two different species) were observed.
The soil chemical profiles indicate that plantations present lower levels of important nutrients in the topsoil, for instance Ca, Mg and S, and also lower levels of V% and CEC. On the other hand, they had higher levels of P, which could be due to fertilizer addition at the moment of planting. Lower levels of Ca and Mg in the topsoil under plantations (and V% in the case of TM) could be due to the movement of the bases from soil to the aboveground biomass due to fast growth of the plantations.
In early stages of the secondary succession, sites with higher fertility can exhibit higher diversity ([13]). However, lower levels of nutrients only hardly could explain a lower diversity under S. molle, which had lower diversity and density of woody recruits than T. micrantha plantation, in spite of equivalent levels of nutrients in the topsoil (except for P, which was larger in S. molle sites).
Moreover, the diversity tends to increase along the secondary succession, in spite of reduction of nutrients in the topsoil ([10]). In tropical mature forests, differences in floristic composition and diversity could be related to soil fertility, with a negative correlation between diversity and nutrient levels ([18], [27]).
The light intensity under S. molle was intermediate, so that factor was probably not the cause of its lower diversity and abundance of saplings. Two alternative explanations to the low diversity and abundance of woody species could be invoked, namely: the differences in the seed rain and the existence of allelopathy. Total number of propagules in seed rain was more than six times higher under T. micrantha than under S. molle, which partially could have contributed to the higher diversity in the first.
Low diversity under S. molle could also be related to allelopathy. S. molle presents high contents of allelochemicals with noteworthy biological activities ([14]) and in vitro allelopathy ([23], [39]). The increment in fertility combined with inhibitory effects (and low plant diversity) was also observed under plantations of Pinus taeda L. and Eucalyptus viminalis Labill. ([16]). Notwithstanding, allelopathic effects in the field are a matter of controversy, and usually difficult to be distinguished from other mechanisms of negative interference, such as competition for nutrients and water ([32]). New experimental research on the allelopathic potential of fast-growth pioneer tree species is decisive to understand the secondary succession and to the perspective of tree plantations as a strategy to improve forest restoration.
The seed rain intensity was higher under T. micrantha plantation, which demonstrates that species can differ in their attractiveness to the seed dispersers. The zoochory was the predominant mechanism in regeneration of the experimental area (79% of the species), slightly larger to other reports from Seasonal Semi-deciduous Forests from southern Brazil (63-77 % - [25], [15], [33]). Nevertheless, the observed rate of 23 seeds m-2 year-1 can be considered low. In the same ecoregion, perches installed in an abandoned pasture presented 106 seeds m-2 year-1 ([29]). In other ecoregion of the Atlantic Forest, a seed rain intensity of 181 seeds m-2 year-1 was observed under perches ([38]).
As a low-cost alternative to restoration, spontaneous secondary succession could be recommended to sites without hampering factors, such as weed invasion or absence of seeds source. On the other hand, the use of pioneer tree species such as T. micrantha could facilitate the establishment of other tree species, especially the late successional ones. Whenever possible, the combination of plantation with facilitation properties and abandonment, side by side, could promote higher diversity in degraded areas.
Conclusions
The diversity of woody plants under the plantations of S. molle was lower than spontaneous regeneration and under T. micrantha plantation, which configures an inhibition.
T. micrantha could favor the recruitment of certain late successional woody species, and its plantation could contribute to accelerate the secondary succession.
T. micrantha present higher values of litterfall and mineral content in the leaves when compared with S. molle, but the topsoil under T. micrantha present lower levels of P, indicating that the process of transference of mineral nutrients from the soil to the aboveground biomass could overcome the return via litterfall.
T. micrantha presents higher values of seed rain of woody species when compared with S. molle.
Acknowledgements
César O. Sartori and Jorge Schirmer (IRDeR, UNIJUÍ) helped with logistical support. Mara Rejane Ritter and Angelo Schneider (UFRGS) collaborated with taxonomic assistance. Universidade Regional do Noroeste do Estado do Rio Grande do Sul - UNIJUÍ provided work support and infrastructure to the investigation activities of the authors. Universidade Federal do Rio Grande do Sul - UFRGS helped with the chemical analyses. Andressa P. Felipin has reviewed the English.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
UNIJUÍ, Rua do Comércio 3000, Universitário, 98700 000, Ijuí, Rio Grande do Sul (Brazil)
Universidade Federal da Fronteira Sul, Av. Presidente Getúlio Vargas 609-N, 2° andar, Centro, 89812-000, Chapecó, Santa Catarina (Brazil)
Corresponding author
Paper Info
Citation
Trindade DFV, Coelho GC (2012). Woody species recruitment under monospecific plantations of pioneer trees - facilitation or inhibition?. iForest 5: 1-5. - doi: 10.3832/ifor0601-009
Academic Editor
Roberto Tognetti
Paper history
Received: Sep 01, 2011
Accepted: Nov 28, 2011
First online: Feb 06, 2012
Publication Date: Feb 27, 2012
Publication Time: 2.33 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2012
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 56639
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 47666
Abstract Page Views: 3388
PDF Downloads: 4156
Citation/Reference Downloads: 20
XML Downloads: 1409
Web Metrics
Days since publication: 5101
Overall contacts: 56639
Avg. contacts per week: 77.72
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2012): 7
Average cites per year: 0.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Effect of restoration methods on natural regeneration in the Brazilian Atlantic Forest
vol. 18, pp. 23-29 (online: 15 February 2025)
Research Articles
Rewilding beech-dominated temperate forest ecosystems: effects on carbon stocks and biodiversity indicators
vol. 18, pp. 1-9 (online: 02 February 2025)
Research Articles
Can the dynamics of forest restoration reduce landscape fragmentation in the Atlantic forest?
vol. 18, pp. 61-68 (online: 04 April 2025)
Research Articles
The effects of post-pasture woody plant colonization on soil and aboveground litter carbon and nitrogen along a bioclimatic transect
vol. 6, pp. 238-246 (online: 13 June 2013)
Research Articles
First results on early post-fire succession in an Abies cephalonica forest (Parnitha National Park, Greece)
vol. 5, pp. 6-12 (online: 06 February 2012)
Research Articles
Net ecosystem production of a tropical secondary forest in Jengka, Pahang, Malaysia
vol. 18, pp. 54-60 (online: 04 April 2025)
Research Articles
Scale dependency of the effects of landscape structure and stand age on species richness and aboveground biomass of tropical dry forests
vol. 16, pp. 234-242 (online: 23 August 2023)
Review Papers
Shaping the multifunctional tree: the use of Salicaceae in environmental restoration
vol. 6, pp. 37-47 (online: 21 January 2013)
Research Articles
Outplanting performance of three provenances of Quillaja saponaria Mol. established in a Mediterranean drought-prone site and grown in different container size
vol. 13, pp. 33-40 (online: 21 January 2020)
Research Articles
Effects of water surplus over a secondary forest remain after 16 years in the Amazonia
vol. 18, pp. 234-241 (online: 06 September 2025)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword