Effects of abiotic stress on gene transcription in European beech: ozone affects ethylene biosynthesis in saplings of Fagus sylvatica L.
iForest - Biogeosciences and Forestry, Volume 2, Issue 3, Pages 114-118 (2009)
doi: https://doi.org/10.3832/ifor0495-002
Published: Jun 10, 2009 - Copyright © 2009 SISEF
Research Articles
Collection/Special Issue: COST Action E52 Meeting 2008 - Florence (Italy)
Evaluation of beech genetic resources for sustainable forestry
Guest Editors: Georg von Wühlisch, Raffaello Giannini
Abstract
The influence of ozone (150-190 nl L-1; 8h/d) on transcription levels of genes involved in the biosynthesis of the stress hormone ethylene, and its precursor 1-aminocyclopropane-1-carboxylate (ACC), was analysed in leaves of European beech saplings. Ozone-induced leaf lesions appeared 7 weeks after onset of ozone exposure. Cell lesion formation was preceded by persistent increases in ethylene emission, in the level of its malonylated precursor ACC, and in the transcript levels of specific ACC synthase 1 (ACS1), ACS2, ACC oxidase 1 (ACO1), and ACO2. Our results demonstrate that mechanisms similar to those operating in herbaceous plants may determine beech saplings responses to ozone exposure.
Keywords
Abiotic stress, Ethylene biosynthesis, Fagus sylvatica, Gene expression, Ozone
Introduction
European beech is the most abundant broadleaf tree in Germany ([25]). It is also of major importance for the European forest industry and was therefore chosen as an experimental species for our studies, to obtain deeper molecular insights into the potentially detrimental effects of ozone on European broadleaf forest ecosystems. Plants have long been known to exhibit responses to ozone exposure resembling the hypersensitive response following pathogen attack. Ozone has been qualified as an abiotic elicitor of plant defence reactions ([23]). These responses include phytoalexin production and synthesis of pathogenesis-related (PR) proteins, accompanied by the formation of various aromatic metabolites like lignins and flavonoids ([12]).
Another well-known plant response to ozone exposure is the stimulation of ethylene biosynthesis ([27], [22], [10]). Activation of ethylene biosynthesis is one of the fastest ozone-dependent biochemical responses so far observed in herbaceous plants, occurring within 5 hours, or even more rapidly ([24], [17], [16]). This response was similarly rapid in birch ([28]) and in an ozone-sensitive poplar clone ([5]). ACC synthase (ACS) and ACC oxidase (ACO), the two enzymes of ethylene biosynthesis, are encoded by gene families in herbaceous plants; several ACS and ACO isoforms have been reported to be induced by ozone ([17], [16]). In ozone-sensitive as well as ozone-tolerant birch clones, ozone-induced accumulation of ACS and ACO transcripts was found ([28]). Similarly, ozone fumigation of beech saplings (180-200 nl L-1) showed enhanced transcript levels of ACS2 and ACO1 ([18]). We therefore extended our study by reporting the cloning of ACS2 and ACO2, and analysing over a period of 3 month an ozone-induced expression of the ethylene biosynthesis genes (ACS1, ACS2, ACO1, ACO2), the accumulation of the ethylene precursor free ACC and conjugated ACC, and the emission of the end product of this pathway, ethylene, using beech saplings.
Materials and methods
Plant material and growth conditions
Three-year-old European beech saplings (Fagus sylvatica L., provenance 81024; Schlegel Baumschulen, Riedlingen, Germany) were planted in 14-L pots filled with natural forest soil (site Höglwald, Bavaria, Germany - [2]). Saplings were maintained during winter under a wooden pergola. Before bud burst, the plants were treated with Promanal® (Neudorff, Emmerthal, Germany), to prevent insect infestation. In spring the 4-year-old saplings were transferred to climate-controlled cabinets (39 m2) of a greenhouse covered with UV-permeable glass sheets (⇒ http://www.helmholtz-muenchen.de/eus/index.php - [20], [1]). Each cabinet contained 8 tables (2 m2 per table) and 12 saplings were placed on a single table. The saplings were cultivated until full leaf development and were kept at a relative humidity of 70-80% and temperatures of 22-25 °C during day hours (6:00 to 20:00) and 17-20 °C during the night (20:00 to 6:00). At the beginning of June, saplings were exposed to ozone for 8 h/d (150 nl L-1) or pollutant-free air, and after 13 d the ozone concentration was increased to 190 nl L-1 for a further 70 d ([2]). For pollutant treatments ozone was added to conditioned air by mass flow controllers and injected into the cabinet by a fan (1180 m3 h-1). Additional 6 fans within the cabinet provided an even distribution of the pollutant. Ozone was generated by electrical discharge in dry oxygen (Fischer Ozon Generator 500, Neckenheim, Germany) and continuously monitored in the middle of the cabinet using a computer-controlled system and an UV-type ozone analyser CSI 3100 (Columbia Scientific Industries, USA). Ozone concentrations, temperature, humidity and light intensities were continuously measured and adjusted automatically to the climate setpoints by external shading, water nebulisation and aeration. Temperature and humidity were as described above, and no artificial light exposure was used. At sunny days maximum light intensities were 30 kLux. To minimize changes of ozone concentrations aeration was only opened at a temperature of 28 °C. In addition to the water nebulisation two tables were flooded with water up to 4 cm. Watering of saplings was carried out with deionized water using an automatically droplet watering system (250 ml/d/pot). No additional nutrient supply was carried out. Pest control against beech scale was carried out weekly by hand to spare beneficial insects like larvae of lacewings and hover flies. Thrips were fought off with predatory mites and predatory bugs (Sautter & Stepper, Ammerbuch, Germany). Two to three leaves were collected for RNA analysis from four ozone-treated and four control saplings at 15 different time points beginning at the onset of the ozone treatment (days: 0, 2, 6, 11, 16, 21, 27, 34, 41, 48, 55, 62, 69, 76, 83). The leaves were immediately frozen in liquid N2 and stored at -80 °C until further analyses.
Isolation of RNA and cDNA transcription
Total RNA, was isolated according to the protocol described by [9], treated with RQ1 DNAse (Promega, Mannheim, Germany) and quantified photometrically (NanoDrop system; Kisker, Steinfurt, Germany). For cDNA synthesis, 5 µg of total RNA (14.5 µL), 1 µL of dNTP (10 mM) and 1 µL of oligo-dT(15) primer (5 µg µL-1) was used for first-strand cDNA synthesis. Reverse transcription was carried out for 4 h at 42 °C using Superscript II reverse transcriptase, according to the manufacturer’s instructions (Invitrogen, Karlsruhe, Germany).
Cloning of homologous beech cDNA for ACS2 and ACO2
To obtain the putative cDNA sequence ACS2 from beech, degenerated primers were designed based on conserved coding regions identified from a multiple sequence alignment from different plants using MULTALIN ([4]). Eight identical PCR reactions (25 µL) were prepared containing 2.5 µL reaction buffer (10x), 1 µL MgCl2 (50 mM), 0.5 µL dNTP (10 mM), 0.5 µL (10 µM) of degenerate forward (5’-TCTTTCARGAYTATCATGGMTTGCC-3’) and reverse (5’-GGGVTTCCCTGGMYTYAGRGTYGGC-3’) primer, 0.2 µL cDNA, and 0.1 µL Taq Polymerase (Invitrogen). In the subsequent PCR reaction (1 cycle at 94 °C for 3 min; 35 cycles at 94 °C for 30 s; 45-60 °C for 50 s; 72 °C for 1 min), a temperature gradient was applied to optimise primer annealing. PCR products were cloned into pGEM-T Easy (Promega) and sequenced using an ABI3100 sequencer (Applied Biosystems, Darmstadt, Germany). The resulting cDNA sequence was published under the NCBI accession number AY705445. The cloned ACO2 (Acc. Nr. AM062963) was derived from a recently published ozone-induced cDNA library of beech leaves ([20]).
Quantitative real-time RT-PCR (qRT-PCR)
Tab. 1 - Sequences of primers (5’-3’) used for quantitative real-time RT-PCR.
| Gene | Acc. nr. | Forward primer | Reverse primer |
|---|---|---|---|
| ACS1 | AJ420188 | GGTCTTCACTGAAGGTCTTG | CTGCTCTTTGAGTAGGTGAC |
| ACS2 | AY705445 | TCCAAACCGCATAGTCATGG | TGGTGAGGGCACAAGAAAAG |
| ACO1 | AJ420190 | CTGGTGGGATCATCTTACTC | CAATAGAATGGCGCATAGGG |
| ACO2 | AM062963 | GAAGGAAGTGTGACATAGCT | GAAAACCCTT CTTCTTGTAG |
| 26S rRNA | - | CGGCTCTTCCTATCATTGTG | AACCTGTCTCACGACGGTCT |
qRT-PCR was performed in a volume of 12.5 µL of SYBR Green ROX kit (ABgene, Hamburg, Germany), 0.5 µL gene-specific forward primer (10 µM), 0.5 µL gene-specific reverse primer (10 µM - Tab. 1), 0.5 µL cDNA and 11 µL H2O, using an ABIPrism 7700 Sequence Detector (Applied Biosystems, Darmstadt, Germany). The PCR conditions were as follows: 1 cycle at 50 °C for 2 min and at 95 °C for 10 min; 40 cycles at 95 °C for 15 sec and at 60 °C for 1 min ([1]). As an internal reference, 26S rRNA was used, and the relative expression ratio (R) was calculated according to the “Delta-delta” method (eqn. 1):
Determination of ethylene emission and concentrations of ACC
For ethylene determination, single fresh leaves from 12 saplings were analysed directly upon harvest (days: 1, 3, 6, 15, 20, 24, 29, 35, 42, 49, 65, 63, 70, 77, 84) as described previously ([18]) and determination of free and conjugated ACC from leaf material of 9 pooled saplings (days: 0, 2, 6, 11, 16, 21, 27, 34, 41, 48, 55, 62, 59, 76, 83) was performed according to Tuomainen et al. ([27]).
Results
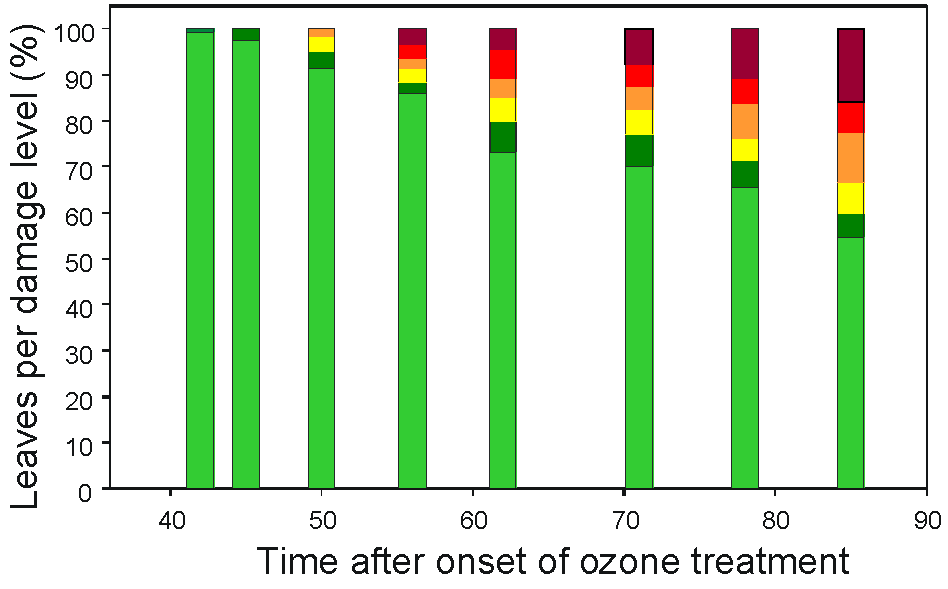
Treatment of beech saplings with ozone (150-190 nl L-1) led to visible leaf symptoms that were classified into six symptom levels (Fig. 1, Tab. 2). Lesions occurred on the upper leaf surface 42-49 d after the onset of treatment (Fig. 2). At the end of the experiment, ozone-treated saplings started to shed the leaves. Nine out of the 12 treated saplings developed severe symptoms, whereas control saplings did not show any damage. Ethylene emission is a frequent ozone response in plants, and it correlates with leaf injury and cell death ([16], [18]). To analyse details of ozone-induced ethylene biosynthesis in our experiment, we started the analysis using clones putatively assigned to ACS2 and ACO2. Pairwise comparison of ACS2 with nine ACS isoforms of tomato showed an identity of only 70%. However, CLUSTALW analysis with a homologous region of 600 bp indicated 82% identity with ACS2 from birch, but only 70% with ACS1 from the same species ([28]). The cloned ACO2 from beech showed about 97% identity to the 3’-open reading frame of ACO1 from beech ([3]). ACO2 had a 73 bp insertion in the C-terminal coding region compared to ACO1, which resulted in a protein sequence extension of 12 amino acids. This allowed us to design a specific primer for ACO2 for qRT-PCR studies. For the known ACO1 of beech we did not find such isoform specificity. Therefore, we could not exclude the possibility that the expression data for ACO1 may mirror the more strongly expressed ACO2.
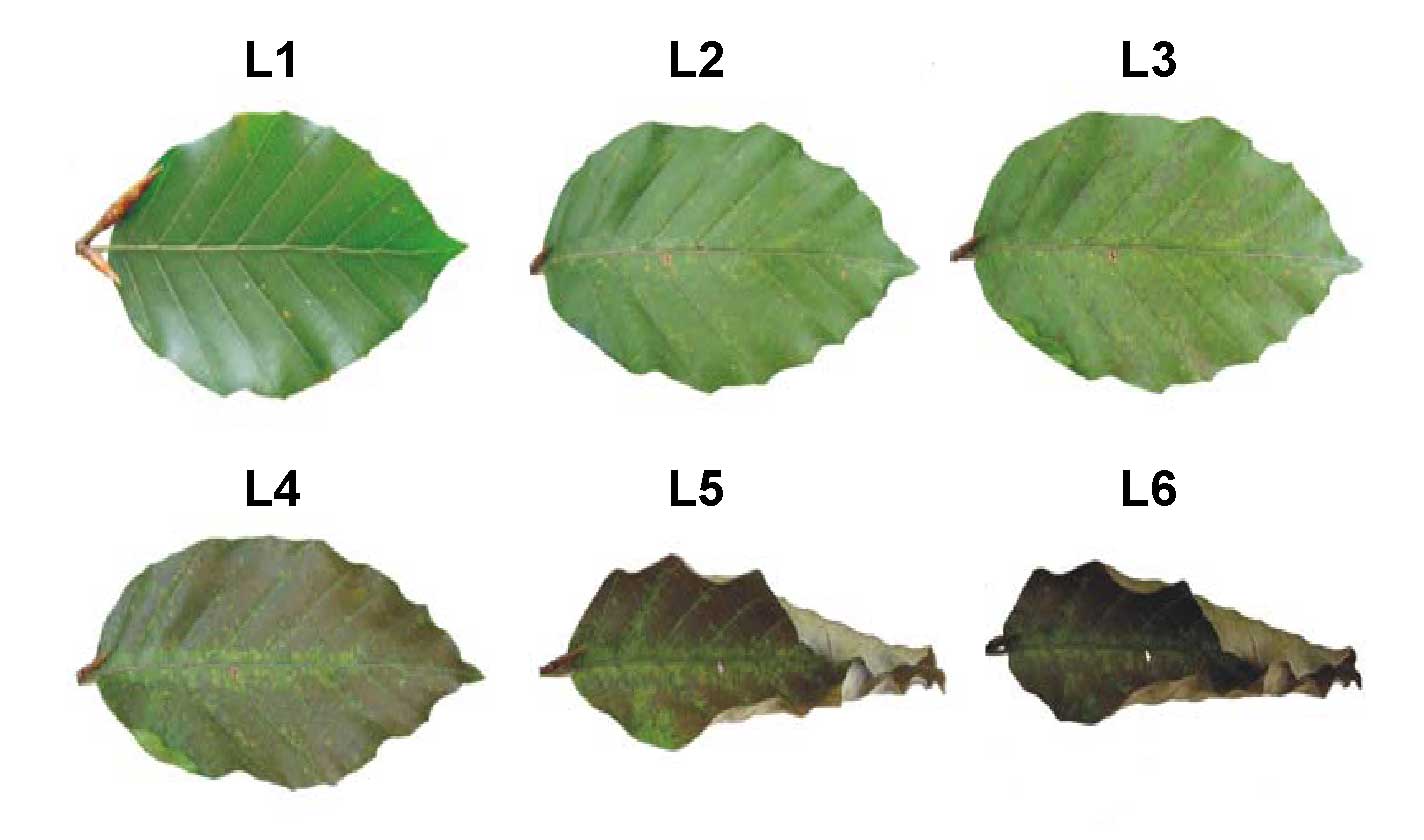
Fig. 1 - Ozone-induced leaf lesions in leaves of European beech saplings. Classification is as in Tab. 2.
Tab. 2 - Classification of ozone symptoms in leaves of European beech saplings.
| Level | Symptoms |
|---|---|
| L1 | No visible lesions |
| L2 | Single punctual lesions |
| L3 | Many small lesions all over the lamina |
| L4 | About 50% lesions of the lamina |
| L5 | About 70% lesions of the lamina (leaves started to roll up) |
| L6 | More then 90% lesions of the lamina (leaves were shed) |
Fig. 2 - Percentage of leaves with ozone-induced lesions corresponding to the classification in Tab. 2 (L1: light green; L2: dark green; L3: yellow; L4: orange; L5: red; L6: dark red).
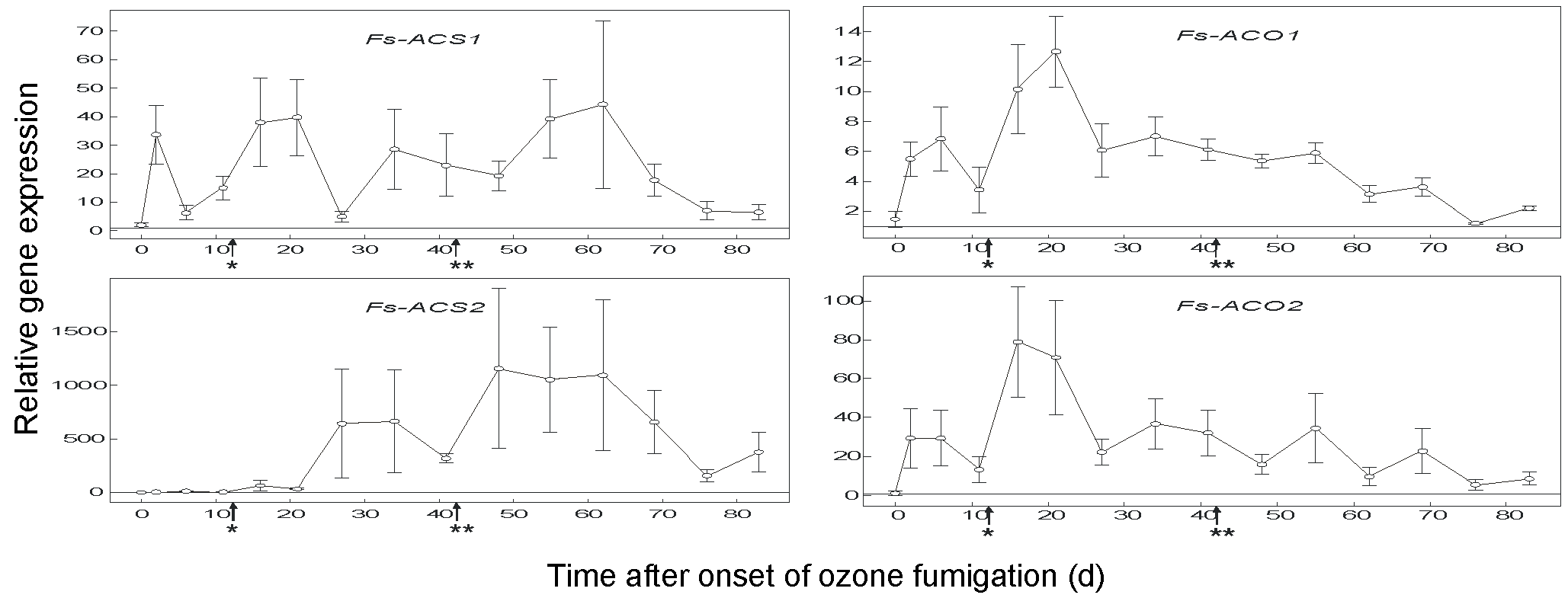
ACS1 expression increased with the onset of ozone fumigation, then decreased, and increased again with increasing ozone concentration from 150 nL L-1 to 190 nL L-1 (Fig. 3). ACS2 showed the highest expression level of all the genes analysed in this study, about 20 orders of magnitude higher then ACS1 (Fig. 3 - please note the different graduation for the relative gene expression charts). ACO1 showed the lowest induction rate of all the genes related to ethylene biosynthesis, and a second increase was observed for ACO1 and ACO2 after the ozone concentration was increased (Fig. 3). The time-dependence of the expression levels of ACO2 and ACO1 were comparable, although the ACO2 levels were much higher (Fig. 3). In contrast to what was observed with ACS isoforms, levels of ACO isoform transcripts did not increase when the macroscopic lesions appeared (Fig. 3).
Fig. 3 - Quantitative real-time RT-PCR analysis of ACS1, ACS2, ACO1 and ACO2 gene transcripts in total RNA isolated from ozone-treated leaves of European beech. 4-year-old European beech saplings were fumigated with ozone (150-190 nl L-1, 8 h/d, 83 d); n: 4 saplings, ± SEM; (*): increasing the ozone concentration from 150 nl L-1 to 190 nl L-1; (**): first ozone-induced leaf lesions.
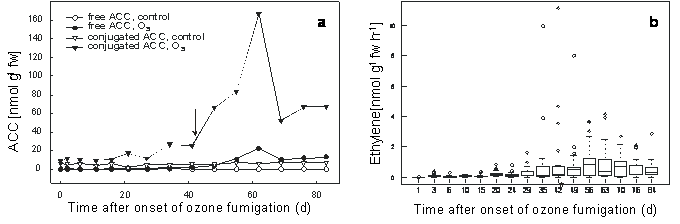
Free and conjugated ACC, the precursor molecules of ethylene, were regularly analysed at 15 time points throughout the 83 d ozone fumigation period (Fig. 4a). Leaf material of 9 ozone-treated and 9 control saplings were pooled and analysed. As the small saplings should not loss more then 1/10 of their leaves, which might mimic pest attack and no ozone effects, single sapling analyses could not be carried out. Levels of both metabolites increased in the course of fumigation, although to different extents. The highest concentration of free ACC was about 20 nmol g-1 fw, whereas conjugated ACC reached 175 nmol g-1 fw on day 62, and then decreased. Control values of free and conjugated ACC were below 0.56 and 6.9 nmol g-1 fw, respectively, throughout the experiment (Fig. 4a). Interestingly, conjugated ACC rapidly increased with the first appearance of macroscopic lesions (Fig. 4a).
Fig. 4 - a) Accumulation of free and conjugated ACC in leaves of control and ozone-treated 4-year-old European beech saplings (150-190 nl L-1, 8 h/d, 83 d); leaf material of 9 ozone-treated and 9 control saplings was pooled; ↓ = first ozone-induced leaf lesions. (b) Boxplot diagram of the mean ethylene emission of ozone-treated 4-year-old European beech saplings (150-190 nl L-1, 8 h/d, 83 d). The upper, middle and lower margins of the plot correspond to 75%, 50% and 25% percentiles of the monitoring; n: 12 saplings; (*): first ozone-induced leaf lesions.
Ethylene emission was analysed from freshly harvested leaves of 12 saplings. The absolute amount of ethylene produced as well as the time course of expression varied greatly between different saplings. Nevertheless, a box blot analysis revealed an apparent correlation between ozone fumigation and ethylene emission (Fig. 4b).
Discussion
Young beech saplings are considered as relatively sensitive to the pollutant ozone, although genetic variabilities are known ([11], [6], [19], [1]). The ozone concentration used in this study resulted in an AOT40 value of about 96 μl L-1 h-1, which is comparable with previous studies ([18], [19]). However it has to kept in mind that a newly developed ozone flux model might be a better risk assessment approach for European forest trees ([14], [21]). There is some information indicating that ozone-induced leaf damage in beech saplings is linked to the formation of ethylene ([11], [18]). In the present study, we showed that the ozone-induced production of ethylene differed widely from tree to tree, but nevertheless could be correlated with the extent of leaf damage and the appearance of ozone symptoms 42 d after onset of ozone exposure (190 nl L-1 - Fig. 4b). Similar strong variation in ethylene formation and symptom development among individuals of a plant species has been reported in herbaceous plants ([15], [26]). However, in contrast to the rapid induction of ethylene production in herbaceous plants ([17], [16]), production was remarkably delayed in beech and displayed a markedly longer persistence (Fig. 4b). This is consistent with findings recently published by other investigators ([18]).
Previous studies with herbaceous plants have shown that the levels of free and conjugated ACC increase upon ozone exposure ([16]). Moreover, in 60-year-old beech trees exposed to two times the ambient ozone levels, conjugated ACC was induced only in shade leaves, but not sun leaves ([18]). In the present greenhouse study with 4-year-old beech saplings, the maximum levels of free and conjugated ACC, as well as maximal ethylene production, occurred about 9 weeks after the onset of ozone exposure (190 nl L-1; 8 h/d - Fig. 4). This delayed increase in ACC may be explained by the fact that beech is better equipped to detoxify ozone than the herbaceous tomato plant ([27], [16]). As the leaves of young beech saplings more closely resemble the sun leaves rather than the shade leaves of a mature tree, this difference in response may reflect an ontogenetic effect. There is also increasing evidence that juvenile trees may differ in physiology and, therefore, in ozone responses compared to mature trees ([13], [19]).
Previous studies with herbaceous plants have also shown that ozone-induced ethylene emission is regulated by differential transcription of the ACS and ACO genes ([27], [16]). We have analysed the transcript levels of two previously known genes (ACS1, ACO1), as well as two genes (ACS2, ACO2) cloned here for the first time. Up-regulation of ACS2 transcription was delayed compared to that of ACS1. However, the relative gene expression was more than 1000-fold and was the highest of all transcripts analysed in this study (Fig. 3). A delayed up-regulation of ACS2 compared to ACS1 has also been shown in tomato after a single ozone pulse ([17]). Tomato ACS1, however, was much more strongly induced compared to ACS2 ([17]). Interestingly, ACS2 was also up-regulated in sun leaves of adult European beech in an open-air fumigation site in the presence of ozone at two times the ambient level ([8]). In a previous study with beech saplings, ACS2 was up-regulated by a factor of only 150 when exposed to ozone at 200 nL L-1 from September to October ([18]). The reason for this difference in induction factor is not obvious. As the present study was carried out from June to September, it may indicate the influence of light conditions, including UV radiation, which have not been considered so far. In 1-year-old clonal birch plants, ACS1 was induced in all clones irrespective of ozone tolerance or sensitivity, whereas ACS2 was induced exclusively in sensitive clones ([28]). Thus, 4-year-old beech saplings can be classified as ozone-sensitive. This idea is further supported by the absence of free SA in sensitive birch clones ([28]), as well as in beech saplings ([2]). ACO1 and ACO2 were strongly induced at the onset of ozone treatment (150-190 nL L-1), but decreased upon continuous exposure (Fig. 3). Ozone induction of ACO1 and ACO2 transcripts has also been reported recently in leaves of 3-year-old beech saplings ([20], [18]) and in moderately ozone-tolerant aspen clones in a free-air ozone fumigation study ([7]). Interestingly, ACO1 and ACO2 were much more strongly induced in ozone-sensitive than in ozone-resistant birch clones, again favouring the notion that beech saplings are ozone-sensitive. As for leaf injury and ethylene production, the relative gene expression levels among individual saplings showed pronounced variation.
