Mini-tunnel and season influence in clonal garden on the production of clonal seedlings for two subtropical clones: Eucalyptus saligna and Corymbia torelliana × Corymbia citriodora
iForest - Biogeosciences and Forestry, Volume 18, Issue 3, Pages 154-162 (2025)
doi: https://doi.org/10.3832/ifor4460-018
Published: Jun 09, 2025 - Copyright © 2025 SISEF
Research Articles
Abstract
Improving technologies for clonal seedling production is essential due to the expansion of forestry production areas. The objective of this study was to assess the impact of managing mini-tunnels in a clonal mini-garden on the productivity and physiological quality of mini-stumps, as well as on the survival and rooting of mini-cuttings of Eucalyptus and Corymbia clones throughout the four seasons. For this purpose, the experiments were conducted in two phases: in the first phase, the clonal mini-garden phase, two clonal mini-gardens were established, one with a mini-tunnel and the other without. Each mini-garden contained mini-stumps of Eucalyptus saligna and hybrid clones of Corymbia torelliana × Corymbia citriodora. During this phase, environmental variables (temperature and relative humidity) were monitored in the cultivation environments (a mini-garden with and without a mini-tunnel), along with productivity and hydrogen peroxide concentration. In the second phase, in the rooting house, survival (%) and rooting (%) were evaluated. The use of mini-tunnels increased environmental variables near the mini-stumps throughout all four seasons. The presence of the mini-tunnel promoted an increase in mini-stump productivity for both genetic materials throughout the year and positively influenced the survival and rooting of mini-cuttings, demonstrating the effectiveness of the structure in mini-stump preconditioning, especially during seasons with lower temperatures. Therefore, the use of mini-tunnels in the clonal mini-garden during autumn and winter is recommended to increase the production of Eucalyptus saligna and Corymbia torelliana × Corymbia citriodora seedlings.
Keywords
Minicuttings, Cuttings Pre-conditioning, Vegetative Propagation, Rooting, Climatic Conditions
Introduction
As global awareness of the need to protect biodiversity in flora and fauna grows, the rising global population has led to increased consumption of goods and services from the forest sector. As a result, the sector has shifted from relying raw materials sourced from native forests to using those obtained from plantations ([21]).
Plantations of fast-growing tree species, such as Eucalyptus and Corymbia, play a significant role in the conservation of native forests worldwide ([6]). Additionally, forests planted with these genera offer benefits related to soil use and conservation, as well as high rates of atmospheric carbon fixation ([3]).
In Brazil, there are more than nine million hectares of plantations, with approximately 75% consisting of species from the genus Eucalyptus ([20]). Given that Brazil is the world’s largest producer of eucalyptus timber ([19]), the increase in planted area and the excellent adaptation of this genus to various edaphic and climatic conditions, makes it of great importance to the national economy ([32]). Among the planted species, clones of Eucalyptus saligna Smith are widely used in forest plantations throughout southern Brazil, primarily due to their adaptability to various conditions and the high quality of their wood. These clones serve as a valuable source of raw material for cellulose production ([16]).
The hybrids of the genus Corymbia have also attracted the interest of companies in the forestry sector. These hybrids are a promising alternative for various areas of forest production, primarily because of their high resistance to pests and diseases, wind resilience, and tolerance to water shortages. Additionally, the quality of their wood serves as a viable raw material alternative to eucalyptus. Despite these advantages, clonal propagation of Corymbia hybrids presents challenges, which has contributed to their limited cultivation ([4]). However, private companies and researchers in the forestry sector are motivated to invest in studies aimed to acquire the suitable quality and quantity of clonal seedlings for large-scale forest plantations.
Environmental conditions such as temperature, relative air humidity, and luminosity directly affect the production of clonal seedlings, particularly during the initial production phase in a clonal mini-garden and during the rooting stage. In addition, studies have shown that in subtropical regions, the highest production of mini-cuttings occurs during warmer seasons, whereas the highest percentages of rooting are observed during colder seasons ([8], [24]).
In this context, studies have focused on improving management techniques at various stages of seedling production through mini-cutting, with the goal of minimizing the negative effects of seasonality on rooting. The use of mini-tunnels in mini-clonal gardens has become prominence in both tropical and subtropical regions ([5], [10]) because they create favorable conditions for the development of mini-stumps. However, there is still a lack of information regarding different genetic materials, especially hybrids of Corymbia, which is an important genus in the forestry sector and is known for its difficulty in rooting. Previous research has reported favorable rooting results for Corymbia hybrids and Eucalyptus clones when mini-tunnels were used in mini-cuttings in greenhouses during colder seasons, demonstrating their effectivenes ([24]).
The objective of the present study was to evaluate the effect of a mini-tunnel in the clonal mini-garden on the productivity, survival, and rooting success of mini-cuttings taken from clones of the genera Eucalyptus and Corymbia across different seasons. Our initial hypotheses were: (i) the use of the mini-tunnel increases the productivity of shoots in mini-stumps of Eucalyptus saligna and Corymbia torelliana × Corymbia citriodora clones during the different seasons of the year; and (ii) mini-cuttings produced in environments containing a mini-tunnel have a higher percentage of rooting.
Material and methods
Study site
The study was conducted at the Forest Nursery of the company Celulose Riograndense (CMPC), located in the Horto Florestal Barba Negra, in the municipality of Barra do Ribeiro, Rio Grande do Sul (30° 20′ 33.63″ S, 51° 14′ 42.29″ W). According to the Köppen climate classification system, the local climate is characterized as humid subtropical (Cfa), with an average annual temperature between 18 and 20 °C ([2]).
Clonal mini-gardens
Two mini-gardens (MG) measuring 20.0 × 0.6 × 0.5 m (length, width, and height, respectively) were built in a greenhouse with a retractable roof. They consisted of masonry beds, with sand as the primary substrate. Two genetic materials were evaluated: a commercial clone of Eucalyptus saligna and a hybrid of Corymbia torelliana × Corymbia citriodora. The selection of these materials was based on the potential of the hybrid for inclusion in the operational line for pulp production. Additionally, considering the difficulties in rooting, the Eucalyptus clone consisted of an established operational material with lower rooting difficulty.
The experimental design in the mini-garden was a randomized block design, with six replications in each of two treatments: one with mini-tunnel coverage (Ce) and the other without (Se). In the E. saligna clone, the repetitions had 72 mini-strains. For C. torelliana × C. citriodora, each replication consisted of eight mini-stumps due to the limited availability of the material.
The E. saligna seedlings used for establishing the clonal mini-garden were selected based on their high vigor, with a height of approximately 15 cm, while the Corymbia torelliana × C. citriodora seedlings had an approximate height of 25 cm.
After planting and initial establishment in the clonal mini-garden, E. saligna plants were pruned approximately 4 cm from the base (neck), whereas those of C. torelliana × C. citriodora were initially bent at the same height, keeping the stem attached to one side until budding began, and then removed to conduct the mini-stump.
The mini-garden was irrigated by dripping, applied three to four times a day, depending on the variation in temperature and relative humidity during each season of the year, with 5 L m-2 day-1 of solution delivered to the mini-stumps and the electrical conductivity maintained at 1.8 mS m-2. The solution applied consisted of nitrate (367.5 mg L-1), monoammonium phosphate (60.2 mg L-1), potassium chloride (239.4 mg L-1), magnesium sulfate (113 .6 mg L-1), iron chelate (5.0 mg L-1), organic boron (0.5 mg L-1), manganese sulfate (1.8 mg L-1), copper (0. 15 mg L-1), and zinc sulfate (0.25 mg L-1) ([5], [24]). In the spring, the water supply in the mini-clonal garden was reduced to limit the production of mini-cuttings. This was done because the seedlings produced in this season will be planted in the summer, which is not an ideal time due to high temperatures and low rainfall.
Mini-tunnel
The mini-tunnel consisted of a metal structure covered with 0.15 mm thick transparent plastic film, measuring 20.0 × 1.0 × 0.6 m (length, width and height, respectively), and was installed over one of the MG.
Based on a pilot study, the management of the mini-tunnel was adapted for each season ([24]). During spring and autumn, the mini-tunnel was opened in the morning (8:30 am) and closed in the afternoon (4:00 pm). During winter, the opening was postponed to 9:30 am and closed at the same time as during spring and autumn (4:00 pm). In the summer, the opening was shifted to 8:00 am, and the closing was postponed to 5:00 pm. Still, it remained open when the temperature was equal to or greater than 32 °C, thus preventing the mini-stumps from being compromised by excessive heat ([5], [24]). Due to the high temperatures recorded during the summer, the mini-tunnel remained open for several nights.
Shoot collection and mini-cutting preparation
Six months after the installation of the mini-garden, mini-cuttings were collected from the apical portion of the shoots. Each cutting was approximately 8 cm in length, with a 50% reduction in leaf area. The collection was carried out early in the morning when temperatures were milder. To maintain turgidity after collection, the mini-cuttings were kept in thermal boxes with water for up to 30 minutes before planting in polypropylene tubes (55 cm3) filled with a substrate composed of 80% of sphagnum peat and 20% of vermiculite, along with the company’s standard base fertilizer, composed of controlled release fertilizer (NPK 19:06:10), simple superphosphate, and PG Mix®, at doses of 1.5 kg, 2.0 kg, and 2.0 kg m-3 of substrate, respectively. After staking, the trays were placed in a rooting house with high humidity, where they remained for approximately 45 days.
Irrigation in the rooting houses was performed using a micro-sprinkler system. For mini-cuttings older than 15 days, irrigation occurred at 10-minute intervals, with 30 seconds of irrigation at a flow rate of 150 mL min-1 in each microsprinkler. For mini-cuttings younger than 15 days, irrigation was performed at intervals of 20 min, maintaining the same flow rate used for the recently staked cuttings.
Experimental design and evaluated attributes
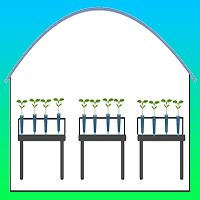
The experiment was conducted using a randomized block design with a split-plot scheme, with two types of mini-garden management approaches allocated to the main plot (environments with and without a mini-tunnel on the MG) and two clones in the subplots (E. saligna and C. torelliana × C. citriodora - Fig. 1). The evaluations were carried out in each of the four seasons (summer, autumn, winter and spring), monitoring the formation of mini-stumps, in the months of February, May, July and October, with average temperatures of 24.3, 17.1, 14.3 and 20.0 °C, respectively.
Fig. 1 - Schematic drawing representing the experimental design in the two phases of the experiment: clonal mini-garden phase (Ce and Se, left) and rooting house phase (right), with evaluated attributes in each phase.
Temperature and relative humidity (RH) were measured using automatic thermo-hygrometers (Datalogger - AK 174®) in the two environments of the MG (Ce and Se) during the entire experimental period (Fig. 1).
The number of mini-stumps was monitored throughout all seasons by recording the number of mini-cuttings per square meter in each collection. This assessment considered all mini-cuttings that were suitable for staking, i.e., those containing two to three pairs of leaves and measuring approximately 8 to 11 cm in height. Once each season, 20 mini-cuttings from each replicate of Eucalyptus saligna and 10 mini-cuttings from each replicate of Corymbia torelliana × Corymbia citriodora were staked, due to the limited availability of plant material in the hybrid.
To evaluate the hydrogen peroxide (H2O2) content, fully expanded leaves were collected from the central mini-stumps on specific days of each season. The leaves were placed in aluminum foil envelopes, and immediately frozen in liquid nitrogen. The samples were then transported to the Plant Physiology laboratory in the Department of Biology at the Federal University of Santa Maria (UFSM), where they were stored in an ultra-freezer at -80 °C for further analysis.
The hydrogen peroxide (H2O2) concentration was determined according to Loreto & Velikova ([25]), using 0.3 g of leaf samples homogenized in 3 mL of 0.1% trichloroacetic acid (w/v) after the homogenate was centrifuged at 12.000 rpm × g for 10 minutes at 4 °C. Subsequently, the supernatant (0.5 mL) was added to 10 mM potassium phosphate buffer (pH 7.0) and 1 mL KI (1M). The H2O2 concentration in the supernatant was determined by comparing the absorbance at 390 nm with that of a standard calibration curve. The H2O2 concentration was expressed in μmol g-1 fresh mass.
Survival was visually assessed 30 days after staking the mini-cuttings, focusing only on the live mini-cuttings that exhibited a green color and a turgid appearance. The percentage of mini-cutting survival (%SUR) was determined using the following equation (eqn. 1):
where SM denotes staked mini-cuttings; and LM indicates live mini-cuttings.
Rooting was assessed 45 days after staking. Mini-cuttings that remained firmly in the container or exhibited a noticeable root system at the container’s base were deemed as rooted. The rooting percentage of the mini-cuttings (%ROT) was determined using the following equation (eqn. 2):
where SM denotes staked mini-cuttings, and RM indicates rooted mini-cuttings.
Statistical analysis
The normality of errors and the homogeneity of variances were evaluated using the Shapiro-Wilk and Bartlett tests, respectively. When these assumptions were not met, the data were transformed using the BOX-COX transformation. Data were then submitted to analysis of variance (ANOVA), and once interaction between the factors was confirmed, the interactions were broken down, and the averages were compared via the Tukey test (α = 0.05) using the statistical package "ExpDes.pt" ([14]) of the R software ([28]).
Finally, Pearson’s correlation analysis was performed to test for association between environmental variables and other variables linked to seedling production.
Results
We observed that the use of a mini-tunnel increased the average temperature near the mini-stumps by approximately 1 °C in all seasons. In the mini-tunnel environment, the temperature ranged from 16 to 27 °C between the coldest and hottest seasons (Fig. 2). In these contrasting seasons, the relative humidity of the air under the mini-tunnel increased by 15 and 7%, varying from 80% to 90% in summer and winter, respectively (Fig. 2).
Fig. 2 - Average temperature and relative humidity in the clonal mini-garden with (Ce) and without (Se) a mini-tunnel throughout all seasons, as measured at Horto Florestal Barba Negra, Barra do Ribeiro, Rio Grande do Sul. Environmental variables were measured during the clonal garden phase. (Temp_Ce): temperature in the environment with the mini-tunnel; (Temp_Se): temperature in the environment without the mini-tunnel; (RH_Ce): relative humidity in the environment with the mini-tunnel; (RH_Se): relative humidity in the environment without the mini-tunnel.
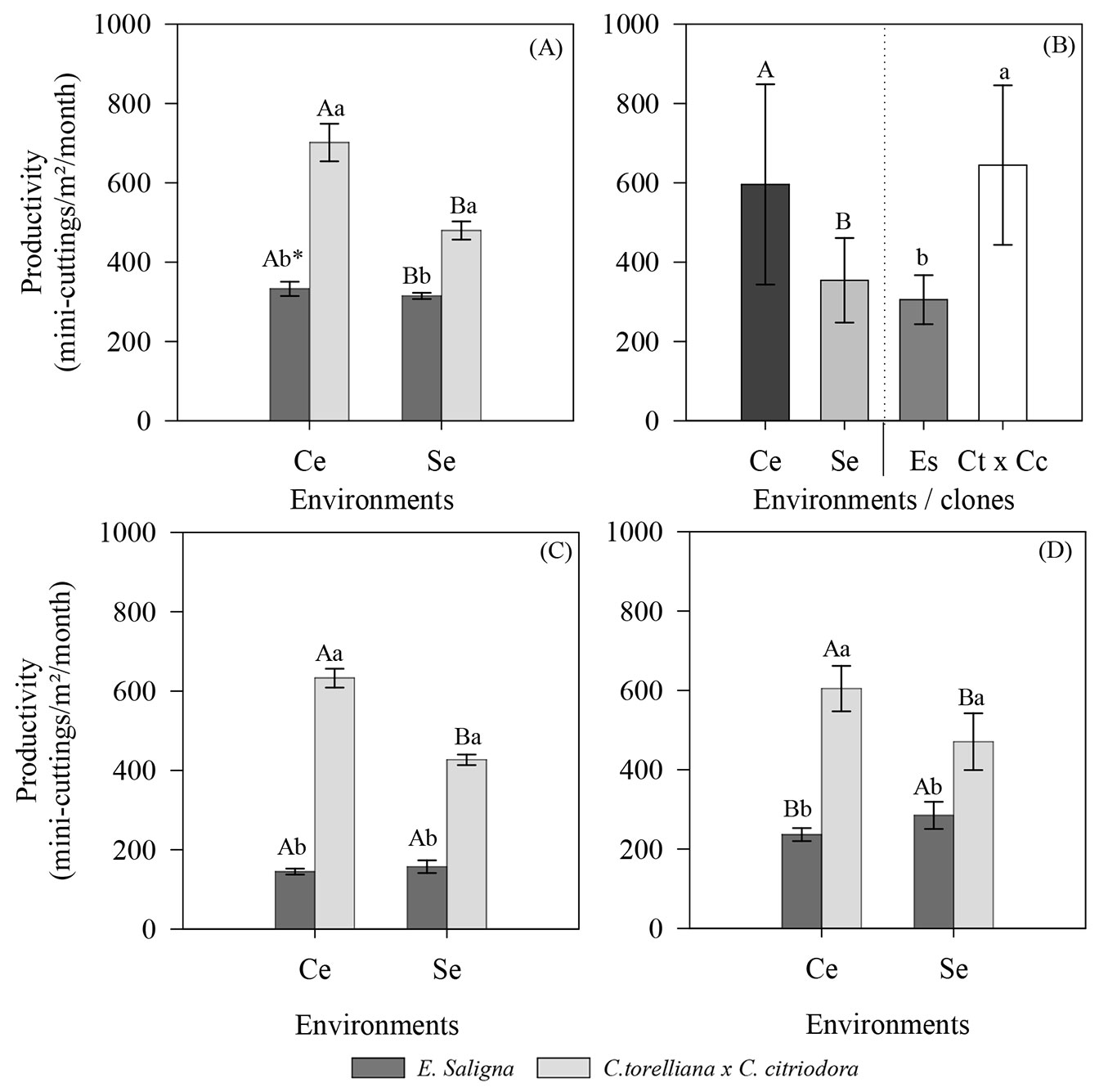
Mini-stump productivity exhibited significant interactions between study factors (environment × clone) in summer (p = 0.0022), winter (p = 0.00009), and spring (p = 0.0015). During the summer and winter seasons, the clones exhibited similar behavior, with increased productivity when the mini-stumps were cultivated in a mini-tunnel environment. Under these conditions, mini-stumps of the C. torelliana × C. citriodora hybrid showed average productivity of 701 and 632 mini-cuttings m-2 month-1 during the winter and summer seasons, respectively. In contrast, the clone of E. saligna had lower productivities of 333 and 144 mini-cuttings m-2 month-1 during the same seasons, respectively (Fig. 3A, Fig. 3C).
Fig. 3 - Productivity of the mini-strains of E. saligna and C. torelliana × C. citriodora, cultivated in different environments of the mini-clonal garden during the summer (A), autumn (B), winter (C), and spring (D) of 2018. Averages followed by the same uppercase (environments) and lowercase (clones) letters do not differ according to Tukey’s test (p > 0.05). Values are presented as mean ± standard error. (Ce): use of a mini-tunnel; (Se): absence of a mini-tunnel; (Es): Eucalyptus saligna; (Ct × Cc): Corymbia torelliana × Corymbia citriodora. Variable analyzed in the clonal garden phase.
In autumn, only single experimental factors showed a significant effect after ANOVA (p = 0.0003 and p = 2.2 × 10-16 for environments and clones, respectively). At this station, the mini-tunnel increased mini-stump productivity by approximately 40 percentage points (p.p.) compared to the environment without the mini-tunnel. As for the productivity of genetic materials, the highest production of mini-cuttings was observed for C. torelliana x C. citriodora (644.83 mini-cuttings m-² month-¹), approximately 47 p.p. more than that of E. saligna (305.67 mini-cuttings m-² month-¹ - Fig. 3B).
Unlike the other seasons, the E. saligna clone during spring showed higher productivity in the environment without the mini-tunnel (284.64 mini-cuttings m-2 month-1). In contrast, C. torelliana × C. citriodora showed higher mini-stump productivity in the mini-tunnel environment (604.49 mini-cuttings m-² month-¹), similar to what was observed during other seasons (Fig. 3D). We also recorded that C. torelliana × C. citriodora showed higher productivity in both environments compared to E. saligna clone (Fig. 3D).
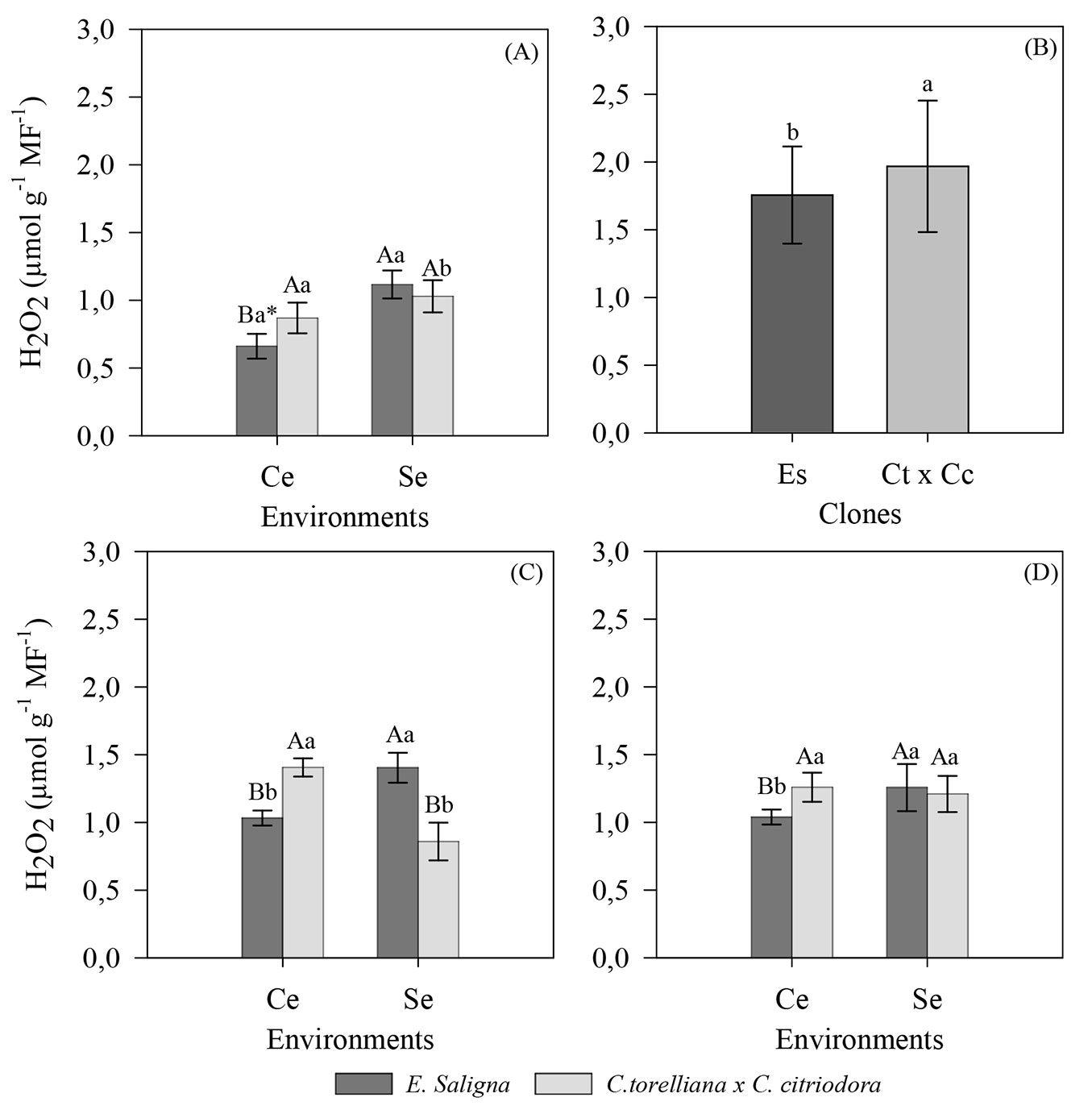
Regarding the concentrations of hydrogen peroxide (H2O2), the clones were significantly influenced by the production environments (interaction environments × clones) during summer (p = 0.0002), winter (p = 2.2 × 10-16), and spring (v = 0.0474). During autumn, there was a significant effect only for the clone factor (p = 4.7 × 10-5).
During summer, the E. saligna clones had the lowest mean H2O2 concentration (0.66 µmol g-1 MF-1) in the mini-tunnel environment, which was approximately 41 p.p. lower than that observed in plants cultivated without the mini-tunnel (Fig. 4A). However, no differences were observed between the environments studied in C. torelliana × C. citriodora (Fig. 4A).
Fig. 4 - Hydrogen peroxide concentration in mini-strains of E. saligna and C. torelliana × C. citriodora cultivated in different environments during (A) summer, (B) autumn, (C) winter, and (D) spring of 2018. Averages followed by the same uppercase (environments) and lowercase (clones) letters do not differ according to Tukey’s test (p > 0.05). Values are presented as mean ± standard error. (Ce): use of a mini-tunnel; (Se): absence of a mini-tunnel; (Es): Eucalyptus saligna; (Ct × Cc): Corymbia torelliana × Corymbia citriodora. Variable analyzed in the clonal garden phase.
During autumn, the cultivation environment did not affect the H2O2 concentrations; only differences between clones were significant (p = 4.7 × 10-5). The highest concentrations of H2O2 were observed in C. torelliana × C. citriodora (2.20 µmol g-1 MF-1), which were 68.8 p.p. higher than those observed for E. saligna (Fig. 4B).
During winter, similar to summer, there was a reduction in the H2O2 concentration in the mini-cuttings placed in the mini-garden with the mini-tunnel for E. saligna, whereas it increased for C. torelliana × C. citriodora (Fig. 4C).
During spring, E. saligna had the lowest concentrations of H2O2 in the mini-tunnel environment (1.03 µmol g-1 MF-1). We did not observe any significant differences in H2O2 concentrations in the hybrid during spring (Fig. 4D).
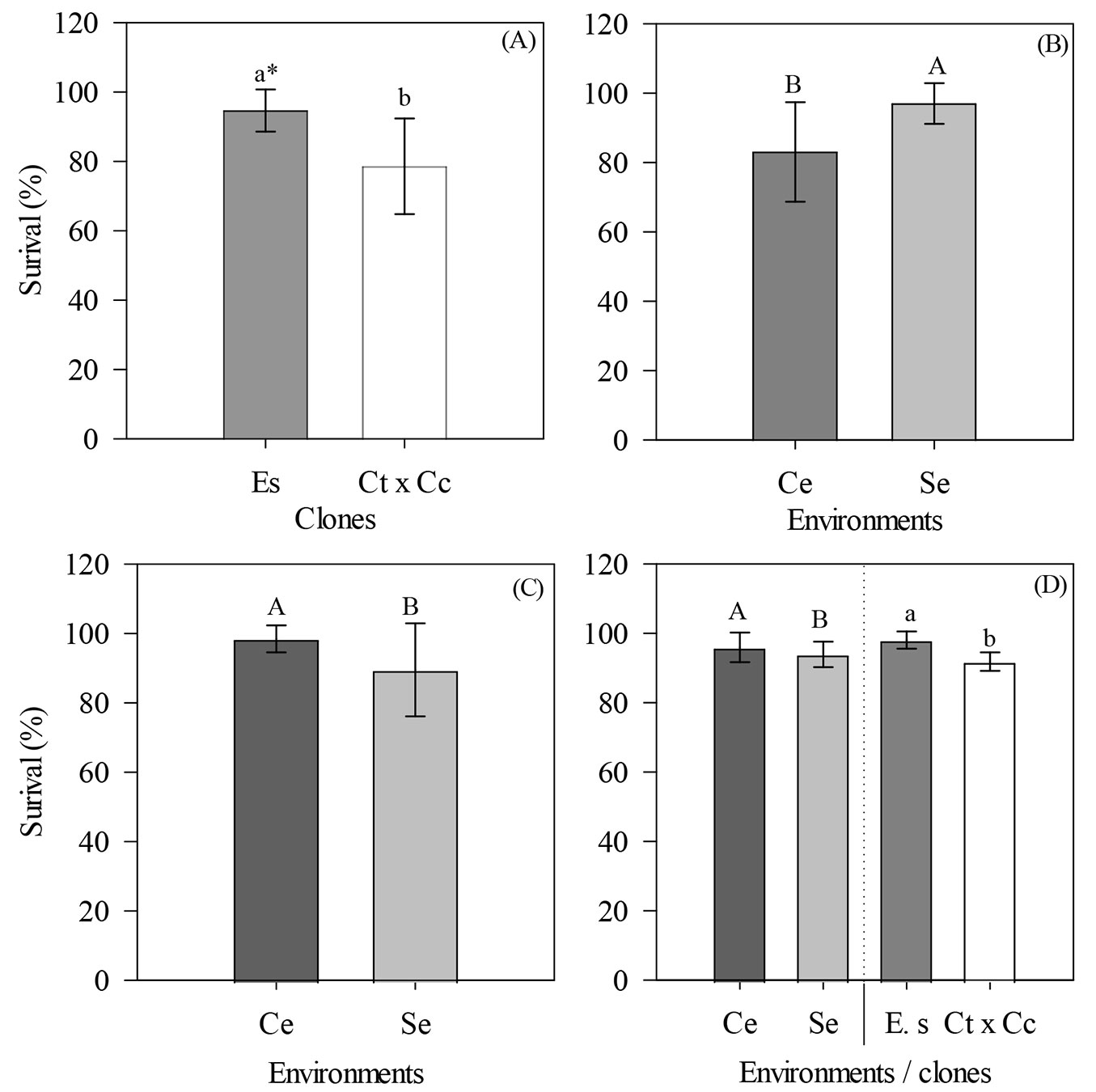
In general, mini-cutting survival during summer, autumn, and spring was higher for the E. saligna clone than for the C. torelliana × C. citriodora hybrid (Fig. 5A, Fig. 5D). During the summer, we determined that E. saligna (94.69%) had the highest percentage of mini-cutting survival, which was approximately 16 p.p. higher than that of C. torelliana × C. citriodora (78.61% - Fig. 5A).
Fig. 5 - Percentage of mini-cuttings of E. saligna and C. torelliana × C. citriodora that survived in different environments during (A) summer, (B) autumn, (C) winter, and (D) spring of 2018. Averages followed by the same uppercase (environments) and lowercase (clones) letters do not differ according to Tukey’s test (p > 0.05). Values are presented as mean ± standard error. (Ce): use of a mini-tunnel; (Se): absence of a mini-tunnel; (Es): Eucalyptus saligna; (Ct × Cc): Corymbia torelliana × Corymbia citriodora. Variable analyzed in the rooting house phase.
During autumn, the environment without the mini-tunnel showed the highest survival rates, close to 100%, which is approximately 14 p.p. higher than that observed in the environment with the mini-tunnel (Fig. 5B). Similarly, during winter, we only detected a significant effect for the environment factor (p = 0.0308), but the highest survival rates were observed for mini-cuttings collected in the mini-garden with the mini-tunnel (98.49%), which was approximately 10 percentage points higher than those collected in the environment without the mini-tunnel (89.52% - Fig. 5C).
During the spring, we observed significant effects of the environment (p = 0.0269) and clones (p = 6 × 10-6) in isolation. The presence of the mini-tunnel increased the survival of mini-cuttings (95.99%) compared to the treatment without mini-tunnel (93.95% - Fig. 5D). The clone E. saligna had superior survival (98.07%) to that of the hybrid C. torelliana × C. citriodora (91.87% - Fig. 5D).
