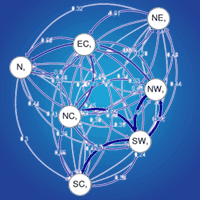
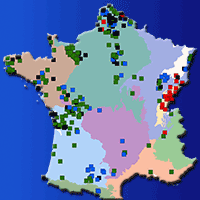
Gene flow is one of the main factors shaping genetic diversity within and among tree populations, and occurs through pollen and seed dispersal. Recent findings of pollen-release asynchronies in distant populations of Scots pine (Pinus sylvestris L.) within Scotland suggest that gene dispersal among more distant populations might be less effective than previously thought. Limited gene dispersal is one of the major factors causing genetic structure for neutral markers, and pollen-release asynchrony could have driven isolation by distance (IBD) among Scottish populations. Previous studies of neutral markers found little differentiation among Scottish populations of Scots pine, however they did not consider IBD over the full Scottish range. We analysed data from 6 nuclear simple sequence repeats (SSR) and 5 chloroplast SSR loci in a total of 540 individuals of Scots pine from 18 populations across Scotland. Our aim was to assess contemporary levels and distribution of genetic variation and to test if the distribution of genetic diversity was consistent with IBD. We also analysed patterns of gene flow that could have contributed to the observed patterns of variation. Levels of genetic diversity were high, for both nuclear and chloroplast markers within populations, and there was no significant differentiation among populations. A weak signal of IBD was present. We found an increase in nuclear diversity towards the East along with greater gene flow in a West-East direction commensurate with the prevailing winds. Our findings suggest that this wind-driven gene flow is dominant over genetic drift and prevents differentiation among the Scottish populations. It may also counteract any pollen-release asynchronies among populations.
Keywords
, , , ,
Citation
González-Díaz P, Cavers S, Iason GR, Booth A, Russell J, Jump AS (2018). Weak isolation by distance and geographic diversity gradients persist in Scottish relict pine forest. iForest 11: 449-458. - doi: 10.3832/ifor2454-011
Academic Editor
Fulvio Ducci
Paper history
Received: Apr 06, 2017
Accepted: Apr 19, 2018
First online: Jul 02, 2018
Publication Date: Aug 31, 2018
Publication Time: 2.47 months
© SISEF - The Italian Society of Silviculture and Forest Ecology 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.

Breakdown by View Type
(Waiting for server response...)
Article Usage
Total Article Views: 50033
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 41584
Abstract Page Views: 4039
PDF Downloads: 3460
Citation/Reference Downloads: 8
XML Downloads: 942
Web Metrics
Days since publication: 2801
Overall contacts: 50033
Avg. contacts per week: 125.04
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2018): 2
Average cites per year: 0.25
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
(1)
Aitken SN, Yeaman S, Holliday JA, Wang T, Curtis-McLane S (2008)Adaptation, migration or extirpation: climate change outcomes for tree populations. Evolutionary Applications 1: 95-111.
CrossRef |
Gscholar
(2)
Auckland LD, Bui T, Zhou Y, Shepard M, Williams CG (2002)Conifer microsatellite handbook. Corporate Press, Raleigh, NC, USA, pp. 57.
Online |
Gscholar
(3)
Bagnoli F, Buonamici A (2009)Is
Cupressus sempervirens native in Italy? An answer from genetic and palaeobotanical data. Molecular Ecology 18: 2276-2286.
CrossRef |
Gscholar
(4)
Belletti P, Ferrazzini D, Piotti A, Monteleone I, Ducci F (2012)Genetic variation and divergence in Scots pine (
Pinus sylvestris L.) within its natural range in Italy. European Journal of Forest Research 131: 1127-1138.
CrossRef |
Gscholar
(5)
Bernhardsson C, Floran V, Ganea SL, García-Gil MR (2016)Present genetic structure is congruent with the common origin of distant Scots pine populations in its Romanian distribution. Forest Ecology and Management 361: 131-143.
CrossRef |
Gscholar
(6)
Birks HJB (1989)Holocene isochrone maps and patterns of tree-spreading in the British Isles. Journal of Biogeography 16: 503-540.
CrossRef |
Gscholar
(7)
Burczyk J, Difazio SP, Adams WT (2004)Gene flow in forest trees: How far do genes really travel? Forest Genetics 11: 179-192.
Online |
Gscholar
(8)
Chagné D, Chaumeil P, Ramboer A, Collada C, Guevara A, Cervera MT, Vendramin GG, Garcia V, Frigerio J-M, Echt C, Richardson T, Plomion C (2004)Cross-species transferability and mapping of genomic and cDNA SSRs in pines. Theoretical and Applied Genetics 109: 1204-1214.
CrossRef |
Gscholar
(9)
Chapuis MP, Estoup A (2007)Microsatellite null alleles and estimation of population differentiation. Molecular Biology and Evolution 24: 621-631.
CrossRef |
Gscholar
(10)
Comps B, Gömöry D, Letouzey J, Thiébaut B, Petit RJ (2001)Diverging trends between heterozygosity and allelic richness during postglacial colonization in the European Beech. Genetics 157: 389-397.
Online |
Gscholar
(11)
Davies SJ, Cavers S, Finegan B, White A, Breed MF, Lowe AJ (2013)Pollen flow in fragmented landscapes maintains genetic diversity following stand-replacing disturbance in a neotropical pioneer tree,
Vochysia ferruginea Mart. Heredity 115: 125-129.
CrossRef |
Gscholar
(12)
Deacon NJ, Cavender-Bares J (2015)Limited pollen dispersal contributes to population genetic structure but not local adaptation in
Quercus oleoides forests of Costa Rica. PLoS ONE 10 (9): e0138783.
CrossRef |
Gscholar
(13)
Dore AJ, Vieno M, Fournier N, Weston KJ, Sutton MA (2006)Development of a new wind-rose for the British Isles using radiosonde data, and application to an atmospheric transport model. Quarterly Journal of the Royal Meteorological Society 132: 2769-2784.
CrossRef |
Gscholar
(14)
Earl DA, Von Holdt BM (2011)STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources 4: 359-361.
CrossRef |
Gscholar
(15)
Eckert CG, Samis KE, Lougheed SC (2008)Genetic variation across species’ geographical ranges: the central-marginal hypothesis and beyond. Molecular Ecology 17: 1170-1188.
CrossRef |
Gscholar
(16)
Ennos RA, Dodson RK (1987)Pollen success, functional gender and assortative mating in an experimental plant population. Heredity 58: 119-126.
CrossRef |
Gscholar
(17)
Ennos RA (1994)Estimating the relative rates of pollen and seed migration among plant populations. Heredity 72: 250-259.
CrossRef |
Gscholar
(18)
Ennos AR (1997)Wind as an ecological factor. Trends in Ecology and Evolution 12: 108-111.
CrossRef |
Gscholar
(19)
Excoffier L, Lischer HEL (2010)Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources 10: 564-567.
CrossRef |
Gscholar
(20)
Fady B, Aravanopoulos FA, Alizoti P, Mátyás C, Von Wühlisch G, Westergren M, Belletti P, Cvjetkovic B, Ducci F, Huber G, Kelleher CT, Khaldi A, Kharrat MBD, Kraigher H, Kramer K, Mühlethaler U, Peric S, Perry A, Rousi M, Sbay H, Stojnic S, Tijardovic M, Tsvetkov I, Varela MC, Vendramin GG, Zlatanov T (2016a)Evolution-based approach needed for the conservation and silviculture of peripheral forest tree populations. Forest Ecology and Management 375: 66-75.
CrossRef |
Gscholar
(21)
Fady B, Cottrell J, Ackzell L, Alía R, Muys B, Prada A, González-Martínez SC (2016b)Forests and global change: what can genetics contribute to the major forest management and policy challenges of the twenty-first Century? Regional Environmental Change 16: 927-939.
CrossRef |
Gscholar
(22)
FAO (2014)The state of the world’s forest genetic resources. Commission on genetic resources for food and agriculture, Rome, Italy, pp. 276.
Gscholar
(23)
Forrest GI (1980)Genotypic variation among native Scots pine populations in Scotland based on monoterpene analysis. Forestry 53: 101-128.
CrossRef |
Gscholar
(24)
Forrest GI (1982)Relationship of some European Scots pine populations to native Scottish woodlands based on monoterpene analysis. Forestry 55: 19-37.
CrossRef |
Gscholar
(25)
García-Gil MR, Floran V, Ostlund L, Mullin TJ, Gull BA (2015)Genetic diversity and inbreeding in natural and managed populations of Scots pine. Tree Genetics and Genomes 11 (2): 41.
CrossRef |
Gscholar
(26)
Gerlach G, Jueterbock A, Kraemer P, Deppermann J, Harmand P (2010)Calculations of population differentiation based on G
ST and D: forget G
ST but not all of statistics. Molecular Ecology 19: 3845-3852.
CrossRef |
Gscholar
(27)
González-Díaz P, Jump AS, Perry A, Wachowiak W, Lapshina E, Cavers S (2017)Ecology and management history drive spatial genetic structure in Scots pine. Forest Ecology and Management 400: 68-76.