Conclusion
The observed differential induction of ACS and ACO isoforms, the accumulation of ACC, and the production of ethylene strongly support the idea that ethylene triggers development of the leaf lesions related to ozone-induced cell death. These findings further support the recently postulated idea that ethylene plays an important role in cell death in leaves of both saplings ([28]) and adult trees ([18]).
Acknowledgements
Excellent technical assistance was provided by Lucia Gößl and Rosina Ludwig. We wish to thank the technical staff of EUS for performing the ozone fumigation. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 607) and in part by the European Community (EVOLTREE, 6th Framework Programme and COST E52 Action).
References
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
E Gerstner
M Olbrich
C Langebartels
W Heller
H Sandermann
D Ernst
Institute of Biochemical Plant Pathology “Helmholtz Zentrum München, German Research Center for Environmental Health, D-85764 Neuherberg (Germany)
Olympus Life Science Research Europa GmbH, Sauerbruchstraße 50, D-81377 München (Germany)
Department of Environmental Engineering, Helmholtz Zentrum München - German Research Center for Environmental Health, D-85764 Neuherberg (Germany)
Ecotox Freiburg, Schubertstr. 1, D-79104 Freiburg (Germany)
Corresponding author
Paper Info
Citation
Betz GA, Gerstner E, Olbrich M, Winkler JB, Langebartels C, Heller W, Sandermann H, Ernst D (2009). Effects of abiotic stress on gene transcription in European beech: ozone affects ethylene biosynthesis in saplings of Fagus sylvatica L.. iForest 2: 114-118. - doi: 10.3832/ifor0495-002
Academic Editor
Marco Borghetti
Paper history
Received: Feb 04, 2009
Accepted: Mar 12, 2009
First online: Jun 10, 2009
Publication Date: Jun 10, 2009
Publication Time: 3.00 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2009
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 54575
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 45199
Abstract Page Views: 3477
PDF Downloads: 4568
Citation/Reference Downloads: 48
XML Downloads: 1283
Web Metrics
Days since publication: 6107
Overall contacts: 54575
Avg. contacts per week: 62.56
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2009): 4
Average cites per year: 0.24
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Changes in the proteome of juvenile European beech following three years exposure to free-air elevated ozone
vol. 4, pp. 69-76 (online: 05 April 2011)
Research Articles
A comparison between stomatal ozone uptake and AOT40 of deciduous trees in Japan
vol. 4, pp. 128-135 (online: 01 June 2011)
Research Articles
Ozone fumigation effects on the morphology and biomass of Norway spruce (Picea abies L.) saplings
vol. 2, pp. 15-18 (online: 21 January 2009)
Research Articles
Soil drench of ethylenediurea (EDU) protects sensitive trees from ozone injury
vol. 4, pp. 66-68 (online: 05 April 2011)
Research Articles
Prediction of ozone effects on net ecosystem production of Norway spruce forest
vol. 11, pp. 743-750 (online: 15 November 2018)
Research Articles
Ambient ozone phytotoxic potential over the Czech forests as assessed by AOT40
vol. 5, pp. 153-162 (online: 25 June 2012)
Short Communications
Ozone flux modelling for risk assessment: status and research needs
vol. 2, pp. 34-37 (online: 21 January 2009)
Review Papers
Monitoring the effects of air pollution on forest condition in Europe: is crown defoliation an adequate indicator?
vol. 3, pp. 86-88 (online: 15 July 2010)
Research Articles
Long-term monitoring of air pollution effects on selected forest ecosystems in the Bucegi-Piatra Craiului and Retezat Mountains, southern Carpathians (Romania)
vol. 4, pp. 49-60 (online: 05 April 2011)
Research Articles
Tracing the acclimation of European beech (Fagus sylvatica L.) populations to climatic stress by analyzing the antioxidant system
vol. 14, pp. 95-103 (online: 01 March 2021)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword