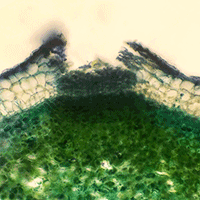
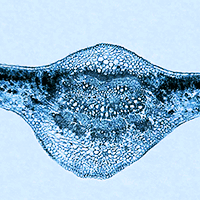
In light of the increase of environmental pollution, we tested the effect of cadmium (Cd) and lead (Pb) soil contamination on ash dieback. The experiment included the inoculation of Hymenoscyphus fraxineus on Fraxinus excelsior and Fraxinus angustifolia seedlings growing on unpolluted soil, soil contaminated with cadmium (Cd), and soil contaminated with lead (Pb). At the end of the experiment, 173 days after soil contamination and 50 days since inoculation, all F. excelsior and F. angustifolia seedlings inoculated with H. fraxineus showed ash dieback symptoms in comparison to their control groups. However, both F. excelsior and F. angustifolia seedlings grown on contaminated soil had significantly increased necrotic lesions in comparison to the seedlings grown on uncontaminated soil. Our results showed for the first time that cadmium (Cd) and lead (Pb) soil contamination can significantly contribute to ash dieback and increase damage to F. excelsior and F. angustifolia seedlings.
Keywords
, , ,
Citation
Vemić A, Popović V, Miletić Z, Radulović Z, Rakonjac L, Lučić A (2023). Effect of cadmium (Cd) and lead (Pb) soil contamination on the development of Hymenoscyphus fraxineus on Fraxinus excelsior and F. angustifolia seedlings. iForest 16: 307-313. - doi: 10.3832/ifor4322-016
Academic Editor
Daniela Baldantoni
Paper history
Received: Feb 06, 2023
Accepted: Sep 11, 2023
First online: Nov 09, 2023
Publication Date: Dec 31, 2023
Publication Time: 1.97 months
© SISEF - The Italian Society of Silviculture and Forest Ecology 2023
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.

Breakdown by View Type
(Waiting for server response...)
Article Usage
Total Article Views: 16504
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 12583
Abstract Page Views: 2270
PDF Downloads: 1308
Citation/Reference Downloads: 2
XML Downloads: 341
Web Metrics
Days since publication: 812
Overall contacts: 16504
Avg. contacts per week: 142.28
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2023): 1
Average cites per year: 0.33
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
(1)
Adamčikova K, Paitny J, Pastirčáková K (2018)Individual resistance of
Fraxinus angustifolia and
F. excelsior clones to
Hymenoscyphus fraxineus. Journal of Plant Protection Research 58 (3): 227-233.
Gscholar
(2)
Aksoy A, Demirezen D (2006)Fraxinus excelsior as a biomonitor of heavy metal pollution. Polish Journal of Environmental Studies 15 (1): 27-33.
Online |
Gscholar
(3)
Bakys R, Vasaitis R, Skovsgaard JP (2009)Patterns and severity of crown dieback in young even-aged stands of European ash (
Fraxinus excelsior L.) in relation to stand density, bud flushing phenotype, and season. Plant Protection Science 49 (3): 120-126.
CrossRef |
Gscholar
(4)
Baxter E, Cooke LR, Spaans F, Grant IR, McCracken AR (2023)The introduction of
Hymenoscyphus fraxineus to Northern Ireland and the subsequent development of ash dieback. Forest Pathology 53 (1): e12789.
CrossRef |
Gscholar
(5)
Bengtsson SBK, Barklund Von P B, Stenlind J (2014)Seasonal pattern of lesion development in diseased
Fraxinus excelsior infected by
Hymenoscyphus pseudoalbidus. PLoS One 9 (4): e76429.
CrossRef |
Gscholar
(6)
Bommarez T, Cools N, De Vos B (2021)Heavy metals in forest floors and topsoils of ICP Forests Level I plots. Forest Soil Coordinating Centre of ICP Forests, Report of the Research Institute for Nature and Forest 2021 (5), Research Institute for Nature and Forest, Brussels, Belgium, pp. 120.
Gscholar
(7)
Bouida L, Rafatullah M, Kerrouche A, Qutob M, Alosaimi AM, Alorfi HS, Hussein MA (2022)A review on cadmium and lead contamination: sources, fate, mechanism, health effects and remediation methods. Water 14 (21): 3432.
CrossRef |
Gscholar
(8)
Chmielowska-Bak J, Deckert J (2021)Plant recovery after metal stress - A review. Plants 10 (3): 450.
CrossRef |
Gscholar
(9)
Coker TLR, Rozsypálek J, Edwards A, Harwood TP, Butfoy L, Buggs RJA (2019)Estimating mortality rates of European ash (
Fraxinus excelsior) under the ash dieback (
Hymenoscyphus fraxineus) epidemic. Plants People Planet 1 (1): 48-58.
CrossRef |
Gscholar
(10)
De Vries W, Bakker DJ (1998)Manual for calculating critical loads of heavy metals for terrestrial ecosystems. Guidelines for critical limits, calculation methods and input data, Report 166, DLO Winand Staring Centre, Wageningen, Netherlands, pp. 173.
Gscholar
(11)
Diminić D, Kajba D, Milotić M, Andrić I, Kranjec J (2017)Susceptibility of
Fraxinus angustifolia clones to
Hymenoscyphus fraxineus in lowland Croatia. Baltic Forestry 23 (1): 233-243.
Gscholar
(12)
Enderle R, Stenlid J, Vasaitis R (2019)An overview of ash (
Fraxinus spp.) and the ash dieback disease in Europe. CAB Reviews 14 (25): 1-12.
CrossRef |
Gscholar
(13)
Gajewska J, Floryszak-Wieczorek J, Sobieszczuk-Nowicka E, Matto A, Arasimowicz-Jelonek M (2022)Fungal and oomycete pathogens and heavy metals: an inglorious couple in the environment. IMA Fungus 13 (6): 1-20.
CrossRef |
Gscholar
(14)
Grosdidier M, Ioos R, Marçais B (2018)Do higher summer temperatures restrict the dissemination of
Hymenoscyphus fraxineus in France? Forest Pathology 48 (4): e12426.
CrossRef |
Gscholar
(15)
Gross A, Holdenrieder O, Pautasso M, Queloz V, Sieber TN (2014)Hymenoscyphus pseudoalbidus, the causal agent of European ash dieback. Molecular Plant Pathology 15 (1): 5-21.
CrossRef |
Gscholar
(16)
Hauptman T, Ogris N, De Groot M, Piškur B, Jurc D (2016)Individual resistance of
Fraxinus angustifolia clones to ash dieback. Forest Pathology 46 (4): 269-280.
CrossRef |
Gscholar
(17)
Havrdová L, Zahradník D, Romportl D, Pešková V, Cerny K (2017)Environmental and silvicultural characteristics influencing the extent of ash dieback in forest stands. Baltic Forestry 23 (1): 168-182.
Gscholar
(18)
Heinze B, Tiefenbacher H, Litschauer R, Kirisits T (2017)Ash dieback in Austria - history, current situation and outlook. In: “Dieback of European ash (
Fraxinus spp.) - Consequences and Guidelines for Sustainable Management” (Vasaitis R, Enderle R eds). Swedish University of Agricultural Sciences, Uppsala, Sweden, pp. 33-52.
Gscholar
(19)
Hietala AM, Borja I, Solheim H, Nagy NE, Timmermann V (2018)Propagule pressure build-up by the invasive
Hymenoscyphus fraxineus following its introduction to an ash forest inhabited by the native
Hymenoscyphus albidus. Frontiers in Plant Science 9: 374.
CrossRef |
Gscholar
(20)
Kiran X, Bharti R, Sharma R (2022)Effect of heavy metals: an overview. Materials Today: Proceedings 51 (1): 880-885.
CrossRef |
Gscholar
(21)
Kirisits T, Matlakova M, Mottinger-Kroupa S, Halmschlager E, Lakatos F (2010)Chalara fraxinea associated with dieback of narrow-leafed ash (
Fraxinus angustifolia). Plant Pathology 59 (2): 411-411.
CrossRef |
Gscholar
(22)
Kowalski T, Holdenrieder O (2009)Pathogenicity of
Chalara fraxinea. Forest Pathology 39 (1): 1-7.
CrossRef |
Gscholar
(23)
Lygis V, Prospero S, Burokiene D, Schoebel CN, Marciulyniene Norkute D G, Rigling D (2017)Virulence of invasive ash pathogen
Hymenoscyphus fraxineus in old and recently established populations. Plant Pathology 66 (5): 783-791.
CrossRef |
Gscholar
(24)
Marçais B, Husson C, Godart L, Caël O (2016)Influence of site and stand factors on
Hymenoscyphus fraxineus-induced basal lesions. Plant Pathology 65 (9): 1452-1461.
CrossRef |
Gscholar
(25)
Matisone I, Matisons R, Kenigsvalde K, Gaitnieks T, Burneviča N (2018)Seasonal development of lesions caused by
Hymenoscyphus fraxineus on young
Fraxinus excelsior trees in Latvia. iForest - Biogeosciences and Forestry 11 (1): 17-23.
CrossRef |
Gscholar
(26)
McKinney LV, Nielsen LR, Collinge DB, Thomsen IM, Hansen JK, Kjr ED (2014)The ash dieback crisis: genetic variation in resistance can prove a long-term solution. Plant Pathology 63 (3): 485-499.
CrossRef |
Gscholar
(27)
Mitchell R, Beaton J, Bellamy P, Broome A, Chetcuti J, Eaton S, Ellis C, Gimona A, Harmer R, Hester A, Hewison R, Hodgetts N, Iason G, Kerr G, Littlewood N, Newey S, Potts J, Pozsgai G, Ray D, Sim D, Stockan J, Taylor A, Woodward S (2014)Ash dieback in the UK: a review of the ecological and conservation implications and potential management options. Biological Conservation 175 (Suppl. 2): 95-109.
CrossRef |
Gscholar
(28)
Morkunas I, Wozniak A, Chung Mai V, Rucinska-Sobkowiak R, Jeandet P (2018)The role of heavy metals in plant response to biotic stress. Molecules 23 (9): 2320.
CrossRef |
Gscholar
(29)
Nas FS, Ali M (2018)The effect of lead on plants in terms of growing and biochemical parameters: a review. MOJ Ecology and Environmental Sciences 3 (4): 265-268.
Online |
Gscholar
(30)
Nawrot-Chorabik K, Sulkowska M, Osmenda M, Mohytych V, Surówka E, Latowski D (2022)The impact of biotic and abiotic stress factors on development of European ash tissue cultures. Forests 13 (1): 59.
CrossRef |
Gscholar
(31)
Niekerk LA, Carelse MF, Bakare OO, Mavumengwana V, Keyster M, Gokul A (2021)The relationship between cadmium toxicity and the modulation of epigenetic traits in plants. International Journal of Molecular Science 22 (13): 7046.
CrossRef |
Gscholar
(32)
Nielsen LR, McKinney LV, Hietala AM, Kjaer ED (2017)The susceptibility of Asian, European and North American
Fraxinus species to the ash dieback pathogen
Hymenoscyphus fraxineus reflects their phylogenetic history. European Journal of Forest Research 136 (1): 59-73.
CrossRef |
Gscholar
(33)
Noble R, Woodhall JW, Dobrovin-Pennington A, Perkins K, Somoza-Valdeolmillos E, Gómez HL, Lu Y, Macarthur R, Henry CM (2019)Control of
Hymenoscyphus fraxineus, the causal agent of ash dieback, using composting. Forest Pathology 49 (6): 429.
CrossRef |
Gscholar
(34)
RS Official Gazette (2019)Regulation on limit values of polluting, harmful, and dangerous substances in the soil. Official Gazette of the Republic of Serbia nos. 30/2018 and 64/2019, Belgrade, Serbia, pp. 10. [in Serbian].
Online |
Gscholar
(35)
Papić S, Buriánek V, Longauer R, Kudláček T, Rozsypálek J (2018)Phenotypic variability of
Fraxinus excelsior L. and
Fraxinus angustifolia Vahl under the ash dieback disease in the Czech Republic. Journal of Forest Science 64 (6): 279-288.
CrossRef |
Gscholar
(36)
Pastirčáková K, Adamčíková K, Barta M, Paitny J, Hotka P, Sarvašová I, Kádasi Horáková M (2020)Host Range of
Hymenoscyphus fraxineus in Slovak Arboreta. Forests 11 (5): 596.
CrossRef |
Gscholar
(37)
Pautasso M, Aas G, Queloz V, Holdenrieder O (2013)European ash (
Fraxinus excelsior) dieback - A conservation biology challenge. Biological conservation 158 (2): 37-49.
CrossRef |
Gscholar
(38)
Pliura A, Lygis V, Marčiulyniene D, Suchockas V, Bakys R (2015)Genetic variation of
Fraxinus excelsior half-sib families in response to ash dieback disease following simulated spring frost and summer drought treatments. iForest - Biogeosciences and Forestry 9 (1): 12-22.
CrossRef |
Gscholar
(39)
Schwanda K, Kirisits T (2016)Pathogenicity of
Hymenoscyphus fraxineus towards leaves of three European ash species:
Fraxinus excelsior, F. angustifolia and
F. ornus. Plant Pathology 65 (7): 1071-1083.
CrossRef |
Gscholar
(40)
Semizer-Cuming D, Finkeldey R, Rostgaard Nielsen R, Kjr ED (2019)Negative correlation between ash dieback susceptibility and reproductive success: good news for European ash forests. Annals of Forest Science 76 (1): 219.
CrossRef |
Gscholar
(41)
Sheikh-Assadi M, Khandan-Mirkohi A, Alemardan A, Moreno-Jiménez E (2015)Mycorrhizal
Limonium sinuatum (L.) Mill. enhances accumulation of lead and cadmium. International Journal of Phytoremediation 17 (6): 556-562.
CrossRef |
Gscholar
(42)
Skovsgaard JP, Thomsen IM, Skovsgaard IM, Martinussen T (2010)Associations among symptoms of dieback in even-aged stands of ash (
Fraxinus excelsior L.). Forest pathology 40 (1): 7-18.
CrossRef |
Gscholar
(43)
Skovsgaard JP, Wilhelm GJ, Thomsen IM, Metzler B, Kirisits T, Havrdová L, Enderle R, Dobrowolska D, Cleary M, Clark J (2017)Silvicultural strategies for
Fraxinus excelsior in response to dieback caused by
Hymenoscyphus fraxineus. Forestry 90 (4): 455-472.
CrossRef |
Gscholar
(44)
Tyler G (1992)Critical concentrations of heavy metals in the mor horizon of Swedish forests. Technical Report 4078, Swedish Environmental Protection Agency, Solna, Sweden, pp. 38.
Gscholar
(45)
Tóth G, Hermann T, Szatmári G, Pásztor L (2016)Maps of heavy metals in the soils of the European Union and proposed priority areas for detailed assessment. Science of the Total Environment 565 (15): 1054-1062.
CrossRef |
Gscholar
(46)
Vemić A, Kerkez-Janković I, Kudláček T, Jung T, Nikolić M, Nonić M, Milenković I (2021)Development of
Hymenoscyphus fraxineus on seedlings from different half-sib lines of
Fraxinus angustifolia in Serbia. Forest Pathology 51 (4): 227.
CrossRef |
Gscholar
(47)
Volke V, Knapp S, Roloff A (2019)Survey of
Hymenoscyphus fraxineus in a central European urban area and exploration of its possible environmental drivers. Urban Forestry and Urban Greening 40: 165-173.
CrossRef |
Gscholar
(48)
Yadov SK (2010)Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South African Journal of Botany 76 (2): 167-179.
CrossRef |
Gscholar