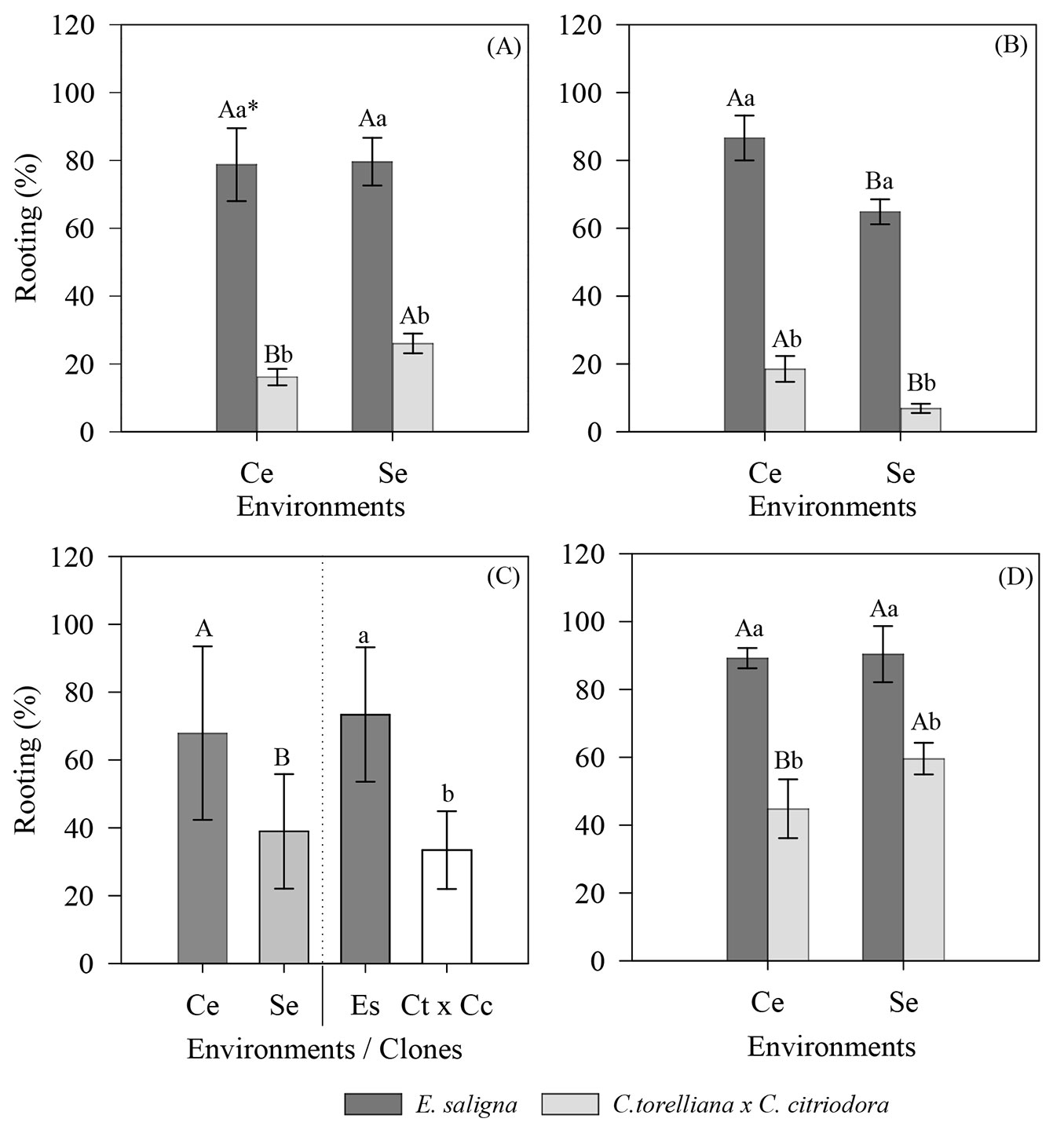
We found significant interactions between the genetic materials and cultivation environments in the percentage of rooting in the summer (p = 0.0088), autumn (p = 0.0187), and spring (p = 0.0025). Noteworthy, the E. saligna clone demonstrated superior rooting compared to the C. torelliana × C. citriodora hybrid in both environments with and without the mini-tunnel across all seasons.
During summer, there were no differences in the rooting of mini-cuttings collected in different environments for E. saligna. In contrast, the hybrid C. torelliana × C. citriodora had the highest percentages of rooting for mini-cuttings cultivated without the mini-tunnel, with an increase of 10 p.p. rooting in relation to those from the mini-tunnel environment (Fig. 6A).
Fig. 6 - Rooting of mini-cuttings of E. saligna and C. torelliana × C. citriodora grown in different environments during the (A) summer, (B) autumn, (C) winter, and (D) spring of 2018. Averages followed by the same uppercase (environments) and lowercase (clones) letters do not differ according to Tukey’s test (p > 0.05). Values are presented as mean ± standard error. (Es): Eucalyptus saligna; (Ct × Cc): Corymbia torelliana × Corymbia citriodora. Variable analyzed in the rooting house phase.
During autumn, the mini-tunnel environment increased rooting for both clones by approximately 12 p.p. for C. torelliana × C. citriodora and 22 p.p. for those cultivated without the mini-tunnel (Fig. 6B). During winter, mini-cuttings produced in the mini-tunnel demonstrated greater rooting success in both clones (67.9% - Fig. 6C). The E. saligna clone showed the highest percentage of rooting (73.4%) in comparison to that observed for the C. torelliana x C. citriodora hybrid (33.4% - Fig. 6C).
During spring, E. saligna showed similar rooting percentages (90%) in both MG environments. In contrast, for C. torelliana × C. citriodora, the environment without the mini-tunnel promoted an increase in rooting of mini-cuttings, which was approximately 15 p.p. superior to the environment with the mini-tunnel (Fig. 6D).
Pearson’s correlation analysis of the E. saligna clones revealed a negative correlation between temperature and RH (r = -0.39). In contrast, productivity was positively correlated with temperature (r = 0.85) and, consequently, negatively correlated with RH (r = -0.36). Also, rooting was positively correlated with RH (0.28). Both environmental variables were negatively correlated with H2O2 concentrations (r = -0.33 and r = -0.30 for temperature and relative humidity, respectively - Tab. 1).
Tab. 1 - Results of Pearson’s correlation analysis between the environmental variables of the different environments of the clonal mini-garden and the production variables for the E. saligna clone. (Temp): temperature; (RH): relative humidity; (Peroxide): hydrogen peroxide; (*): low correlation; (**): medium correlation; (***): high correlation.
| Variable | Temp | RH | Productivity | Surival | Rooting | Peroxide |
|---|---|---|---|---|---|---|
| Temp | 1 | -0.39** | 0.85*** | -0.09 | 0.27 | -0.33* |
| RH | - | 1 | -0.36* | -0.069 | 0.28 | -0.30* |
| Productivity | - | - | 1 | -0.24 | 0.18 | -0.11 |
| Surival | - | - | - | 1 | 0.051 | -0.1 |
| Rooting | - | - | - | - | 1 | -0.041 |
| Peroxide | - | - | - | - | - | 1 |
For the hybrid C. torelliana × C. citriodora, we found a negative correlation between environmental variables (r = -0.39), but a positive correlation between environmental variables and minimum productivity. For rooting, a negative correlation was observed with temperature (r = -0.20), while a positive correlation was found with RH (r = 0.27). Additionally, a strong positive correlation existed between rooting and hydrogen peroxide concentration (r = 0.76 - Tab. 2).
Tab. 2 - Results of Pearson’s correlation analysis between the environmental variables of the different environments of the clonal mini-garden and the production variables for the C. torelliana x C. citriodora hybrid. (Temp): temperature; (RH): relative humidity; (Peroxide): hydrogen peroxide; (*): low correlation; (**): medium correlation; (***): high correlation.
| Variable | Temp | RH | Productivity | Survival | Rooting | Peroxide |
|---|---|---|---|---|---|---|
| Temp | 1 | -0.39** | 0.28 | -0.31* | -0.2 | 0.027 |
| RH | - | 1 | 0.56*** | 0.027 | 0.27 | -0.085 |
| Productivity | - | - | 1 | -0.14 | 0.15 | 0.18 |
| Surival | - | - | - | 1 | 0.2 | 0.11 |
| Rooting | - | - | - | - | 1 | 0.76** |
| Peroxide | - | - | - | - | - | 1 |
Discussion
The productivity of the mini-strains of the C. torelliana x C. citriodora hybrid was significantly higher than that of the E. saligna clone (Fig. 2), particularly when the mini-strains of the hybrid were cultivated in an environment protected by a mini-tunnel. However, this environment did not appear to benefit E. saligna productivity throughout the year.
Nonetheless, the survival of both genetic materials was “slightly” higher when mini-cuttings were produced in MG_Ce. This indicates that the rooting environment was favorable for maintaining the turgidity of propagules, allowing for effective translocation of tissue reserves and induction of roots.
For the C. torelliana × C. citriodora hybrid, we observed higher productivity of mini-stumps in environments with mini-tunnels during the summer, winter, and spring. This was likely due to the increase in temperature within the structure, which promoted root growth and nutrient absorption. As a result, it accelerated mini-stump metabolism, increasing cell division and, consequently, shoot production ([18], [31]).
We found that the productivity of C. torelliana × C. citriodora was higher than that of E. saligna in all seasons of the year. However, the hybrid showed lower percentages of survival and rooting in all seasons evaluated, confirming the recalcitrance to rooting of mini-cuttings.
During the winter and spring seasons, the mini-tunnel promoted an increase in the productivity of the mini-stumps for both genetic materials. This improvement may be due to the conditioning of the mini-stumps during the summer months, which required greater energy expenditure and initiated a “winter dormancy”. This process enhances the quality of the plants, providing a stronger source of propagules and resulting in higher-quality seedlings. Consequently, this leads to an increase in shoot production ([27]).
We did not find any significant pattern in survival between the different environment for producing mini-cuttings. However, the rooting house presented more suitable microclimatic conditions for E. saligna than for the Corymbia hybrid. This suggests the need to group similar genetic materials in specific management practices to avoid losses associated with management, which are primarily caused by high temperatures and low humidity, as observed in summer and autumn.
Rooting showed the same trend as survival but confirmed greater losses for the hybrid despite its high productivity, demonstrating the superiority of the E. saligna clone (Fig. 4). During colder seasons, the mini-tunnel increased rooting percentages in both clones, with emphasis on the hybrid, suggesting that the mini-tunnel is an essential structure for propagating hybrids of the genus Corymbia (Fig. 4C), and supporting its adoption by forestry companies.
In contrast to other regions of Brazil, the southern region is characterized by regular rainfall, which helped maintain the relative air humidity in the clonal mini-garden. As a result, the photosynthetic rates of the mini-stumps increased, leading to higher concentrations of photoassimilates ([31]) and, consequently, an increase in mini-stump shoot productivity ([1], [11]). Summer is characterized by higher temperatures and longer insolation times than winter, during which temperatures are lower and photoperiods are shorter ([26], [35]). In this sense, the variation in solar radiation within the greenhouse throughout different seasons affects the temperature, leading to changes in the metabolic activity of the plants and mini-stumps. This, in turn, results in variations in their productivity and the rooting success of mini-cuttings ([36]).
An increase in air temperature up to 30 °C has been reported to increase in gas exchange ([12]), resulting in greater carbon assimilation and water loss through evapotranspiration. This positively affects the plant productivity, but can reduce the rooting of mini-cuttings, as an excess of light and extreme temperatures may lead to photoinhibition (Fig. 2, Fig. 4).
We found that the mini-tunnel promoted an increase in temperature and productivity during colder seasons (Fig. 1), as infered by the positive correlation between temperature and productivity observed for both studied clones (Fig. 6), particularly for C. torelliana × C. citriodora. Cunha et al. ([11]) obtained similar results in clones of Eucalyptus grandis W. Hill ex Maiden and Eucalytus urophylla S. T. Blake, which presented higher shoot productivity with an increase in temperatures in MG. Alfenas et al. ([1]) noted that optimal temperatures for rooting are between 25 and 30 °C. According to the same authors, an excessive variation in temperature can damage rooting. Using a mini-tunnel enables a more precise temperature control, particularly during mild seasons, and favors a higher concentration of CO2 in the production enviroment ([24], [17]). Genetic materials of subtropical origin tend to respond positively in this controlled MG environment.
Research findings similar to those observed in this study have been reported for clones of the genus Eucalyptus ([9], [5]). These studies indicate that mini-stump productivity increases during warmer seasons. However, higher temperatures can lead to decreased survival and rooting rates of mini-cuttings ([7]).
Plants exposed to high temperatures typically show increased stomatal conductance, reduced photosynthetic rates, and increased transpiration ([31]). In clonal mini-gardens, despite frequent irrigation, the water potential tends to decrease with increasing temperature. This decline can lead to reduced turgor pressure in plant cells, which negatively affects the rooting process ([15]). In this study, we observed that warmer temperatures during the growing season may have hindered rooting. Conversely, low temperatures slow down metabolic processes, requiring mini-cuttings to take longer to initiate root development ([34]).
It should be noted that the highest rooting averages were recorded for the E. saligna clone, which demonstrates better adaptation to local environmental conditions and is easier to root than the studied hybrid. The clone of C. torelliana x C. citriodora showed a high productivity when grown in a clonal mini-garden with a mini-tunnel. However, it requires further research regarding its recalcitrance to rooting. It is essential to prioritize the rejuvenation of the plant material used ([33]) and to adopt specific management practices in the rooting house, focusing on temperature, relative humidity, and irrigation depth.
It was also verified that during summer, the relative air humidity was lower than during other seasons, with the highest values occurring during winter in both management environments (Fig. 2). Hartmann et al. ([18]) highlighted the importance of controlling the relative humidity of the air, as its excess can cause the emergence of pathogens. Lima et al. ([24]) and Griebeler et al. ([17]) found that a mini-tunnel over a clonal mini-garden increases the relative humidity throughout all seasons, and this enhanced rooting of different Eucalyptus clones and Corymbia hybrids.
In this study, a lower mini-stump productivity was observed in spring, despite temperatures and humidity levels similar to those of summer. It is important to note that during spring, irrigation is reduced in the MG, intentionally aimed at decreasing the number of mini-cuttings. This is because the seedlings produced during spring would be ready for field planting in summer, a time considered unsuitable for planting in southern Brazil due to low precipitation and high temperatures ([35]).
During autumn, the highest productivity was achieved in the mini-tunnel environment with higher average temperatures, promoting an increase in mini-stump production. According to Assis ([4]), a mini-tunnel increases the temperature and relative humidity of the air, which in turn enhances the production of sprouts from mini-stumps.
High temperatures favor the production of hydrogen peroxide in plants ([30]). This study confirmed that the production of H2O2 increased during autumn due to fluctuations in environmental factors, primarily temperature. Although H2O2 is an oxidizing agent, it can be beneficial for plant production. The increase in hydrogen peroxide in plants can reduce stomatal conductance and, consequently, transpiration ([13]), favoring the maintenance of cell turgor. In this study, the increase in H2O2 in the mini-stumps enabled the maintenance of turgidity in the hybrid mini-cuttings, thus fostering their rooting rate. During the season with lower temperatures, we found that the mini-tunnel increased the H2O2 concentration for the hybrid (1.3 µmol g-1 MF-1), which may have favored an increased productivity of mini-stumps and rooting of mini-cuttings for C. torelliana × C. citriodora. Indeed, an increase in H2O2 is directly related to a higher concentration of auxins ([22], [23]), which promotes an increase in rooting percentage ([29]). This is corroborated by the results of the correlation analysis, which showed the highest rooting averages in C. torelliana × C. citriodora to be significantly associated with increases in H2O2 concentrations up to certain levels (Tab. 2).
Conclusions
In subtropical regions, the use of a mini-tunnel in a clonal mini-garden increased the productivity of mini-stumps of Corymbia torelliana × Corymbia citriodora during all four seasons of the year.
During autumn and winter, the use of the mini-tunnel promoted an increase in the rooting percentages of mini-cuttings of the studied clones, demonstrating the effectiveness of the structure in colder seasons, with particular emphasis on the Corymbia torelliana × Corymbia citriodora clone.
We suggest paying more attention to the management of opening and closing mini-tunnels in clonal mini-gardens, especially considering their efficiency during colder periods (16 °C). During the autumn and spring seasons, the greater efficiency of using the mini-tunnel was evident, increasing the production of clonal seedlings of Eucalyptus saligna and Corymbia torelliana × Corymbia citriodora.
We emphasize the importance of characterizing genetic materials to understand the differences between cultivation conditions and environments, aiming to improve the production and rooting processes of mini-cuttings for each clone.
Acknowledgements
The Coordination for the Improvement of Higher Education Personnel (Capes) granted a research grant to the first author, and CNPq granted a productivity grant to Professor Maristela Machado Araujo (Process 31294/2022-4). Celulose Riograndense provided structural and logistical support. Thanks to Master Luiza Somavilla for her collaboration in setting up, conducting the experiments, and collecting the data used in this work.
List of abbreviations
The abbreviations listed below have been used throughout the text:
- MG - mini-garden
- Ce - mini-garden with mini-tunnel coverage
- Se - mini-garden without mini-tunnel
- ES: Eucalytpus saligna
- CTxCC: Corymbia torelliana × Corymbia citriodora
Declaration
The authors declare no conflict of interest.
References
Gscholar
CrossRef | Gscholar
Online | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Maristela Machado Araujo 0000-0003-3751-8754
Suelen Carpenedo Aimi 0000-0002-2502-6325
asparin Ezequiel 0000-0001-6362-2964
Department of Forest Sciences, Federal University of Santa Maria, Santa Maria, Rio Grande do Sul (Brazil)
Department of Forest Sciences, Federal University of Paraná, Curitiba, Paraná (Brazil)
Federal Rural University of the Amazon, Capitão Poço, Pará (Brazil)
Osmarino Pires Dos Santos 0000-0003-3036-8861
CMPC - Celulose Riograndense, Guaíba, Rio Grande do Sul (Brazil)
Eldorado Brasil Celulose S.A., Três Lagoas, Mato Grosso do Sul (Brazil)
Corresponding author
Paper Info
Citation
Costella C, Araujo MM, Aimi SC, Berghetti ÁLP, Griebeler AM, De Lima MS, E, Dos Santos OP, Valente BMDRT (2025). Mini-tunnel and season influence in clonal garden on the production of clonal seedlings for two subtropical clones: Eucalyptus saligna and Corymbia torelliana × Corymbia citriodora. iForest 18: 154-162. - doi: 10.3832/ifor4460-018
Academic Editor
Pierluigi Paris
Paper history
Received: Aug 25, 2023
Accepted: Feb 12, 2025
First online: Jun 09, 2025
Publication Date: Jun 30, 2025
Publication Time: 3.90 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2025
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 5311
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 2197
Abstract Page Views: 1267
PDF Downloads: 1709
Citation/Reference Downloads: 5
XML Downloads: 133
Web Metrics
Days since publication: 263
Overall contacts: 5311
Avg. contacts per week: 141.36
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
(No citations were found up to date. Please come back later)
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Effect of family, crown position, number of winter buds, fresh weight and the length of needle on rooting ability of Pinus thunbergii Parl. cuttings
vol. 9, pp. 370-374 (online: 11 January 2016)
Research Articles
Conservation of Betula oycoviensis, an endangered rare taxon, using vegetative propagation methods
vol. 13, pp. 107-113 (online: 23 March 2020)
Technical Reports
De novo adventitious root formations in mini-cuttings of Azadirachta indica in response to different rooting media and auxin treatments
vol. 8, pp. 558-564 (online: 09 December 2014)
Research Articles
Variation of wood and bark density and production in coppiced Eucalyptus globulus trees in a second rotation
vol. 9, pp. 270-275 (online: 08 September 2015)
Research Articles
Wood production and nutritional status of Pinus taeda L. in response to fertilization and liming: a meta-analysis of the Americas
vol. 16, pp. 195-201 (online: 25 July 2023)
Research Articles
Interaction between planting spacing and wood properties of Eucalyptus clones grown in short rotation
vol. 14, pp. 12-17 (online: 02 January 2021)
Review Papers
Moisture in modified wood and its relevance for fungal decay
vol. 11, pp. 418-422 (online: 05 June 2018)
Research Articles
Impact of rotation length of Eucalyptus globulus Labill. on wood production, kraft pulping, and forest value
vol. 15, pp. 433-443 (online: 20 October 2022)
Research Articles
Use of alternative containers for promoting deep rooting of native forest species used for dryland restoration: the case of Acacia caven
vol. 10, pp. 776-782 (online: 02 September 2017)
Review Papers
Should the silviculture of Aleppo pine (Pinus halepensis Mill.) stands in northern Africa be oriented towards wood or seed and cone production? Diagnosis and current potentiality
vol. 12, pp. 297-305 (online: 27 May 2019)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword