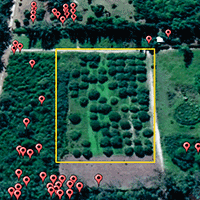
The ever-increasing use of paternity analysis to estimate the dispersal capability of forest trees calls for a quantitative evaluation of potential errors due to sampling design. Previous studies on optimal sampling strategies for seed trapping experiments suggested a link between sampling effort and error rate in the reconstruction of the seed dispersal kernel. We considered 92 papers on paternity analysis to quantitatively assess the sampling strategy used to study the characteristics of pollen dispersal patterns (pollen immigration rate, distribution of male reproductive success and estimates of pollen dispersal kernel parameters). For each studied stand we report data on the sampling effort (the total number of sampled seeds, the number of mother trees and the number of seeds per mother tree) and additional information on the studied species and characteristics of the sampling areas. The reviewed papers used a median of 8 mother trees (acting as pollen traps in paternity analysis studies), a median of 29 seeds per mother tree and a median of 240 total sampled seeds. These are values (especially the number of mother trees) lower than usually found in classical seed trapping studies, for which accuracy and precision of seed dispersal estimates had already been assessed. These findings underline the need of evaluating the consequences of realistic sampling efforts on the estimation of parameters describing the pollen dispersal pattern to provide the basis for meaningful guidelines to paternity analysis.
Keywords
, , , ,
Citation
Leonarduzzi C, Leonardi S, Menozzi P, Piotti A (2012). Towards an optimal sampling effort for paternity analysis in forest trees: what do the raw numbers tell us?. iForest 5: 18-25. - doi: 10.3832/ifor0606-009
Academic Editor
Marco Borghetti
Paper history
Received: Dec 06, 2011
Accepted: Feb 01, 2012
First online: Feb 27, 2012
Publication Date: Feb 27, 2012
Publication Time: 0.87 months
© SISEF - The Italian Society of Silviculture and Forest Ecology 2012
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.

Breakdown by View Type
(Waiting for server response...)
Article Usage
Total Article Views: 60632
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 49984
Abstract Page Views: 3911
PDF Downloads: 5285
Citation/Reference Downloads: 38
XML Downloads: 1414
Web Metrics
Days since publication: 5085
Overall contacts: 60632
Avg. contacts per week: 83.47
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2012): 12
Average cites per year: 0.86
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
(1)
Adams WT (1992)Gene dispersal within forest tree populations. New Forests 6: 217-240.
CrossRef |
Gscholar
(2)
Apsit VJ, Hamrick JL, Nason JD (2001)Breeding population size of a fragmented population of a Costa Rican dry forest tree species. Journal of Heredity 92: 415-420.
CrossRef |
Gscholar
(3)
Ashley MV (2010)Plant parentage, pollination, and dispersal: how DNA microsatellites have altered the landscape. Critical Reviews in Plant Sciences 29: 148-161.
CrossRef |
Gscholar
(4)
Bacles CFE, Burczyk J, Lowe AJ, Ennos RA (2005)Historical and contemporary mating patterns in remnant populations of the forest tree
Fraxinus excelsior L. Evolution 59: 979-990.
CrossRef |
Gscholar
(5)
Bacles CFE, Ennos RA (2008)Paternity analysis of pollen-mediated gene flow for
Fraxinus excelsior L. in a chronically fragmented landscape. Heredity 101: 368-380.
CrossRef |
Gscholar
(6)
Bai WN, Zeng Y-F, Zhang D-Y (2007)Mating patterns and pollen dispersal in a heterodichogamous tree,
Junglans mandshurica (Junglandaceae). New Phytologist 176: 699-707.
CrossRef |
Gscholar
(7)
Bittencourt JVM, Sebbenn AM (2007)Patterns of pollen and seed dispersal in a small, fragmented population of the wind-pollinated tree in southern Brazil. Heredity 99: 580-591.
CrossRef |
Gscholar
(8)
Bittencourt JVM, Sebbenn AM (2008)Pollen movement within a continuous forest of wind-pollinated , inferred from paternity and TwoGener analysis. Conservation Genetics 9: 855-868.
CrossRef |
Gscholar
(9)
Boshier D, Chase MR, Bawa KS (1995)Population genetics of (Boraginaceae), a Neotropical tree. 3. Gene flow, neighborhood, and population substructure. American Journal of Botany 82: 484-490.
CrossRef |
Gscholar
(10)
Braga AC, Collevatti RG (2011)Temporal variation in pollen dispersal and breeding structure in a bee-pollinated Neotropical tree. Heredity 106: 911-919.
CrossRef |
Gscholar
(11)
Buiteveld J, Bakker EG, Bovenschen J, de Vries SMG (2001)Paternity analysis in a seed orchard of
Quercus robur L. and estimation of the amount of background pollination using microsatellite markers. Forest Genetics 8: 331-337.
Gscholar
(12)
Burczyk J, Adams WT, Shimizu JY (1996)Mating patterns and pollen dispersal in a natural knobcone pine (
Pinus attenuata Lemmon.) stand. Heredity 77: 251-260.
CrossRef |
Gscholar
(13)
Burczyk J, Adams WT, Moran GF, Griffin AR (2002)Complex patterns of mating revealed in a seed orchard using allozyme markers and the neighbourhood model. Molecular Ecology 11: 2379-2391.
CrossRef |
Gscholar
(14)
Burczyk J (2004)Local pollen dispersal and distant gene flow in Norway spruce ( Karst.). Forest Ecology and Management 197: 39-48.
CrossRef |
Gscholar
(15)
Burczyk J, DiFazio SP, Adams WT (2004)Gene flow in forest trees: how far do genes really travel? Forest Genetics 11: 179-192.
Gscholar
(16)
Burczyk J, Adams WT, Birkes DS, Chybicki IJ (2006)Using genetic markers to directly estimate gene flow and reproductive success parameters in plants on the basis of naturally regenerated seedlings. Genetics 173: 363-372.
CrossRef |
Gscholar
(17)
Buschbom J, Yanbaev Y, Degen B (2011)Efficient long-distance gene flow into an isolated relict oak stand. Journal of Heredity 102: 464-472.
CrossRef |
Gscholar
(18)
Byrne M, Elliott CP, Yates C, Coates DJ (2007)Extensive pollen dispersal in a bird-pollinated shrub,
Calothamnus quadrifidus, in a fragmented landscape. Molecular Ecology 16: 1303-1314.
CrossRef |
Gscholar
(19)
Byrne M, Elliott CP, Yates C, Coates DJ (2008)Maintenance of high pollen dispersal in
Eucalyptus wandoo, a dominant tree of the fragmented agricultural region in Western Australia. Conservation Genetics 9: 97-105.
CrossRef |
Gscholar
(20)
Carneiro FS, Degen B, Kanashiro M, de Lacerda AEB, Sebbenn AM (2009)High levels of pollen dispersal detected through paternity analysis from a continuous
Symphonia globulifera population in the Brazilian Amazon. Forest Ecology and Management 258: 1260-1266.
CrossRef |
Gscholar
(21)
Chaix G, Gerber S, Razafimaharo V, Vigneron P, Verhaegen D, Hamon S (2003)Gene flow estimation with microsatellites in a Malagasy seed orchard of
Eucalyptus grandis. Theoretical and Applied Genetics 107: 705-712.
CrossRef |
Gscholar
(22)
Chase MR, Moller C, Kesseli R, Bawa KS (1996)Distant gene flow in tropical trees. Nature 383: 398-399.
CrossRef |
Gscholar
(23)
Chybicki IJ, Burczyk J (2010a)NM+: software implementing parentage-based models for estimating gene dispersal and mating patterns in plants. Molecular Ecology Resources 10: 1071-1075.
CrossRef |
Gscholar
(24)
Chybicki IJ, Burczyk J (2010b)Realized gene flow within mixed stands of L. and (Matt.) L. revealed at the stage of naturally established seedlings. Molecular Ecology 19: 2137-2151.
CrossRef |
Gscholar
(25)
Cloutier D, Hardy OJ, Caron H, Ciampi AY, Degen B, Kanashiro M, Schoen DJ (2007)Low inbreeding and high pollen dispersal distances in populations of two Amazonian Forest tree species. Biotropica 39: 406-415.
CrossRef |
Gscholar
(26)
Cottrell JE, Vaughan SP, Connolly T, Sing L, Moodley DJ, Russell K (2009)Contemporary pollen flow, characterization of the maternal ecological neighbourhood and mating patterns in wild cherry ( L.). Heredity 103: 118-28.
CrossRef |
Gscholar
(27)
Craft KJ, Ashley MV (2010)Pollen-mediated gene flow in isolated and continuous stands of bur oak, Quercus macrocarpa (Fagaceae). American Journal of Botany 97: 1999-2006.
CrossRef |
Gscholar
(28)
Curtu AL, Gailing O, Finkeldey R (2009)Patterns of contemporary hybridization inferred from paternity analysis in a four-oak-species forest. BMC Evolutionary Biology 9: 284.
CrossRef |
Gscholar
(29)
de Lacerda AEB, Kanashiro M, Sebbenn AM (2008)Long-pollen movement and deviation of random mating in a low-density continuous population of a tropical tree in the Brazilian Amazon. Biotropica 40: 462-470.
CrossRef |
Gscholar
(30)
de Moraes MLT, Sebbenn AM (2011)Pollen dispersal between isolated trees in the Brazilian savannah: a case study of the neotropical tree . Biotropica 43: 192-199.
CrossRef |
Gscholar
(31)
Dick CW (2001)Genetic rescue of remnant tropical trees by an alien pollinator. Proceedings of the Royal Society of London Series B-Biological Sciences 268: 2391-2396.
CrossRef |
Gscholar
(32)
Dow BD, Ashley MV (1998)High levels of gene flow in Bur oak revealed by paternity analysis using microsatellites. Journal of Heredity 89: 62-70.
CrossRef |
Gscholar
(33)
Dunphy BK, Hamrick JL (2005)Gene flow among established Puerto Rican populations of the exotic tree species, . Heredity 94: 418-425.
CrossRef |
Gscholar
(34)
Dunphy BK, Hamrick JL (2007)Estimation of gene flow into fragmented populations of (Burseraceae) in the dry-forest life zone of Puerto Rico. American Journal of Botany 94: 1786-1794.
CrossRef |
Gscholar
(35)
Fuchs EJ, Hamrick JL (2011)Mating system and pollen flow between remnant populations of the endangered tropical tree, (Zygophyllaceae). Conservation Genetics 12: 175-185.
CrossRef |
Gscholar
(36)
Fukue Y, Kado T, Lee SL, Ng KKS, Muhammad N, Tsumura Y (2007)Effects of flowering tree density on the mating system and gene flow in (Dipterocarpaceae) in Peninsular Malaysia. Journal of Plant Research 120: 413-420.
CrossRef |
Gscholar
(37)
Funda T, Chen CC, Liewlaksaneeyanawin C, Kenawy AM, El-Kassaby YA (2008)Pedigree and mating system analyses in a western larch (
Larix occidentalis Nutt.) experimental population. Annals of Forest Science 65 (7): 705-705.
CrossRef |
Gscholar
(38)
Gaino APSC, Silva AM, Moraes MA, Alves, PF, Moraes MLT, Freitas MLM, Sebbenn AM (2010)Understanding the effects of isolation on seed and pollen flow, spatial genetic structure and effective population size of the dioecious tropical tree species
Myracrodruon urundeuva. Conservation Genetics 11: 1631-1643.
CrossRef |
Gscholar
(39)
García C, Arroyo JM, Godoy JA, Jordano P (2005)Mating patterns, pollen dispersal, and the ecological maternal neighbourhood in a
Prunus mahaleb L. population. Molecular Ecology 14: 1821-1830.
CrossRef |
Gscholar
(40)
Geng Q, Lian C, Goto S, Tao J, Kimura M, Islam MS, Hogetsu T (2008)Mating system, pollen and propagule dispersal, and spatial genetic structure in a high-density population of the mangrove tree . Molecular Ecology 17: 4724-4739.
CrossRef |
Gscholar
(41)
Gerber S, Chabrier P, Kremer A (2003)FAMOZ: a software for parentage analysis using dominant, codominant and uniparentally inherited markers. Molecular Ecology Notes 3: 479-481.
CrossRef |
Gscholar
(42)
Ghosh P (1951)Random distance within a rectangle and between two rectangles. Bulletin of Calcutta Mathematical Society 43: 17-24
Gscholar
(43)
Goto S, Shimatani K, Yoshimaru H, Takahashi Y (2006)Fat-tailed gene flow in the dioecious canopy tree species var. revealed by microsatellites. Molecular Ecology 15: 2985-2996.
CrossRef |
Gscholar
(44)
Gregorius HR, Kownatzki D, Höltken AM (2011)Spatial patterns of mating relations in wild cherry ( L.). Perspectives in Plant Ecology, Evolution and Systematics 13: 37-45.
