Carbon, nitrogen and phosphorus stoichiometry controls interspecific patterns of leaf litter-derived dissolved organic matter biodegradation in subtropical plantations of China
iForest - Biogeosciences and Forestry, Volume 14, Issue 1, Pages 80-85 (2021)
doi: https://doi.org/10.3832/ifor3609-013
Published: Feb 19, 2021 - Copyright © 2021 SISEF
Research Articles
Abstract
Leaching of leaf litter is the primary source of dissolved organic matter (DOM) in forest soils. However, the interspecific variations of litter-derived DOM characteristics and biodegradation and their controlling factors remain unclear in subtropical plantations. Using fresh leaf litter of two broadleaf trees (Liquidambar formosana and Schima superba) and two coniferous trees (Pinus massoniana and P. elliottii) in subtropical plantations of China, we assessed the effects of tree species on the amounts and properties of litter-derived DOM with a short-term leaching experiment, and examined the interspecific variation of DOM biodegradation using a 56-day laboratory incubation method. Broadleaf tree litter generally leached higher amounts of dissolved organic carbon (DOC), dissolved total nitrogen (DTN), and dissolved total phosphorus (DTP) than coniferous tree litter. Compared with coniferous trees, broadleaf trees had higher DOM aromaticity and molecular weight, but lower DOC:DTP and DTN:DTP ratios in the litter leachates. Despite greater DOM aromaticity and molecular weight, broadleaf trees had higher litter-derived DOM biodegradation than coniferous trees because of the relatively lower DOC:DTP and DTN:DTP ratios. These results indicate the distinct patterns of litter-derived DOM characteristics and biodegradation between broadleaf and coniferous trees, and also highlight the predominant role of C:N:P stoichiometry in driving the interspecific variation of litter-derived DOM biodegradation in subtropical plantations of China.
Keywords
Broadleaf Trees, Coniferous Trees, DOM Aromaticity, DOM Molecular Weight, Leaching
Introduction
Dissolved organic matter (DOM) often represents the most labile organic matter fraction in soils and plays an essential role in maintaining ecosystem services and functions in forests ([15], [25], [18], [13]). In forest soils, leaching of soluble compounds from plant litter is the primary source of DOM, especially in the early stage of litter decomposition ([2], [11]). Although the importance of litter-derived DOM has recently attracted considerable attention ([4], [20], [1]), the amounts and chemical composition (e.g., aromaticity and molecular weight) of DOM leaching from different tree litter are highly variable and the controls causing these interspecific variations are elusive. Accordingly, these uncertainties about tree litter-derived DOM will limit our understanding of key ecological processes in forest ecosystems.
In forests, litter-derived DOM is either degraded by heterotrophic organisms or absorbed by soil minerals to form stable soil organic matter or delivered directly to groundwater via leaching ([18], [28], [3]). Thus, microbial degradation is a crucial factor controlling the fate of litter-derived DOM, and knowledge about the controls on DOM biodegradation is a prerequisite for understanding DOM dynamics in forest soils. Because microbial growth and activity are often limited by energy and nutrients ([26]), DOM biodegradation is believed to be influenced by carbon (C) quality and stoichiometric ratios between C and nutrients. In empirical studies, however, the controls on litter-derived DOM biodegradation remain unclear in forests ([30], [20], [10]). Several studies have observed that DOM biodegradation was tightly correlated with C quality such as aromaticity or humification index ([4], [31]), while other studies have found the predominant role of C:nitrogen (N):phosphorus (P) stoichiometry in regulating DOM biodegradation ([24]). Moreover, most previous studies have been performed in boreal and temperate forests, and little is known about the interspecific patterns of litter-derived DOM biodegradation in sub/tropical forests. Considering that leaching makes a substantial contribution to litter decomposition in sub/tropical forests ([2], [19]), additional studies are needed to clarify the biodegradation of litter-derived DOM and its underlying mechanisms in these forest ecosystems.
