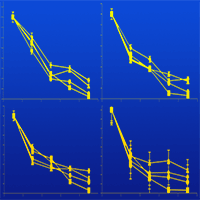
The influence of stand age on litter quality, decomposition rate and nutrient release was examined in pure stands of Kazdagi fir (Abies nordmanniana subsp. equi-trojani [Steven] Spach) differing in age (Fir38, Fir60, Fir90 and Fir100 years). The needle litters were collected and analysed for initial total carbon, cellulose, hemicellulose, lignin and nutrient concentrations (N, P, K, Ca, S, Mg, Mn and Fe). Initial litter quality parameters varied significantly among the four stand age classes. The Fir60 and Fir100 stands had higher total C than the Fir38 and Fir90 stands, while the Fir38 and Fir100 stands had higher N than the Fir60 and Fir90 stands. Mean cellulose and hemicellulose concentrations were highest in the Fir90 stand, while mean lignin concentration was highest in the Fir38 stand. Fir90 stand showed the highest ratios of C/N and Lignin/N. In general, the older fir stands showed higher Ca, Mg and K concentrations and lower P and S concentrations than the younger stands. The litter, however, showed higher a Mn concentration under the Fir60. Mean Fe concentration was highest under the Fir38 stand and lowest under the Fir60 stand. Litter decomposition was studied in the field using the litterbag technique. The litterbags were placed on the soil under each stand age class and sampled every 6 months for 2 years. The interaction of stand age and time on the mass loss was significant (p<0.01). The repeated measures ANOVA showed that the main effect of time on the mass loss was also significant (p<0.001). Needle litters under Fir100 and Fir60 stands decomposed faster than the needle litters under Fir90 and Fir38 stands. The calculated times required for 50% mass loss were higher under Fir38 (1.35 y) and Fir90 (1.27 y) stands than under Fir100 (1.05 y) and Fir60 (1.06 y) stands. The litters in Fir38 and Fir90 stands need approximately 4 years for 95% mass loss compared to the litters in Fir60 and Fir100 stands, which need 3 years. In general, Ca, Mg and S concentrations increased over time, whereas K and Mn decreased. These results illustrate that stand age is a key factor to be considered when studying litter decomposition dynamics.
Keywords
, , , ,
Citation
Savaci G, Sariyildiz T (2020). Effects of stand age on litter quality, decomposition rate and nutrient release of Kazdagi fir (Abies nordmanniana subsp. equi-trojani). iForest 13: 396-403. - doi: 10.3832/ifor3306-013
Academic Editor
Daniela Baldantoni
Paper history
Received: Dec 02, 2019
Accepted: Jun 22, 2020
First online: Sep 03, 2020
Publication Date: Oct 31, 2020
Publication Time: 2.43 months
© SISEF - The Italian Society of Silviculture and Forest Ecology 2020
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.

Breakdown by View Type
(Waiting for server response...)
Article Usage
Total Article Views: 40412
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 33899
Abstract Page Views: 2992
PDF Downloads: 2782
Citation/Reference Downloads: 3
XML Downloads: 736
Web Metrics
Days since publication: 1997
Overall contacts: 40412
Avg. contacts per week: 141.65
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2020): 6
Average cites per year: 1.00
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
(1)
Aber JD, Melillo JM, McClaugherty CA (1990)Predicting long term pattern of mass loss, nitrogen dynamics and soil organic matter formation from fine litter chemistry in temperate forest ecosystems. Canadian Journal of Botany 68: 2201-2269.
CrossRef |
Gscholar
(2)
Aerts R (1997)Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79: 439-449.
CrossRef |
Gscholar
(3)
Albert CH, Thuiller W, Yoccoz NG, Soudant A, Boucher F, Saccone P, Lavorel S (2010)Intraspecific functional variability: extent, structure and sources of variation. Journal of Ecology 98: 604-613.
CrossRef |
Gscholar
(4)
Allen SE (1989)Chemical analysis of ecological materials. Blackwell Scientific Publications. Oxford, London, pp. 368.
Gscholar
(5)
Baldantoni D, Bellino A, Manes F, Alfani A (2013)Ozone fumigation of
Quercus ilex L. slows down leaf litter decomposition with no detectable change in leaf composition. Annals of Forest Science 70: 571-578.
CrossRef |
Gscholar
(6)
Berendse F, Berg B, Bosatta E (1987)The effect of lignin and nitrogen on the decomposition of litter in nutrient-poor ecosystems: a theoretical approach. Canadian Journal of Botany 65: 1116-1120.
CrossRef |
Gscholar
(7)
Berg B, De Marco A, Davey M, Emmett B, Hobbie S, Liu C, McClaugherty C, Norell L, Johansson M-B, Rutigliano F, Vesterdal L, Virzo De Santo A (2010)Limit values for foliar litter decomposition - pine forests. Biogeochemistry 100: 57-73.
CrossRef |
Gscholar
(8)
Berg B, Meentemeyer V (2002)Litter quality in European transect
versus carbon storage potential. Plant and Soil 242 (1): 83-92.
CrossRef |
Gscholar
(9)
Bocock KL, Gilbert OJ (1957)The disappearance of litter under different woodland conditions. Plant and Soil 9: 179-185.
CrossRef |
Gscholar
(10)
Bouyoucos GJ (1962)Hydrometer method improved for making particle size analyses of soils. Agronomy Journal 54: 595-622.
CrossRef |
Gscholar
(11)
Brais S, Camiré C, Bergeron Y, Paré D (1995)Changes in nutrient availability and forest floor characteristics in relation to stand age and forest composition in the southern part of the boreal forest of northwestern Quebec. Forest Ecology and Management 76 (1-3): 181-189.
CrossRef |
Gscholar
(12)
Brun CB, Aström ME, Peltola P, Johansson MB (2008)Trends in major and trace elements in decomposing needle litters during a long-term experiment in Swedish forests. Plant and Soil 306 (1-2): 199-210.
CrossRef |
Gscholar
(13)
Bubb KA, Xu ZH, Simpson JA, Saffigna PG (1998)Some nutrient dynamics associated with litterfall and litter decomposition in hoop pine plantations of southeast Queensland, Australia. Forest Ecology and Management 110 (1-3): 343-352.
CrossRef |
Gscholar
(14)
Carreiro MM, Howe K, Parkhurst DF, Pouyat RV (1999)Variation in quality and decomposability of red oak leaf litter along an urban-rural gradient. Biology and Fertility of Soils 30: 258-268.
CrossRef |
Gscholar
(15)
Chauvat M, Zaitsev AS, Gabriel E, Wolters V (2009)How do soil fauna and soil microbiota respond to beech forest growth? Current Zoology 55 (4): 272-278.
CrossRef |
Gscholar
(16)
Chen J, Saunders SC, Crow TR, Naiman RJ, Brosofske KD, Mroz GD, Brookshire BL, Franklin JF (1999)Microclimate in forest ecosystem and landscape ecology: variations in local climate can be used to monitor and compare the effects of different management regimes. BioScience 49 (4): 288-297.
CrossRef |
Gscholar
(17)
Couteaux MM, Bottner P, Berg B (1995)Litter decomposition, climate and litter quality. Trends in Ecology and Evolution 10: 63-66.
CrossRef |
Gscholar
(18)
Currie WS, Harmon ME, Burke IC, Hart C, Parton JW, Silver W (2010)Cross-biome transplants of plant litter show decomposition models extend to a broader climatic range but lose predictability at the decadal time scale. Global Change Biology 16 (6): 1744-1761.
CrossRef |
Gscholar
(19)
Edmonds RL (1980)Litter decomposition and nutrient release in Douglas-fir, red alder, western hemlock, and Pacific silver fir ecosystems in western Washington. Canadian Journal of Forest Research 10 (3): 327-337.
CrossRef |
Gscholar
(20)
Fogel R, Cromack JRK (1977)Effect of habitat and substrate quality on Douglas fir litter decomposition in western Oregon. Canadian Journal of Botany 55 (12): 1632-1640.
CrossRef |
Gscholar
(21)
Gosz JR, Likens GE, Bormann FH (1973)Nutrient release from decomposing leaf and branch litter in the Hubbard Brook Forest, New Hampshire. Ecological Monographs 43 (2): 173-191.
CrossRef |
Gscholar
(22)
Hättenschwiler S, Hagerman AE, Vitousek PM (2003)Polyphenols in litter from tropical montane forests across a wide range in soil fertility. Biogeochemistry 64 (1): 129-148.
CrossRef |
Gscholar
(23)
Heal OW, Anderson JM, Swift MJ (1997)Plant litter quality and decomposition: an historical overview. In: “Driven by Nature: Plant Litter Quality and Decomposition” (Cadisch G, Giller KE eds). CAB International, Wallingford, UK, pp. 3-45.
Gscholar
(24)
Hobbie SE (2000)Interactions between litter lignin and soil nitrogen availability during leaf litter decomposition in a Hawaiian Montane forest. Ecosystems 3 (5): 484-494.
CrossRef |
Gscholar
(25)
Hobbie SE, Reich PB, Oleksyn J, Ogdahl M, Zytkowiak R, Hale C, Karolewski P (2006)Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 87 (9): 2288-2297.
CrossRef |
Gscholar
(26)
Inagaki Y, Miura S, Kohzu A (2004)Effects of forest type and stand age on litterfall quality and soil N dynamics in Shikoku district, southern Japan. Forest Ecology and Management 202: 107-117.
CrossRef |
Gscholar
(27)
Jackson ML (1962)Soil chemical analysis. Constable & Co. Ltd, London, UK, pp. 42-47.
Gscholar
(28)
Jagodzinski AM, Dyderski MK, Gesikiewicz K, Horodecki P (2019)Tree and stand level estimations of
Abies alba Mill. aboveground biomass. Annals of Forest Science 76 (2): 56.
CrossRef |
Gscholar
(29)
Joly FX, Milcu A, Scherer-Lorenzen M, Jean LK, Bussotti F, Dawud SM, Müller S, Pollastrini M, Raulund-Rasmussen K, Vesterdal L, Hättenschwiler S (2017)Tree species diversity affects decomposition through modified micro-environmental conditions across European forests. New Phytologist 214 (3): 1281-1293.
CrossRef |
Gscholar
(30)
Klaus JA (2018)Influence of stand age, micro-climate, and litter composition on the decomposition of ten litter types in white spruce plantation forests in Nova Scotia, Canada. MSc thesis, Dalhousie University Halifax, Nova Scotia, Canada, pp. 180.
Online |
Gscholar
(31)
Klopatek JM (2008)Litter decomposition contrasts in second-and old-growth Douglas-fir forests of the Pacific Northwest, USA. Plant Ecology 196 (1): 123-133.
CrossRef |
Gscholar
(32)
Laskowski R, Berg B (2006)Litter decomposition: guide to carbon and nutrient turnover. Advances in Ecological Research, Amsterdam, Netherlands, pp. 428.
Online |
Gscholar
(33)
Laskowski R, Berg B, Johansson MB, McClaugherty C (1995b)Release pattern for potassium from decomposing forest needle and leaf litter. Long-term decomposition in a Scots pine forest. IX. Canadian Journal of Botany 73 (12): 2019-2027.
CrossRef |
Gscholar
(34)
Laskowski R, Niklinska M, Maryanski M (1995a)The dynamics of chemical elements in forest litter. Ecology 76 (5): 1393-1406.
CrossRef |
Gscholar
(35)
Lohbeck M, Poorter L, Martínez-Ramos M, Rodriguez-Velázquez J, Breugel M, Bongers F (2014)Changing drivers of species dominance during tropical forest succession. Functional Ecology 28 (4): 1052-1058.
CrossRef |
Gscholar
(36)
Mataraci T (2012)Pinaceae. In: “Türkiye Bitkileri Listesi (Damarli Bitkiler) [Checklist of the Flora of Turkey (Vascular Plants)]” (Güner A, Aslan S, Ekim T, Vural M, Babaç MT eds). Research Association Press, Nezahat Gökyigit Botanical Garden and Flora, Istanbul, Turkey, pp. 14-17. [in Turkish]
Gscholar
(37)
Melillo JM, Aber J, Muratore JF (1982)Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63 (3): 621-626.
CrossRef |
Gscholar
(38)
Olson JS (1963)Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44 (2): 322-331.
CrossRef |
Gscholar
(39)
Palosuo T, Liski J, Trofymow JA, Titus BD (2005)Litter decomposition affected by climate and litter quality-testing the Yasso model with litterbag data from the Canadian intersite decomposition experiment. Ecological Modelling 189 (1-2): 183-198.
CrossRef |
Gscholar
(40)
Pérez-Suárez M, Arredondo-Moreno JT, Huber-Sannwald E (2012)Early stage of single and mixed leaf-litter decomposition in semiarid forest pine-oak: the role of rainfall and microsite. Biogeochemistry 108: 245-258.
CrossRef |
Gscholar
(41)
Rowland AP, Roberts JD (1994)Lignin and cellulose fractionation in decomposition studies using Acid Detergent Fibre methods. Communications in Soil Science and Plant Analysis 25: 269-277.
CrossRef |
Gscholar
(42)
Ryan MG, Binkley D, Fownes JH (1997)Age-related decline in forest productivity: pattern and processes. Advances in Ecological Research 27: 213-262.
CrossRef |
Gscholar
(43)
Sanger LJ, Cox P, Splatt P, Whelan M, Anderson JM (1998)Variability in the quality and potential decomposability of
Pinus sylvestris litter from sites with different soil characteristics: acid detergent fibre (ADF) and carbohydrate signatures. Soil Biology and Biochemistry 30 (4): 455-461.
CrossRef |
Gscholar
(44)
Sariyildiz T (2000)Biochemical and environmental controls of litter decomposition. PhD thesis, University of Exeter, UK, pp. 257.
Gscholar
(45)
Sariyildiz T (2015)Effects of tree species and topography on fine and small root decomposition rates of three common tree species (
Alnus glutinosa, Picea orientalis and
Pinus sylvestris) in Turkey. Forest Ecology and Management 335: 71-86.
CrossRef |
Gscholar
(46)
Sariyildiz T, Anderson JM (2003a)Interactions between litter quality, decomposition and soil fertility: a laboratory study. Soil Biology and Biochemistry 35 (3): 391-399.
CrossRef |
Gscholar
(47)
Sariyildiz T, Anderson JM (2003b)Decomposition of sun and shade leaves from three deciduous tree species, as affected by their chemical composition. Biology and Fertility of Soils 37 (3): 137-146.
CrossRef |
Gscholar
(48)
Sariyildiz T, Anderson JM (2005)Variation in the chemical composition of green leaves and leaf litters from three deciduous tree species growing on different soil types. Forest Ecology and Management 210 (1-3): 303-319.
CrossRef |
Gscholar
(49)
Sariyildiz T, Anderson JM, Kucuk M (2005)Effects of tree species and topography on soil chemistry, litter quality and decomposition in northeast Turkey. Soil Biology and Biochemistry 37 (9): 1695-1706.
CrossRef |
Gscholar
(50)
Sariyildiz T, Küçük M (2008)Litter mass loss rates in deciduous and coniferous trees in Artvin, northeast Turkey: relationships with litter quality, microclimate, and soil characteristics. Turkish Journal of Agriculture and Forestry 32 (6): 547-559.
Online |
Gscholar
(51)
Satti P, Mazzarino MJ, Gobbi M, Funes F, Roselli L, Fernandez H (2003)Soil N dynamics in relation to leaf litter quality and soil fertility in north-western Patagonian forests. Journal of Ecology 91: 173-181.
CrossRef |
Gscholar
(52)
Scott NA, Binkley D (1997)Foliage litter quality and annual net N mineralization: comparison across North American forest sites. Oecologia 111: 151-159.
CrossRef |
Gscholar
(53)
Sharma E, Ambasht RS (1987)Litterfall, decomposition and nutrient release in an age sequence of
Alnus nepalensis plantation stands in the eastern Himalaya. The Journal of Ecology 75 (4): 997.
CrossRef |
Gscholar
(54)
Sharma E, Ambasht RS, Sing MP (1985)Chemical soil properties under five age series of
Alnus nepalensis plantations in the Eastern Himalayas. Plant and Soil 84 (1): 105-113.
CrossRef |
Gscholar
(55)
Shen H, Wang X, Jiang Y, You W (2012)Spatial variations of throughfall through secondary succession of evergreen broad-leaved forests in eastern China. Hydrological Processes 26 (11): 1739-1747.
CrossRef |
Gscholar
(56)
Singh KP, Singh PK, Tripathi SK (1999)Litterfall, litter decomposition and nutrient release patterns in four native tree species raised on coal mine spoil at Singrauli, India. Biology and Fertility Soils 29 (4): 371-378.
CrossRef |
Gscholar
(57)
Sinsabaugh RL, Antibus RK, Linkins AE, McClaugherty CA, Rayburn L, Repert D, Wei-land T (1993)Wood decomposition: nitrogen and phosphorus dynamics in relation to extracellular enzyme activity. Ecology 74 (5): 1586-1593.
CrossRef |
Gscholar
(58)
Smithwick EA, Turner MG, Metzger KL, Balser TC (2005)Variation in NH
4+ mineralization and microbial communities with stand age in lodgepole pine (
Pinus contorta) forests, Yellowstone National Park (USA). Soil Biology and Biochemistry 37 (8): 1546-1559.
CrossRef |
Gscholar
(59)
Trap J, Bureau F, Vinceslas-Akpa M, Chevalier R, Aubert M (2009)Changes in soil N mineralization and nitrification pathways along a mixed forest chronosequence. Forest Ecology and Management 258: 1284-1292.
CrossRef |
Gscholar
(60)
Trap J, Hättenschwiler S, Gattin I, Aubert M (2013)Forest ageing: an unexpected driver of beech leaf litter quality variability in European forests with strong consequences on soil processes. Forest Ecology and Management 302: 338-345.
CrossRef |
Gscholar
(61)
Trogisch S, He JS, Hector A, Scherer-Lorenzen M (2016)Impact of species diversity, stand age and environmental factors on leaf litter decomposition in subtropical forests in China. Plant and Soil 400 (1-2): 337-350.
CrossRef |
Gscholar
(62)
Wang J, You Y, Tang Z, Liu S, Sun OJ (2014)Variations in leaf litter decomposition across contrasting forest stands and controlling factors at local scale. Journal of Plant Ecology 8 (3): 261-272.
CrossRef |
Gscholar
(63)
Wei L, Danli Y, Ji L, Yongmei HE (2020)Medium and long term decomposition process of litter in Abies fabri forest. Journal of Landscape Research 12 (1): 127-136.
CrossRef |
Gscholar
(64)
Welke SE, Hope GD (2005)Influences of stand composition and age on forest floor processes and chemistry in pure and mixed stands of Douglas-fir and paper birch in interior British Columbia. Forest Ecology and Management 219 (1): 29-42.
CrossRef |
Gscholar
(65)
Wu F, Yang W, Zhang J, Deng R (2010)Litter decomposition in two subalpine forests during the freeze-thaw season. Acta Oecologica 36 (1): 135-140.
CrossRef |
Gscholar
(66)
Wu Q, Wu F, Yang W, Zhao Y, He W, Tan B (2014)Foliar litter nitrogen dynamics as affected by forest gap in the Alpine forest of eastern Tibet Plateau. PLoS One 9 (5): e97112.
CrossRef |
Gscholar
(67)
Yu S, Wang D, Dai W, Li P (2014)Soil carbon budget in different-aged Chinese fir plantations in south China. Journal of Forestry Research 25 (3): 621-626.
CrossRef |
Gscholar