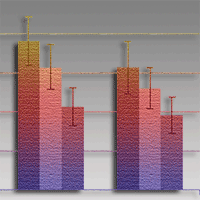
Application of fungicides and urea for control of ash dieback
Tine Hauptman (1) , Franci Aco Celar (2), Maarten de Groot (1), Dušan Jurc (1)
iForest - Biogeosciences and Forestry, Volume 8, Issue 2, Pages 165-171 (2015)
doi: https://doi.org/10.3832/ifor1272-008
Published: Aug 13, 2014 - Copyright © 2015 SISEF
Research Articles
Abstract
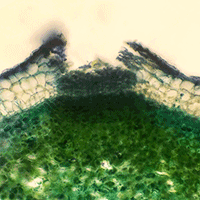
Ash dieback is caused by a highly pathogenic fungus Hymenoscyphus pseudoalbidus (anamorph Chalara fraxinea). Possibilities for disease control are limited, and treatment of fallen leaf debris to prevent sporulation of the pathogen is one of the possible options to control the disease. In some cases chemical treatments could be used, but data on effective chemical agents for control of the ash dieback are lacking. The purpose of this study was to examine the effect of different chemical fungicides and urea on the pathogen. Out of eight tested fungicides, mycelial growth in Petri plates as well as development of H. pseudoalbidus apothecia on ash leaf petioles were most efficiently inhibited by carbendazim. Urea also proved to be effective in prevention of apothecial formation. In addition to inhibition of the pathogen, urea accelerates the degradation of treated leaf debris. Therefore, the use of urea for treatment of infected ash leaf debris could be more effective than the use of fungicides and also more environmentally acceptable.
Keywords
Hymenoscyphus pseudoalbidus, Chemical Control, Mycelial Growth, Leaf Petioles, Apothecia Formation
Authors’ Info
Authors’ address
Maarten de Groot
Dušan Jurc
Department of Forest Protection, Slovenian Forestry Institute, Večna pot 2, SI-1000 Ljubljana (Slovenia)
Agronomy Department, Biotechnical Faculty, University of Ljubljana, Jamnikarjeva 101, SI-1000 Ljubljana (Slovenia)
Corresponding author
Paper Info
Citation
Hauptman T, Celar FA, de Groot M, Jurc D (2015). Application of fungicides and urea for control of ash dieback. iForest 8: 165-171. - doi: 10.3832/ifor1272-008
Academic Editor
Alberto Santini
Paper history
Received: Feb 20, 2014
Accepted: May 07, 2014
First online: Aug 13, 2014
Publication Date: Apr 01, 2015
Publication Time: 3.27 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 56026
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 46341
Abstract Page Views: 3959
PDF Downloads: 4241
Citation/Reference Downloads: 29
XML Downloads: 1456
Web Metrics
Days since publication: 4208
Overall contacts: 56026
Avg. contacts per week: 93.20
Citation Metrics
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 12
Average cites per year: 1.09
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
References
Effect of autumn application of urea on saprotrophic fungi in off-season leaf litter of sour cherry and evaluation of fungal isolates to reduce primary inoculum of Blumeriella jaapii. Journal of Plant Diseases and Protection 113: 107-112.
Online | Gscholar
The current situation of ash dieback caused by Chalara fraxinea in Austria. In: Proceedings of the “IUFRO Working party 7.02.02” (Dogmus-Lehtijärvi T eds). Egirdir (Turkey) 11-16 May 2009. Süleyman Demirel University, SDU Faculty of Forestry Journal, Special Issue, pp. 97-119.
Gscholar
The pesticide manual: a world compendium. British Crop Protection Council, Hampshire, UK, pp. 1344.
Gscholar