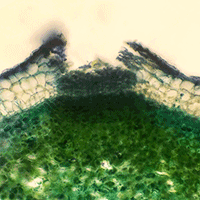
Common ash (Faxinus excelsior L.) in Europe is declining on a continental scale due to the action of Hymenoscyphus fraxineus, an invasive forest pathogen that causes ash dieback disease leading to the collapse and eventual death of ash trees through shoot infection in the crown and through stem collar infection. This study confirms for the first time lenticels as entry points for pathogens to enter shoot bark. Results show the impact of lenticel infection at a very early stage of invasion by H. fraxineus in a F. excelsior provenance trial and its correlation values with other factors such as shoot dieback, canker-like lesions and bud burst. No significant provenance effects were observed for incidence of shoot dieback, lenticel necrosis or canker-like lesions on shoots, but provenance effects were significant for bud burst phenology. The strongest correlation was observed between lenticel necrosis and canker-like lesions on the lenticels of shoots. Boheremia spp. were most frequently isolated from necrotic ash lenticels and confirmed by ITS sequencing, but also species of Diaporthe, Epicoccum, Aspergillus, Neonectria, Didymella and Hymenoscyphus fraxineus. Finally, lenticel density was similar in sets of ash genotypes that were characterized as having a high and low susceptibility to ash dieback.
Keywords
, , ,
Citation
Nemesio-Gorriz M, McGuinness B, Grant J, Dowd L, Douglas GC (2019). Lenticel infection in Fraxinus excelsior shoots in the context of ash dieback. iForest 12: 160-165. - doi: 10.3832/ifor2897-012
Academic Editor
Alberto Santini
Paper history
Received: Jun 15, 2018
Accepted: Jan 12, 2019
First online: Mar 04, 2019
Publication Date: Apr 30, 2019
Publication Time: 1.70 months
© SISEF - The Italian Society of Silviculture and Forest Ecology 2019
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.

Breakdown by View Type
(Waiting for server response...)
Article Usage
Total Article Views: 49094
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 39892
Abstract Page Views: 4628
PDF Downloads: 3622
Citation/Reference Downloads: 22
XML Downloads: 930
Web Metrics
Days since publication: 2544
Overall contacts: 49094
Avg. contacts per week: 135.09
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2019): 21
Average cites per year: 3.00
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
(1)
Bakys R, Vasaitis R, Barklund P, Ihrmark K, Stenlid J (2009)Investigations concerning the role of
Chalara fraxinea in declining
Fraxinus excelsior. Plant Pathology 58 (2): 284-292.
CrossRef |
Gscholar
(2)
Bakys R, Vasaitis R, Skovsgaard JP (2013)Patterns and severity of crown dieback in young even-aged stands of European ash (
Fraxinus excelsior L.) in relation to stand density, bud flushing phenotype, and season. Plant Protection Science 49 (3): 120-126.
CrossRef |
Gscholar
(3)
Baral HO, Queloz V, Hosoya T (2014)Hymenoscyphus fraxineus, the correct scientific name for the fungus causing ash dieback in Europe. IMA Fungus 5 (1): 79-80.
CrossRef |
Gscholar
(4)
Botella L, Cermáková V, Bačová A, Dvorák M (2016)ADA, a fast-growth medium for
Hymenoscyphus fraxineus. Forest Pathology 46 (1): 85-87.
CrossRef |
Gscholar
(5)
Chandelier A, Helson M, Dvorak M, Gischer F (2014)Detection and quantification of airborne inoculum of
Hymenoscyphus pseudoalbidus using real-time PCR assays. Plant Pathology 63 (6): 1296-1305.
CrossRef |
Gscholar
(6)
Chandelier A, Gerarts F, San Martin G, Herman M, Delahaye L (2016)Temporal evolution of collar lesions associated with ash dieback and the occurrence of
Armillaria in Belgian forests. Forest Pathology 46 (4): 289-297.
CrossRef |
Gscholar
(7)
Enderle R, Peters F, Nakou A, Metzler B (2013)Temporal development of ash dieback symptoms and spatial distribution of collar rots in a provenance trial of
Fraxinus excelsior. European Journal of Forest Research 132 (5-6): 865-876.
CrossRef |
Gscholar
(8)
Enderle R, Sander F, Metzler B (2017a)Temporal development of collar necroses and butt rot in association with ash dieback. iForest - Biogeosciences and Forestry 10: 529-536.
CrossRef |
Gscholar
(9)
Enderle R, Busskamp J, Metzler B (2017b)Growth performance of dense natural regeneration of
Fraxinus excelsior under attack of the ash dieback agent
Hymenoscyphus fraxineus. Baltic Forestry 23 (1): 218-228.
Online |
Gscholar
(10)
Flack NJ, Swinburne TR (1977)Host range of
Nectria galligena Bres. and the pathogenicity of some Northern Ireland isolates. Transactions of the British Mycological Society 68 (2): 185-192.
CrossRef |
Gscholar
(11)
Garbelotto M (2004)Sudden oak death: a tale of two continents. Outlooks on pest management 15 (2): 85.
CrossRef |
Gscholar
(12)
Griffith GS, Boddy L (1988)Fungal communities in attached ash (
Fraxinus excelsior) twigs. Transactions of the British Mycological Society 91: 599-606.
CrossRef |
Gscholar
(13)
Gross A, Holdenrieder O, Pautasso M, Queloz V, Sieber TN (2014)Hymenoscyphus pseudoalbidus, the causal agent of European ash dieback. Molecular Plant Pathology 15 (1): 5-21.
CrossRef |
Gscholar
(14)
Hauptman T, Ogris N, Groot M, Piškur B, Jurc D (2016)Individual resistance of
Fraxinus angustifolia clones to ash dieback. Forest Pathology 46 (4): 269-280.
CrossRef |
Gscholar
(15)
Havrdová L, Novotná K, Zahradník D, Buriánek V, Pešková V, Srutka P, Cerny K (2016)Differences in susceptibility to ash dieback in Czech provenances of
Fraxinus excelsior. Forest Pathology 46 (4): 281-288.
CrossRef |
Gscholar
(16)
Havrdová L, Zahradník D, Romportl D, Pešková V, Cerny K (2017)Environmental and silvicultural characteristics influencing the extent of ash dieback in forest stands. Baltic Forestry 23 (1): 168-182.
Online |
Gscholar
(17)
Hietala AM, Timmermann V, Borja I, Solheim H (2013)The invasive ash dieback pathogen
Hymenoscyphus pseudoalbidus exerts maximal infection pressure prior to the onset of host leaf senescence. Fungal Ecology 6 (4): 302-308.
CrossRef |
Gscholar
(18)
Husson C, Cael O, Grandjean JP, Nageleisen LM, Marcais B (2012)Occurrence of
Hymenoscyphus pseudoalbidus on infected ash logs. Plant Pathology 61 (5): 889-895.
CrossRef |
Gscholar
(19)
Janse J (1981)The bacterial disease of ash (
Fraxinus excelsior), caused by
Pseudomonas syringae subsp.
savastanoi pv.
fraxini. Forest Pathology, 11 (5-6), 306-315.
CrossRef |
Gscholar
(20)
Janse J (1982)Pseudomonas syringae subsp.
savastanoi (ex Smith) subsp. nov., nom. rev., the bacterium causing excrescences on Oleaceae and
Nerium oleander L. International Journal of Systematic and Evolutionary Microbiology 32 (2): 166-169.
CrossRef |
Gscholar
(21)
Jung T, Blaschke M (2004)Phytophthora root and collar rot of alders in Bavaria: distribution, modes of spread and possible management strategies. Plant Pathology 53 (2): 197-208.
CrossRef |
Gscholar
(22)
Kirisits T, Matlakova M, Mottinger-Kroupa S, Halmschlager E, Lakatos F (2010)Chalara fraxinea associated with dieback of narrow-leafed ash (
Fraxinus angustifolia). Plant Pathology 59 (2): 411-411.
CrossRef |
Gscholar
(23)
Kosawang C, Amby DB, Bussaban B, McKinney LV, Xu J, Kjr ED, Collinge DB, Nielsen LR (2018)Fungal communities associated with species of
Fraxinus tolerant to ash dieback, and their potential for biological control. Fungal Biology 122 (2-3): 110-120.
CrossRef |
Gscholar
(24)
Kowalski T (2001)O zamieraniu jesionów [Dieback of ash]. Trybuna Lesnika 4 (359): 6-7. [in Polish]
Gscholar
(25)
Kowalski T (2006)Chalara fraxinea sp. nov. associated with dieback of ash (
Fraxinus excelsior) in Poland. Forest Pathology 36 (4): 264-270.
CrossRef |
Gscholar
(26)
Kowalski T, Holdenrieder O (2009)Pathogenicity of
Chalara fraxinea. Forest Pathology 39 (1): 1-7.
CrossRef |
Gscholar
(27)
Kowalski T, Bartnik C (2010)Morphologial variation in colonies of
Chalara fraxinea isolated from ash (
Fraxinus excelsior L.) stems with symptoms of dieback and effects of temperature on colony growth and structure. Acta Agrobotanica 63 (1): 99-106.
CrossRef |
Gscholar
(28)
Kowalski T, Kraj W, Bednarz B (2016)Fungi on stems and twigs in initial and advanced stages of dieback of European ash (
Fraxinus excelsior) in Poland. European Journal of Forest Research 135 (3): 565-579.
CrossRef |
Gscholar
(29)
Kowalski T, Bilanski P, Kraj W (2017)Pathogenicity of fungi associated with ash dieback towards
Fraxinus excelsior. Plant Pathology 66 (8): 1228-1238.
CrossRef |
Gscholar
(30)
Kräutler K, Treitler R, Kirisits T (2015)Hymenoscyphus fraxineus can directly infect intact current-year shoots of
Fraxinus excelsior and artificially exposed leaf scars. Forest Pathology 45 (4): 274-280.
CrossRef |
Gscholar
(31)
Laue BE, Steele H, Green S (2014)Survival, cold tolerance and seasonality of infection of European horse chestnut (
Aesculus hippocastanum) by
Pseudomonas syringae pv.
aesculi. Plant Pathology 63 (6): 1417-1425.
CrossRef |
Gscholar
(32)
Marcais B, Husson C, Godart L, Cael O (2016)Influence of site and stand factors on
Hymenoscyphus fraxineus-induced basal lesions. Plant Pathology 65 (9): 1452-1461.
CrossRef |
Gscholar
(33)
McKinney LV, Thomsen IM, Kjaer ED, Nielsen LR (2012)Genetic resistance to
Hymenoscyphus pseudoalbidus limits fungal growth and symptom occurrence in
Fraxinus excelsior. Forest Pathology 42 (1): 69-74.
CrossRef |
Gscholar
(34)
McKinney LV, Nielsen LR, Collinge DB, Thomsen IM, Hansen JK, Kjaer ED (2014)The ash dieback crisis: genetic variation in resistance can prove a long-term solution. Plant Pathology 63 (3): 485-499.
CrossRef |
Gscholar
(35)
McMullan M, Rafiqi M, Kaithakottil G, Clavijo BJ, Bilham L, Orton E, Percival-Alwyn L, Ward BJ, Edwards A, Saunders DG, Accinelli GG (2018)The ash dieback invasion of Europe was founded by two genetically divergent individuals. Nature Ecology and Evolution 2 (6): 1000.
CrossRef |
Gscholar
(36)
Muñoz F, Marçais B, Dufour J, Dowkiw A (2016)Rising out of the ashes: additive genetic variation for crown and collar resistance to
Hymenoscyphus fraxineus in
Fraxinus excelsior. Phytopathology 106 (12): 1535-1543.
CrossRef |
Gscholar
(37)
Nielsen LR, McKinney LV, Hietala AM, Kjaer ED (2017a)The susceptibility of Asian, European and North American
Fraxinus species to the ash dieback pathogen
Hymenoscyphus fraxineus reflects their phylogenetic history. European Journal of Forest Research 136 (1): 59-73.
CrossRef |
Gscholar
(38)
Nielsen LR, Mckinney LV, Kjaer ED (2017b)Host phenological stage potentially affects dieback severity after
Hymenoscyphus fraxineus infection in
Fraxinus excelsior seedlings. Baltic Forestry 23 (1): 229-232.
Gscholar
(39)
Pliura A, Lygis V, Suchockas V, Bartkevicius E (2011)Performance of twenty four European
Fraxinus excelsior populations in three Lithuanian progeny trials with a special emphasis on resistance to
Chalara fraxinea. Baltic Forestry 17 (1): 17-34.
Online |
Gscholar
(40)
Qin RQ, LeBoldus JM (2014)The infection biology of
Sphaerulina musiva: clues to understanding a forest pathogen. PLoS ONE 9 (7): e1034 77.
CrossRef |
Gscholar
(41)
Schlegel M, Dubach V, Von Buol L, Sieber TN (2016)Effects of endophytic fungi on the ash dieback pathogen. FEMS Microbiology Ecology 92 (9): fiw142.
CrossRef |
Gscholar
(42)
Schumacher J, Kehr R, Leonhard S (2010)Mycological and histological investigations of
Fraxinus excelsior nursery saplings naturally infected by
Chalara fraxinea. Forest Pathology 40 (5): 419-429.
CrossRef |
Gscholar
(43)
Schwanda K, Kirisits T (2016)Pathogenicity of
Hymenoscyphus fraxineus towards leaves of three European ash species:
Fraxinus excelsior, F. angustifolia and
F. ornus. Plant Pathology 65 (7): 1071-1083.
CrossRef |
Gscholar
(44)
Skovsgaard JP, Thomsen IM, Skovgaard I, Martinussen T (2010)Associations among symptoms of dieback in even-aged stands of ash (
Fraxinus excelsior L.). Forest Pathology 40 (1): 7-18.
CrossRef |
Gscholar
(45)
Sønstebø JH, Vivian-Smith A, Adamson K, Drenkhan R, Solheim H, Hietala A (2017)Genome-wide population diversity in
Hymenoscyphus fraxineus points to an eastern Russian origin of European Ash dieback. bioRxiv: 154492.
CrossRef |
Gscholar
(46)
Steele H, Laue BE, MacAskill GA, Hendry SJ, Green S (2010)Analysis of the natural infection of European horse chestnut (
Aesculus hippocastanum) by
Pseudomonas syringae pv.
aesculi. Plant Pathology 59 (6): 1005-1013.
CrossRef |
Gscholar
(47)
Tang ZC, Kozlowski TT (1984)Water relations, ethylene production, and morphological adaptation of
Fraxinus pennsylvanica seedlings to flooding. Plant and Soil 77 (2-3): 183-192.
CrossRef |
Gscholar
(48)
Yamamoto F, Sakata T, Terazawa K (1995)Physiological, morphological and anatomical responses of
Fraxinus mandshurica seedlings to flooding. Tree Physiology 15 (11): 713-719.
CrossRef |
Gscholar