CrossRef |
Gscholar
(28)
Goudet J (1995)Computer note. Journal of Heredity 86: 485-486.
CrossRef |
Gscholar
(29)
Graudal L, Aravanopoulos F, Bennadji Z, Changtragoon S, Fady B, Kjr ED, Loo J, Ramamonjisoa L, Vendramin GG (2014)Global to local genetic diversity indicators of evolutionary potential in tree species within and outside forests. Forest Ecology and Management 333: 35-51.
CrossRef |
Gscholar
(30)
Hamrick JL, Godt MJW, Sherman-Broyles SL (1992)Factors influencing levels of genetic diversity in woody plant species. New Forests 6: 95-124.
CrossRef |
Gscholar
(31)
Hardy OJ, Vekemans X (2002)Spagedi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Molecular Ecology Notes 2: 618-620.
CrossRef |
Gscholar
(32)
Hewitt GM (1999)Post-glacial re-colonization of European biota. Biological Journal of the Linnean Society 68: 87-112.
CrossRef |
Gscholar
(33)
Holderegger R, Kamm U, Gugerli F (2006)Adaptive
vs. neutral genetic diversity: implications for landscape genetics. Landscape Ecology 21: 797-807.
CrossRef |
Gscholar
(34)
Keenan K, Mcginnity P, Cross TF, Crozier WW, Prodöhl PA (2013)DiveRsity: an R package for the estimation and exploration of population genetics parameters and their associated errors. Methods in Ecology and Evolution 4: 782-788.
CrossRef |
Gscholar
(35)
Kelleher CT, Hodkinson TR, Kelly DL, Douglas GC (2004)Characterisation of chloroplast DNA haplotypes to reveal the provenance and genetic structure of oaks in Ireland. Forest Ecology and Management 189: 123-131.
CrossRef |
Gscholar
(36)
Kinloch B, Westfall RD, Forrest GI (1986)Caledonian Scots pine: origins and genetic structure. New Phytologist 104: 703-729.
CrossRef |
Gscholar
(37)
Lendvay B, Hohn M, Brodbeck S, Mindrescu M, Gugerli F (2014)Genetic structure in
Pinus cembra from the Carpathian mountains inferred from nuclear and chloroplast microsatellites confirms post-Glacial range contraction and identifies introduced individuals. Tree Genetics and Genomes 10: 1419-1433.
CrossRef |
Gscholar
(38)
Liewlaksaneeyanawin C, Ritland CE, El-Kassaby YA, Ritland K (2004)Single-copy, species-transferable microsatellite markers developed from loblolly pine ESTs. Theoretical and Applied Genetics 109: 361-369.
CrossRef |
Gscholar
(39)
Lindgren D, Paule L, Xihuan S, Yazdani R, Segerström U, Wallin J-E, Lejdebro ML (1995)Can viable pollen carry Scots pine genes over long distances? Grana 34: 64-69.
CrossRef |
Gscholar
(40)
Mason WL, Hampson A, Edwards C (2004)Managing the pinewoods of Scotland. Forestry Commission, Edinburgh, UK, pp. 234.
Online |
Gscholar
(41)
Naydenov KD, Naydenov MK, Tremblay F, Alexandrov A, Aubin-Fournier LD (2011)Patterns of genetic diversity that result from bottlenecks in Scots Pine and the implications for local genetic conservation and management practices in Bulgaria. New Forests 42: 179-193.
CrossRef |
Gscholar
(42)
Nei M (1987)Molecular evolutionary genetics. Columbia University Press, New York, pp. 512.
Gscholar
(43)
Nowakowska JA, Zachara T, Konecka A (2014)Genetic variability of Scots pine (
Pinus sylvestris L.) and Norway spruce (
Picea abies L. Karst.) natural regeneration compared with their maternal stands. Forest Research Papers 75: 47-54.
CrossRef |
Gscholar
(44)
Petit J, Csaikl UM, Bordács S, Burg K, Coart E, Cottrell J, Van Dam B, Deans JD, Dumolin-Lapègue S, Fineschi S, Finkeldey R, Gillies A, Glaz I, Goicoechea P, Jensen J, König A, Lowe AJ, Madsen SF, Matyas G, Munro RC, Olalde M, Pemonge M-H, Popescu F, Slade D, Tabbener H, Taurchini D, De Vries S, Ziegenhagen B, Kremer A (2002)Chloroplast DNA variation in European white oaks. Phylogeography and patterns of diversity based on data from over 2600 populations. Forest Ecology and Management 156: 5-26.
CrossRef |
Gscholar
(45)
Pritchard JK, Stephens M, Donnelly P (2000)Inference of population structure using multilocus genotype data. Genetics 155: 945-959.
Online |
Gscholar
(46)
Provan J, Soranzo N, Wilson NJ, McNicol JW, Forrest GI, Cottrell JE, Powell W (1998)Gene-pool variation in caledonian and European Scots pine (
Pinus sylvestris L.) revealed by chloroplast simple-sequence repeats. Proceedings of the The Royal Society, Biological sciences 265: 1697-1705.
CrossRef |
Gscholar
(47)
Provan J, Beatty GE, Hunter AM, McDonald RA, McLaughlin E, Preston SJ, Wilson S (2007)Restricted gene flow in fragmented populations of a wind-pollinated tree. Conservation Genetics 9: 1521-1532.
CrossRef |
Gscholar
(48)
Robledo-Arnuncio JJ, Alia R, Gil L (2004a)High levels of genetic diversity in a long-term European glacial refugium of
Pinus sylvestris L. Forest Genetics 11: 239-248.
Online |
Gscholar
(49)
Robledo-Arnuncio JJ, Smouse PE, Gil L, Alia R (2004b)Pollen movement under alternative silvicultural practices in native populations of Scots pine (
Pinus sylvestris L.) in central Spain. Forest Ecology and Management 197: 245-255.
CrossRef |
Gscholar
(50)
Rousset F (1997)Genetic differentiation and estimation of gene flow from F-Statistics under isolation by distance. Genetics 145: 1219-1228.
Online |
Gscholar
(51)
Salmela MJ (2011)Adaptive genetic variation in Scots pine (
Pinus sylvestris L.) in Scotland. Phd Thesis, The University of Edinburgh, Edinburgh, UK, pp. 159.
Online |
Gscholar
(52)
Scalfi M, Piotti A, Rossi M, Piovani P (2009)Genetic variability of Italian southern Scots pine (
Pinus sylvestris L.) populations: the rear edge of the range. European Journal of Forest Research 128: 377-386.
CrossRef |
Gscholar
(53)
Sinclair WT, Morman JD, Ennos RA (1998)Multiple origins for Scots pine (
Pinus sylvestris L.) in Scotland: evidence from mitochondrial DNA variation. Heredity 80: 233-240.
CrossRef |
Gscholar
(54)
Sjölund MJ, González-Díaz P, Moreno-Villa JJ, Jump AS (2017)Understanding the legacy of widespread population translocations on the post-glacial genetic structure of the European beech,
Fagus sylvatica L. Journal of Biogeography 44: 2475-2487.
CrossRef |
Gscholar
(55)
Slatkin M (1985)Gene flow in natural populations. Annual Review of Ecology, Evolution, and Systematics 16: 393-430.
CrossRef |
Gscholar
(56)
Soranzo N, Provan J, Powell W (1998)Characterization of microsatellite loci in
Pinus sylvestris L. Molecular Ecology 7: 1260-1261.
Gscholar
(57)
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004)Micro-Checker: software for identifying and correcting genotyping errors in microsatellite data. Molecular Ecology Notes 4: 535-538.
CrossRef |
Gscholar
(58)
Varis S, Pakkanen A, Galofré A, Pulkkinen P (2009)The extent of south-north pollen transfer in finnish Scots pine. Silva Fennica 43: 717-726.
CrossRef |
Gscholar
(59)
Wachowiak W, Salmela MJ, Ennos RA, Iason G, Cavers S (2011)High genetic diversity at the extreme range edge: nucleotide variation at nuclear loci in Scots pine (
Pinus sylvestris L.) in Scotland. Heredity 106: 775-787.
CrossRef |
Gscholar
(60)
Wachowiak W, Iason GR, Cavers S (2013)Among population differentiation at nuclear genes in native Scots pine (
Pinus sylvestris L.) in Scotland. Flora 208: 79-86.
CrossRef |
Gscholar
(61)
Wahlund S (1928)The combination of populations and the appearance of correlation examined from the standpoint of the study of heredity. Hereditas 11: 65-106.
CrossRef |
Gscholar
(62)
Whittet R, Cavers S, Cottrell J, Rosique-Esplugas C, Ennos R (2017)Substantial variation in the timing of pollen production reduces reproductive synchrony between distant populations of Pinus sylvestris L. in Scotland. Ecology and Evolution 7: 5754-5765.
CrossRef |
Gscholar
(63)
Wright S (1943)Isolation by distance. Genetics 28: 114-138.
Gscholar