CrossRef |
Gscholar
(45)
Grosser C, Potts B, Vaillantcourt RE (2010)Microsatellite based paternity analysis in a clonal
Eucalyptus nitens seed orchard. Silvae Genetica 59: 57-62.
Gscholar
(46)
Hanaoka S, Yuzurihara J, Asuka Y, Tomaru N, Tsumura Y, Kakubari Y, Mukai Y (2007)Pollen-mediated gene flow in a small, fragmented natural population of
Fagus crenata. Canadian Journal of Botany 85: 404-413.
CrossRef |
Gscholar
(47)
Hansen OK, Kjær ED (2006)Paternity analysis with microsatellites in a Danish
Abies nordmanniana clonal seed orchard reveals dysfunctions. Canadian Journal of Forest Research 36: 1054-1058.
CrossRef |
Gscholar
(48)
Hanson TR, Brunsfeld SJ, Bryan F, Waits LP (2008)Pollen dispersal and genetic structure of the tropical tree in a fragmented Costa Rican landscape. Molecular Ecology 17: 2060-2073.
CrossRef |
Gscholar
(49)
Hardy OJ (2009)How fat is the tail? Heredity 103: 437-438.
CrossRef |
Gscholar
(50)
Hasegawa Y, Suyama Y, Seiwa K (2009)Pollen donor composition during the early phases of reproduction revealed by DNA genotyping of pollen grains and seeds of
Castanea crenata. New Phytologist 182 (4): 994-1002.
CrossRef |
Gscholar
(51)
Hoebee SE, Arnold U, Düggelin C, Gugerli F, Brodbeck S, Rotach P, Holderegger R (2007)Mating patterns and contemporary gene flow by pollen in a large continuous and a small isolated population of the scattered forest tree . Heredity 99: 47-55.
CrossRef |
Gscholar
(52)
Hufford KM, Hamrick JL, Rathbun SL (2009)Male reproductive success at three early life stages in the tropical tree . International Journal of Plant Sciences 170: 724-734.
CrossRef |
Gscholar
(53)
Irwin AJ, Hamrick JL, Godt MJW, Smouse PE (2003)A multiyear estimate of the effective pollen donor pool for . Heredity 90: 187-194.
CrossRef |
Gscholar
(54)
Isagi Y, Kanazashi T, Suzuki W, Tanaka H, Abe T (2004)Highly variable pollination patterns in revealed by microsatellite paternity analysis. International Journal of Plant Sciences 165: 1047-1053.
CrossRef |
Gscholar
(55)
Jones FA, Chen J, Weng G, Hubbell SP (2011)A genetic evaluation of seed dispersal in the neotropical tree (Bignoniaceae). The American Naturalist 166 (5): 543-555.
CrossRef |
Gscholar
(56)
Jones FA, Muller-Landau HC (2008)Measuring long-distance seed dispersal in complex natural environments: an evaluation and integration of classical and genetic methods. Journal of Ecology 96: 642-652.
CrossRef |
Gscholar
(57)
Jones ME, Shepherd M, Henry R, Delves A (2008)Pollen flow in determined by paternity analysis using microsatellite markers. Tree Genetics and Genomes 4: 37-47.
CrossRef |
Gscholar
(58)
Jones AG, Small CM, Paczolt KA, Ratterman NL (2010)A practical guide to methods of parentage analysis. Molecular Ecology Resources 10: 6-30.
CrossRef |
Gscholar
(59)
Kalinowski ST, Taper ML, Marshall TC (2007)Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Molecular Ecology 16: 1099-1006.
CrossRef |
Gscholar
(60)
Kameyama Y, Isagi Y, Naito K, Nakagoshi N (2000)Microsatellite analysis of pollen flow in var. . Ecological Research 15: 263-269.
CrossRef |
Gscholar
(61)
Kamm U, Rotach P, Gugerli F, Siroky M, Edwards P, Holderegger R (2009)Frequent long-distance gene flow in a rare temperate forest tree () at the landscape scale. Heredity 103: 476-482.
CrossRef |
Gscholar
(62)
Kaufman SR, Smouse PE, Alvarez-Buylla ER (1998)Pollen-mediated gene flow and differential male reproductive success in a tropical pioneer tree, Bertol. (Moraceae): a paternity analysis. Heredity 81: 164-173.
CrossRef |
Gscholar
(63)
Kenta T, Isagi Y, Nakagawa M, Yamashita M, Nakashizuka T (2004)Variation in pollen dispersal between years with different pollination conditions in a tropical emergent tree. Molecular Ecology 13: 3575-3584.
CrossRef |
Gscholar
(64)
Konuma A, Tsumura Y, Lee CT, Lee SL, Okuda T (2000)Estimation of gene flow in the tropical-rainforest tree (Dipterocarpaceae), inferred from paternity analysis. Molecular Ecology 9: 1843-1852.
CrossRef |
Gscholar
(65)
Lander TA, Boshier DH, Harris SA (2010)Fragmented but not isolated: contribution of single trees, small patches and long-distance pollen flow to genetic connectivity for , an endangered Chilean tree. Biological Conservation 143: 2583-2590.
CrossRef |
Gscholar
(66)
Larsen AS, Kjaer ED (2009)Pollen mediated gene flow in a native population of and its implications for contemporary gene conservation management. Conservation Genetics 10: 1637-1646.
CrossRef |
Gscholar
(67)
Latouche-Hallé C, Ramboer A, Bandou E, Caron H, Kremer A (2004)Long-distance pollen flow and tolerance to selfing in a neotropical tree species. Molecular Ecology 13: 1055-1064.
CrossRef |
Gscholar
(68)
Lee SL, Ng KKS, Saw LG, Lee CT, Muhammad N, Tani N, Tsumura Y, Koskela J (2006)Linking the gaps between conservation research and conservation management of rare dipterocarps: a case study of . Biological Conservation 131: 72-92.
CrossRef |
Gscholar
(69)
Leinemann L, Hattemer H (2006)Genetic variation and mating pattern in a stand of yew ( L.). Allgemeine Forst und Jagdzeitung 177: 217-224.
Gscholar
(70)
Nakanishi A, Tomaru N, Yoshimaru H, Kawahara T, Manabe T, Yamamoto S (2004)Patterns of pollen flow and genetic differentiation among pollen pools in
Quercus salicina in a warm temperate old-growth evergreen broad-leaved forest. Silvae Genetica 53: 258-264.
Online |
Gscholar
(71)
Oddou-Muratorio S, Houot M-L, Demesure-Musch B, Austerlitz F (2003)Pollen flow in the wildservice tree,
Sorbus torminalis (L.) Crantz. I. Evaluating the paternity analysis procedure in continuous populations. Molecular Ecology 12: 3427-3439.
CrossRef |
Gscholar
(72)
Oddou-Muratorio S, Klein EK, Austerlitz F (2005)Pollen flow in the wildservice tree,
Sorbus torminalis (L.) Crantz. II. Pollen dispersal and heterogeneity in mating success inferred from parent-offspring analysis. Molecular Ecology 14: 4441-4452.
CrossRef |
Gscholar
(73)
Pairon M, Jonard M, Jacquemart AL (2006)Modeling seed dispersal of black cherry, an invasive forest tree: how microsatellites may help? Canadian Journal of Forest Research 36: 1385-1394.
CrossRef |
Gscholar
(74)
Pakkad G, Al Mazrooei S, Blakesley D, James C, Elliott S, Luoma-aho T, Koskela J (2008a)Genetic variation and gene flow among D. Don populations in northern Thailand: analysis of a rehabilitated site and adjacent intact forest. New Forests 35: 33-43.
CrossRef |
Gscholar
(75)
Pakkad G, Ueno S, Yoshimaru H (2008b)Gene flow pattern and mating system in a small population of Roxb. (Fagaceae). Forest Ecology and Management 255: 3819-3826.
CrossRef |
Gscholar
(76)
Pielaat A, Lewis M, Lele S, Decaminobeck T (2006)Sequential sampling designs for catching the tail of dispersal kernels. Ecological Modelling 190: 205-222.
CrossRef |
Gscholar
(77)
Piotti A, Leonardi S, Buiteveld J, Geburek T, Gerber S, Kramer K, Vettori C, Vendramin GG (2012)Comparison of pollen gene flow among four European beech ( L.) populations characterized by different management regimes. Heredity 108: 322-331.
CrossRef |
Gscholar
(78)
Piotti A, Leonardi S, Piovani P, Scalfi M, Menozzi P (2009)Spruce colonization at treeline: where do those seeds come from? Heredity 103: 136-145.
CrossRef |
Gscholar
(79)
Pluess AR, Sork VL, Dolan B, Davis FW, Grivet D, Merg K, Papp J, Smouse PE (2009)Short distance pollen movement in a wind-pollinated tree, (Fagaceae). Forest Ecology and Management 258: 735-744.
CrossRef |
Gscholar
(80)
Pollegioni P, Woeste K, Mugnozza GS, Malvolti ME (2009)Retrospective identification of hybridogenic walnut plants by SSR fingerprinting and parentage analysis. Molecular Breeding 24: 321-335.
CrossRef |
Gscholar
(81)
Rathmacher G, Niggemann M, Köhnen M, Ziegenhagen B, Bialozyt R (2009)Short-distance gene flow in L. accounts for small-scale spatial genetic structures: implications for conservation measures. Conservation Genetics 11: 1327-1338.
CrossRef |
Gscholar
(82)
Ribbens E, Silander JA, Pacala SW (1994)Seedling recruitment in forests: calibrating models to predict patterns of tree seedling dispersion. Ecology 75: 1794-1806.
CrossRef |
Gscholar
(83)
Ritland K (2002)Extensions of models for the estimation of mating systems using n indipendent loci. Heredity 88: 221-228.
CrossRef |
Gscholar
(84)
Robledo-Arnuncio JJ, Gil L (2005)Patterns of pollen dispersal in a small population of L. revealed by total-exclusion paternity analysis. Heredity 94: 13-22.
CrossRef |
Gscholar
(85)
Robledo-Arnuncio JJ, Garcia C (2007)Estimation of the seed dispersal kernel from exact identification of source plants. Molecular Ecology 16: 5098-5109.
CrossRef |
Gscholar
(86)
Robledo-Arnuncio JJ, Austerlitz F, Smouse PE (2007)POLDISP: a software package for indirect estimation of contemporary pollen dispersal. Molecular Ecology Notes 7: 763-766.
CrossRef |
Gscholar
(87)
Robledo-Arnuncio JJ (2010)Wind pollination over mesoscale distances: an investigation with Scots pine. New Phytologist 190: 222-233.
CrossRef |
Gscholar
(88)
Salvini D, Bruschi P, Fineschi S, Grossoni P, Kjær ED, Vendramin GG (2009)Natural hybridisation between (Matt.) Liebl. and Willd. within an Italian stand as revealed by microsatellite fingerprinting. Plant Biology 11: 758-765.
CrossRef |
Gscholar
(89)
Savolainen O, Pyhajarvi T, Knurr T (2007)Gene flow and local adaptation in trees. Annual Review of Ecology, Evolution, and Systematics 38: 595-619.
CrossRef |
Gscholar
(90)
Schnabel A, Hamrick JL (1995)Understanding the population genetic structure of L.: the scale and pattern of pollen gene flow. Evolution 49: 921-931.
CrossRef |
Gscholar
(91)
Schuster WSF, Mitton JB (2000)Paternity and gene dispersal in limber pine ( James). Heredity 84: 348-361.
CrossRef |
Gscholar
(92)
Setsuko S, Ishida K, Ueno S, Tsumura Y, Tomaru N (2007)Population differentiation and gene flow within a metapopulation of a threatened tree, (Magnoliaceae). American Journal of Botany 94: 128-136.
CrossRef |
Gscholar
(93)
Silva MB, Kanashiro M, Ciampi AY, Thompson I, Sebbenn AM (2008)Genetic effects of selective logging and pollen gene flow in a low-density population of the dioecious tropical tree in the Brazilian Amazon. Forest Ecology and Management 255: 1548-1558.
CrossRef |
Gscholar
(94)
Silva CRS, Albuquerque PSB, Ervedosa FR, Mota JWS, Figueira A, Sebbenn AM (2011)Understanding the genetic diversity, spatial genetic structure and mating system at the hierarchical levels of fruits and individuals of a continuous population from the Brazilian Amazon. Heredity 106: 973-985.
CrossRef |
Gscholar
(95)
Skarpaas O, Shea K, Bullock JM (2005)Optimizing dispersal study design by Monte Carlo simulation. Journal of Applied Ecology 42: 731-739.
CrossRef |
Gscholar
(96)
Slavov GT, Howe GT, Adams WT (2005)Pollen contamination and mating patterns in a Douglas-fir seed orchard as measured by simple sequence repeat markers. Canadian Journal of Forest Research 35: 1592-1603.
CrossRef |
Gscholar
(97)
Slavov GT, Leonardi S, Burczyk J, Adams WT, Strauss SH, DiFazio SP (2009)Extensive pollen flow in two ecologically contrasting populations of . Molecular Ecology 18: 357-373.
CrossRef |
Gscholar
(98)
Stacy EA, Hamrick JL, Nason JD, Hubbell SP, Foster RB, Condit R (1996)Pollen dispersal in low-density populations of three Neotropical tree species. American Naturalist 148: 275-298.
CrossRef |
Gscholar
(99)
Stoyan D, Wagner S (2001)Estimating the fruit dispersion of anemochorous forest trees. Ecological Modelling 145: 35-47.
CrossRef |
Gscholar
(100)
Streiff R, Ducousso A, Lexer C, Steinkellner H, Gloessl J, Kremer A (1999)Pollen dispersal inferred from paternity analysis in a mixed oak stand of L. and (Matt.) Liebl. Molecular Ecology 8: 831-841.
CrossRef |
Gscholar
(101)
Tabbener H (2003)The use of PCR based DNA markers to study the paternity of poplar seedlings. Forest Ecology and Management 179: 363-376.
CrossRef |
Gscholar
(102)
Tani N, Tsumura Y, Kado T, Taguchi Y, Lee SL, Muhammad N, Ng KKS, Numata S, Nishimura S, Konuma A, Okuda T (2009)Paternity analysis-based inference of pollen dispersal patterns, male fecundity variation, and influence of flowering tree density and general flowering magnitude in two dipterocarp species. Annals of Botany 104: 1421-1434.
CrossRef |
Gscholar
(103)
Vanden Broeck A, Cottrell J, Quataert P, Breyne P, Storme V, Boerjan W, Van Slycken J (2006)Paternity analysis of L. offspring in a Belgian plantation of native and exotic poplars. Annals of Forest Science 63: 783-790.
CrossRef |
Gscholar
(104)
Wang KS (2004)Gene flow in European beech ( L.). Genetica 122: 105-113.
CrossRef |
Gscholar
(105)
Wang J, Ye Q, Kang M, Huang H (2008)Novel polymorphic microsatellite loci and patterns of pollen-mediated gene flow in an ex situ population of (Sapindaceae) as revealed by categorical paternity analysis. Conservation Genetics 9: 559-567.
CrossRef |
Gscholar
(106)
Wang J, Kang M, Gao P, Huang H (2010a)Contemporary pollen flow and mating patterns of a subtropical canopy tree in a fragmented agricultural landscape. Forest Ecology and Management 260: 2180-2188.
CrossRef |
Gscholar
(107)
Wang H, Sork VL, Wu J, Ge J (2010b)Effect of patch size and isolation on mating patterns and seed production in an urban population of Chinese pine ( Carr.). Forest Ecology and Management 260: 965-974.
CrossRef |
Gscholar
(108)
White GM, Boshier DH, Powell W (2002)Increased pollen flow counteracts fragmentation in a tropical dry forest, an example from Zuccarini. Proceedings of the National Academy of Sciences 99: 2038-2042.
CrossRef |
Gscholar
(109)
Williams CG (2005)Framing the issues on transgenic forests. Nature Biotechnology 23: 530-532.
CrossRef |
Gscholar
(110)
Williams CG (2010)Long-distance pine pollen still germinates after meso-scale dispersal. American Journal of Botany 97: 846-855.
CrossRef |
Gscholar
(111)
Willson MF (1993)Dispersal mode, seed shadows, and colonization patterns. Vegetatio 107/108: 261-280.
Gscholar
(112)
Xie C-Y, Knowles P (1994)Mating system and effective pollen immigration in a Norway spruce (
Picea abies (L.) Karst) plantation. Silvae Genetica 43: 48-52.
Gscholar
(113)
Yehili J-L, N’Guetta Assanvo S-P, Gnagne M, Blanc G, Rodier-Goud M, Clément-Demange A, Sequin M, Fanjavola M (2007)Flux de gènes dans un verger à graines d’hévéas sauvages (
Hevea brasiliensis Müll. Arg.). Cahiers Agricultures 16 : 177-184.
Gscholar
(114)
Zhang J-J, Ye Q-G, Yao X-H, Huang H-W (2010)Spontaneous interspecific hybridization and patterns of pollen dispersal in ex situ populations of a tree species (
Sinojackia xylocarpa) that is extinct in the wild. Conservation Biology 24: 246-255.
CrossRef |
Gscholar