To clarify the effect of tree species on litter-derived DOM dynamics, we investigated the differences in the amount, chemical composition, and biodegradation of leaf litter-derived DOM between broadleaf trees (Liquidambar formosana and Schima superba) and coniferous trees (Pinus massoniana and P. elliottii) in subtropical plantations of southern China. Considering that broadleaf trees can produce leaf litter with higher nutrient concentrations and lower lignin concentration than coniferous trees ([6], [21]), we raised the following three hypotheses: (1) broadleaf tree litter would yield larger amounts of DOM than coniferous tree litter during leaching; (2) compared with broadleaf trees, coniferous trees would have greater DOM aromaticity and molecular weight in the litter leachates; and (3) litter-derived DOM biodegradation would be higher for broadleaf trees than for coniferous trees.
Materials and methods
Site description
The experiment was performed in the Long-term Forest Restoration Experimental Station of Jiangxi Agricultural University (26° 44′ N, 115° 04′ E) located in the Luoxi Town, Taihe County, Jiangxi Province, China (Fig. 1). The climate of the study site is a humid subtropical monsoon climate. The average annual precipitation is 1726 mm, and the average annual air temperature is 18.6 °C. The soil is red soil developed from Quaternary red clay, and is classified as Ferric Acrisols in the FAO classification system ([12]). In the study site, the soil is severely degraded due to the intensive anthropogenic activities such as grazing, firewood collection, and cultivation. Accordingly, soil organic matter content is extremely low, and the lands are sparsely covered by the drought-tolerant grasses such as Imperata koenigii, Cymbopogon goeringii, and Searia viridis ([7]). In 1991, the long-term forest restoration experiment was conducted on these severely degraded lands, and the main afforestation tree species included L. formosana, S. superba, P. massoniana, and P. elliottii, Vernicia fordii, Paulownia fortune, Eucalyptus robusta, Lespedeza bicolor, and Acacia mearnsii. The detailed information on the study site was shown in Gong et al. ([7]).
Leaf litter sampling and measurement
In this study, we collected leaf litter from two broadleaf trees (L. formosana and S. superba) and two coniferous trees (P. massoniana and P. elliottii) in these plantations. For each plantation, we randomly established six plots (20 × 20 m) as replicates and set up five litter traps (1 × 1 m) in each plot in September 2018. The mean thickness of litter layer in the L. formosana, S. superba, P. massoniana, and P. elliottii plantations was about 0.4, 4.7, 1.1, and 2.4 cm, respectively. From October to November 2018, freshly fallen leaves in the litter traps were collected semimonthly, oven-dried at 65 °C, and used to determine initial chemical properties and litter-derived DOM. Litter organic C and N concentrations were measured with the dry combustion method on a TOC analyzer (multi N/C 2100s®, Analytik, Jena, Germany), total P concentration was measured colorimetrically on an AMS Alliance SmartChem® 140 spectrophotometer (AMS, Frepillon, France) after acid digestion, and total polyphenol concentration were measured by the Folin-Ciocaileu method with gallic acid as the standard ([27]). In this study, litter C:N, C:P, and N:P ratios were expressed as atomic ratios. The initial properties of tree leaf litter were shown in Tab. 1.
Tab. 1 - Initial properties of tree leaf litter in subtropical plantations of China. Data are means ± standard errors (n=6). In the same column, different lowercase letters indicate significant differences (p<0.05) among the four species.
| Species | Organic C (mg g-1) |
Total N (mg g-1) |
Total P (mg g-1) |
Total polyphenol (mg g-1) |
C:N ratio |
C:P ratio |
N:P ratio |
Water holding capacity (%) |
|---|---|---|---|---|---|---|---|---|
| L. formosana | 438 ± 3 c | 9.52 ± 0.17 a | 1.45 ± 0.05 a | 145 ± 3 a | 54 ± 1 c | 785 ± 27 c | 14.6 ± 0.6 a | 131 ± 1 a |
| S. superba | 457 ± 3 b | 5.70 ± 0.09 b | 0.85 ± 0.04 c | 101 ± 3 b | 94 ± 2 b | 1399 ± 66 b | 14.9 ± 0.6 a | 94 ± 3 b |
| P. massoniana | 481 ± 3 a | 4.85 ± 0.04 c | 0.96 ± 0.03 b | 88 ± 2 c | 116 ± 1 a | 1305 ± 47 b | 11.3 ± 0.4 b | 72 ± 2 c |
| P. elliottii | 441 ± 3 b | 4.32 ± 0.06 d | 0.66 ± 0.03 c | 86 ± 2 c | 119 ± 2 a | 1737 ± 91 a | 14.6 ± 0.9 a | 72 ± 3 c |
Litter-derived DOM was extracted with a short-term leaching experiment ([4]). Oven-dried leaf litter (3 g) per plot was placed in 200 mL of deionized water in the 500 mL Mason jars and soaked in the dark at room temperature (20 °C) for 48 hours. Afterward, litter leachates were filtered through 0.7 μm Whatman GF/F glass microfiber filters and immediately used to measure DOM properties. Dissolved organic C (DOC) and dissolved total N (DTN) concentrations were measured on a TOC analyzer, and dissolved total P (DTP) concentration was measured with the peroxodisulfate oxidation method ([5]). The total extractable amounts of DOC, DTN, and DTP were obtained from the respective amounts of DOC, DTN, and DTP in the litter leachates and the initial litter dry mass. In addition, the stoichiometric ratios among DOC, DTN, and DTP were expressed as atomic ratios.
The absorbances of DOM were measured from 250 nm to 700 nm on an ultraviolet-visible dual-beam spectrophotometer (UV 600SC®, Jinghua Instruments, China) with 1-cm quartz cells. Before measurement, litter leachates were diluted when necessary. In this study, the specific ultraviolet absorbances at 254 nm (SUVA254), 280 nm (SUVA280), 350 nm (SUVA350), and 370 nm (SUVA370) were used to indicate the aromaticity of DOM, and the higher values were associated with greater aromatic content ([29], [8]); SR was used to indicate DOM molecular weight, and higher SR values showed lower molecular weight ([9], [8]). For each litter leachate, SUVA254, SUVA280, SUVA350, and SUVA370 were obtained by dividing the ultraviolet absorbances at 254, 280, 350, and 370 nm by the DOC concentration, respectively ([29], [8]). To calculate SR values, we firstly obtained S275-295 and S350-400 values by fitting absorption spectrum to an exponential decay function over the wavelength range of 275-295 nm and 350-400 nm, respectively, and then calculated SR values as the ratio of spectral slope S275-295 to spectral slope S350-400 ([9]).
Dissolved organic matter biodegradation was measured by a 56-day standard laboratory incubation method ([23]). To prepare the inoculum, 40-g fresh soils (0-10 cm depth) from a mixed coniferous-broadleaf plantation were placed in 1000 mL deionized water in the dark at 20 °C for 12 hours. The inoculum suspension was filtered through 0.7 μm glass microfiber filters, and stored for the laboratory incubation experiment. In the leaf litter leachates, DOC concentration was diluted to 10-20 mg DOC L-1 to prevent excessive microbial growth. Afterward, 100-mL diluted litter leachate per treatment was placed in 250 mL glass jars, inoculated with 5 mL microbial inoculum, sealed, and incubated in the dark at 20 °C. Meanwhile, six glass jars containing 100 mL deionized water and 5 mL inoculum were used as blanks. There were 150 glass jars: five treatments (L. formosana, S. superba, P. massoniana, P. elliottii, and blank) × six replicates × five sampling times (7, 14, 28, 42, and 56 days). Following 7, 14, 28, 42, and 56 days of incubation, the water sample in six jars per treatment was collected and filtered to determine DOC concentration and SR value. In the filters, DOC concentration and SR value were obtained with the method mentioned above. For each litter leachate, DOM biodegradation (%) was calculated from the difference in cumulative DOC consumption between litter leachates and the blank over the incubation period, and was expressed as the percentage of the initial DOC concentration ([23]).
Statistical analyses
The statistical analyses were conducted using the SPSS® statistical software (version 19.0) for Windows, and the statistically significant level of 0.05 was accepted. Before statistical analyses, the normality of the data was examined with the Levene test, and all data followed a normal distribution. One-way ANOVA was used to assess the effect of tree species on DOM properties in the litter leachates, and post-hoc Tukey’s HSD test was adopted to compare the significant differences in DOM properties among the four tree species. Repeated measures of ANOVA were used to examine the effect of tree species on DOM biodegradation and SR values over the incubation periods. We also used the linear regression to assess the relationship between DOM biodegradation and initial DOC:DTN, DOC:DTP, and DTN:DTP ratios in the litter leachates.
Results
Initial litter properties varied significantly with tree species (Tab. 1). Broadleaf tree (L. formosana and S. superba) leaf litter had higher total N concentration, total polyphenol concentration, and water holding capacity, but lower C:N ratio than coniferous tree (P. massoniana and P. elliottii) leaf litter (Tab. 1). Among the four tree species, L. formosana litter had highest total N, total P, and total polyphenol concentrations, whereas P. elliottii litter had lowest total N, total P, and total polyphenol concentrations (Tab. 1). Consequently, L. formosana litter had lowest C:N and C:P ratios, whereas P. elliottii litter had highest C:N and C:P ratios among the tree species (Tab. 1).
During leaching, the extractable amounts of DOC, DTN, and DTP were significantly influenced by tree species (Tab. 2). In the litter leachates, two broadleaf trees (L. formosana and S. superba) generally had higher amounts of DOC, DTN, and DTP than two coniferous trees (P. massoniana and P. elliottii - Tab. 2). For broadleaf trees, L. formosana litter released greater DOC amounts during leaching than S. superba litter, and for coniferous trees, P. massoniana had lower extractable DOC amounts than P. elliottii (Tab. 2). In addition, L. formosana litter produced higher amounts of DTN and DTP during leaching than the other three tree species (Tab. 2).
Tab. 2 - Total extractable amounts of dissolved organic C (DOC), dissolved total N (DTN), and dissolved total P (DTP) after 48-hour leaching of tree leaf litter in subtropical plantations of China. Data are means± standard errors (n=6). In the same column, different lowercase letters indicate significant differences (p<0.05) among the four species.
| Tree species | DOC amount (μg C g-1 litter) |
DTN amount (μg N g-1 litter) |
DTP amount (μg P g-1 litter) |
DOC:DTN ratio |
DOC:DTP ratio |
DTN:DTP ratio |
|---|---|---|---|---|---|---|
| L. formosana | 13.922 ± 109 a | 223 ± 5 a | 66.3 ± 2.8 a | 72.8 ± 1.7 a | 548 ± 24 c | 7.6 ± 0.4 c |
| S. superba | 10.798 ± 199 b | 170 ± 6 b | 12.2 ± 0.3 b | 74.6 ± 2.3 a | 2302 ± 81 b | 31.0 ± 1.2 b |
| P. massoniana | 8.207 ± 90 d | 151 ± 4 b | 10.0 ± 0.4 b | 63.6 ± 1.6 b | 2139 ± 88 b | 33.7 ± 1.3 b |
| P. elliottii | 10.120 ± 85 c | 158 ± 5 b | 7.4 ± 0.5 b | 75.1 ± 2.9 a | 3622 ± 261 a | 48.1 ± 2.3 a |
In the litter leachates, both DOC:DTN:DTP stoichiometry and DOM optical properties differed significantly among tree species (Tab. 2). Among the four species, L. formosana had lowest DOC:DTP and DTN:DTP ratios, while P. elliottii had highest DOC: DTP and DTN:DTP ratios (Tab. 2). In addition, P. massoniana had lower DOC:DTN ratio than the other three tree species (Tab. 2). In the litter leachates, broadleaf trees (L. formosana and S. superba) had greater SUVA254, SUVA280, SUVA350, and SUVA370 values, but lower SR values than coniferous trees (P. massoniana and P. elliottii - Tab. 3). However, no significant differences in the selected absorbance optical properties were observed between L. formosana and S. superba or between P. massoniana and P. elliottii (Tab. 3).
Tab. 3 - Absorbance optical properties of dissolved organic matter in the leachates of tree leaf litter in subtropical plantations of China. Data are means ± standard errors (n=6). In the same column, different lowercase letters indicate significant differences (p<0.05) among the four species.
| Tree species | SUVA254 (L mg-C-1 m-1) |
SUVA280 (L mg-C-1 m-1) |
SUVA350 L mg-C-1 m-1) |
SUVA370 L mg-C-1 m-1) |
Sr values |
|---|---|---|---|---|---|
| L. formosana | 8.26 ± 0.32 a | 7.37 ± 0.36 a | 1.35 ± 0.06 a | 1.01 ± 0.05 a | 1.14 ± 0.02 b |
| S. superba | 8.09 ± 0.35 a | 8.19 ± 0.32 a | 1.44 ± 0.05 a | 1.00 ± 0.04 a | 1.28 ± 0.06 b |
| P. massoniana | 1.75 ± 0.05 b | 1.63 ± 0.08 b | 0.35 ± 0.01 b | 0.27 ± 0.01 b | 2.32 ± 0.14 a |
| P. elliottii | 1.69 ± 0.08 b | 1.36 ± 0.15 b | 0.20 ± 0.02 b | 0.15 ± 0.02 b | 2.51 ± 0.24 a |
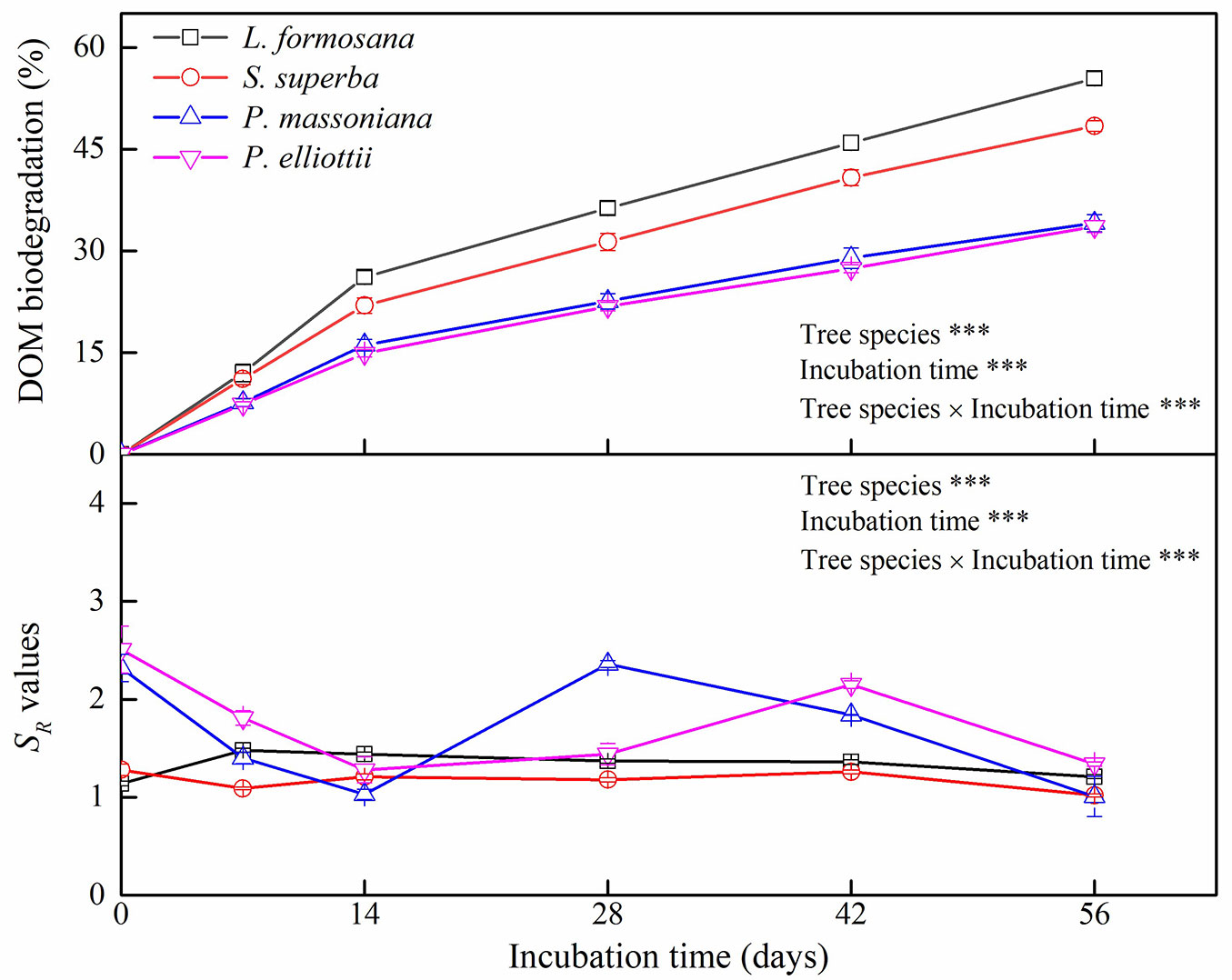
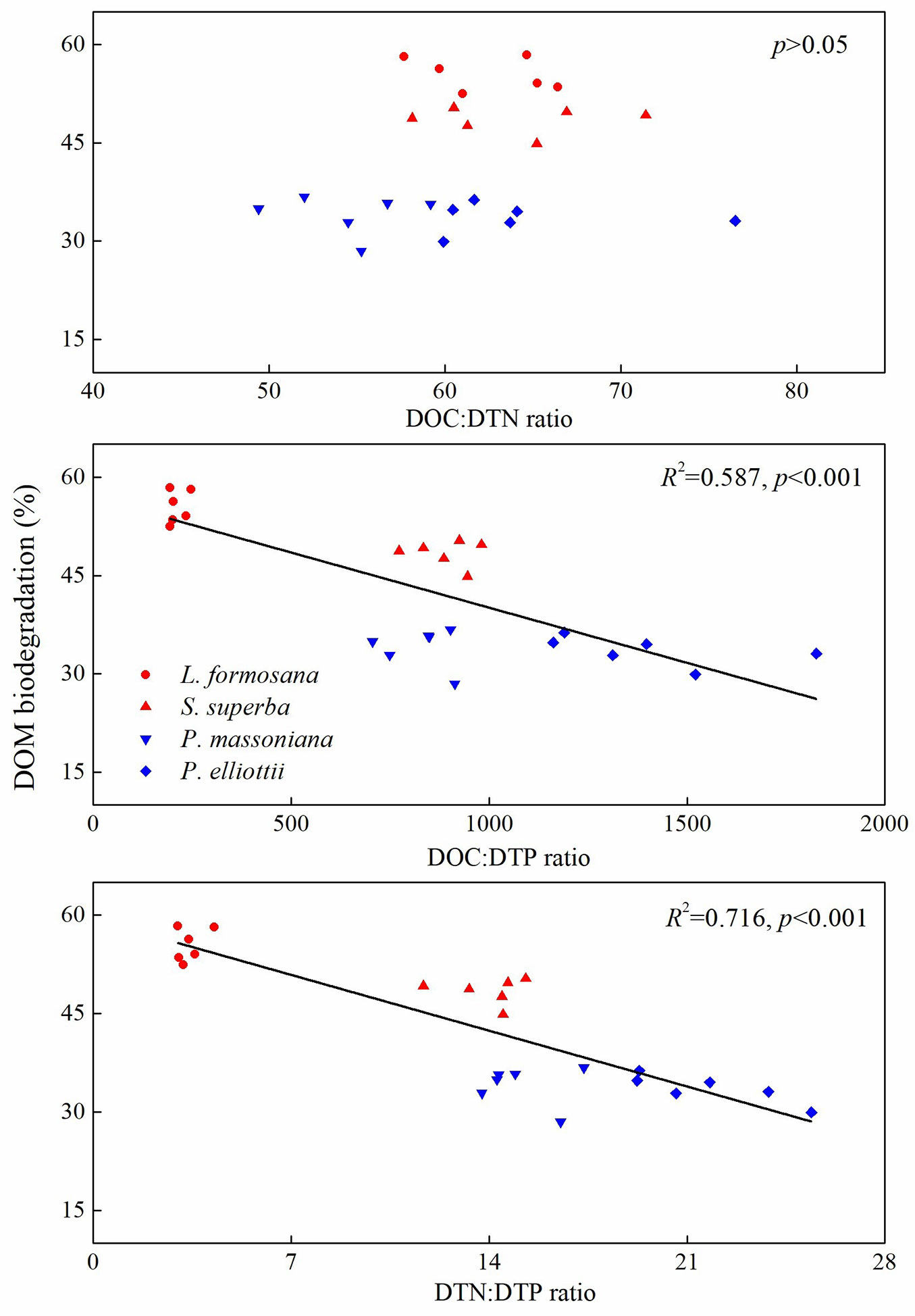
Tree species, incubation time, and their interaction significantly affected biodegradation and SR values of DOM during 56 days of incubation (Fig. 2). Litter-derived DOM biodegradation was greater for broadleaf trees than coniferous trees over the incubation period (Fig. 2). Moreover, litter-derived DOM biodegradation was always higher for L. formosana than for S. superba, whereas no significant difference in litter-derived DOM biodegradation was observed between P. massoniana and P. elliottii (Fig. 2). In addition, SR values of DOM varied with tree species in the initial 42 days of incubation, but showed no significant difference among the tree species by the end of incubation (Fig. 2). After 56-day incubation, DOM biodegradation correlated negatively with DOC:DTP ratio (R2 = 0.587, p<0.001) and DTN:DTP ratio (R2 = 0.716, p<0.001), but did not exhibit a significant relationship with DOC:DTN ratio (Fig. 3).
Fig. 2 - Leaf litter-leached dissolved organic matter (DOM) biodegradation and changes in SR values during 56 days of incubation in subtropical plantations of China. (***): p<0.001.
Fig. 3 - Relationship between dissolved organic matter (DOM) biodegradation and stoichiometric ratios of dissolved organic C (DOC), dissolved total N (DTN), and dissolved total P (DTP) in subtropical plantations of China.
Discussion
In line with the first hypothesis, L. formosana and S. superba generally yielded greater amounts of DOC, DTN, and DTP per gram of dry leaf litter than P. massoniana and P. elliottii following short-term leaching. Previous studies also observed similar patterns of litter-derived DOC or/and dissolved nutrient amounts between broadleaf and coniferous trees in boreal and temperate forests ([4], [17], [14], [10]). In this study, the substantial differences in DOC quantity in the litter leachates would be explained by the distinct leaf litter physical and chemical properties among broadleaf and coniferous trees. First, broadleaf litter might contain higher amounts of soluble C and nutrient fractions such as sugar and secondary metabolic compounds than coniferous litter ([14], [21]). Second, compared with coniferous species, broadleaf species produced leaf litter with a relatively thinner epidermic and hypodermic layer, lower toughness, and flatter surface structure, which would enable leaf litter to be easily broken and leached ([4], [11], [10]). Third, relative to coniferous litter, the relatively higher water holding capacity of broadleaf litter (Tab. 1) could permit a larger amount of water to enter leaf litter, and thus enhance leaching of soluble compounds ([14]). Considering that most previous studies have been conducted in temperate and boreal forests, our result confirms the generality of interspecific patterns in leaf litter-derived DOM amounts between broadleaf and coniferous trees to subtropical forests.
Contrary to the second hypothesis, both L. formosana and S. superba had higher SUVA254, SUVA280, SUVA350, and SUVA270 values, but lower SR values in the litter leachates than the selected two pine trees. Given that litter-derived DOM chemical make-up is primarily influenced by litter chemistry ([4], [17], [28], [20], [14]), the higher litter total polyphenol concentration of broadleaf trees (Tab. 1) would account for the greater aromaticity and molecular weight of DOM in the litter leachates relative to coniferous trees in this study. In general, DOM aromaticity and molecular weight are observed to be tightly correlated with heterotrophic growth and metabolism, pollutant mobilization and transportation, and groundwater quality ([16], [8], [14]). Accordingly, these findings will help explain the spatial variations of ecosystem services between broadleaf and coniferous tree plantations in subtropical regions.
Consistent with the third hypothesis, litter-derived DOM biodegradation was greater for broadleaf trees than coniferous trees during 56-day incubation. In contrast, several studies found that leaf litter-derived DOM biodegradation showed a slight difference between broadleaf and coniferous trees in temperate and boreal forests ([20], [10]). Don & Kalbitz ([4]) even observed much greater DOM biodegradation in the fresh litter leachates for coniferous trees than for broadleaf trees. These inconsistent results implied that the interspecific patterns of litter-derived DOM biodegradation between broadleaf and coniferous trees in subtropical forests might be different from that in temperate and boreal forests. In general, DOM biodegradation correlated positively with nutrient availability, but negatively with DOM aromaticity and molecular weight ([16], [30], [22]). Despite greater DOM aromaticity and molecular weight, broadleaf species often had lower DOC:DTP and DTN:DTP ratios in the litter leachates than coniferous species. Considering that DOC biodegradation was limited by P availability in subtropical regions ([22]), broadleaf tree litter produced DOM with greater biodegradability than coniferous trees. These results suggest that C:N:P stoichiometry is an overriding factor controlling the interspecific patterns of litter-derived DOM biodegradation between broadleaf and coniferous trees in subtropical plantations.
Interestingly, the magnitudes of the changes in SR values of DOM varied substantially with species. Over the entire incubation period, SR values of DOM declined for P. massoniana and P. elliottii, but remained unchanged for L. formosana and S. superba (Fig. 2). Although the mechanisms causing these contrasting patterns were unclear, we speculated that the intrinsic differences in DOM chemical composition might account for the divergent shifts in molecular weight among tree species. The decreased SR values indicated that, for coniferous species, microorganisms might predominantly utilize the labile fractions of DOM with relatively low molecular weight, resulting in the net accumulation of organic compounds with a high molecular weight in the leachates ([9], [8]). In contrast, SR values of DOM did not change with incubation time, probably due to the relatively high DOM aromaticity and complexity in the litter leachates of broadleaf trees. Accordingly, SR values of DOM did not differ between broadleaf and coniferous species by the end of incubation. These results suggest that leaf litter-derived DOM chemical composition will become convergent with microbial degradation proceeds in subtropical plantations.
Conclusions
In summary, broadleaf trees produced larger amounts of leaf litter-derived DOM with greater aromaticity and molecular weight than coniferous trees in subtropical plantations of China. Over 56 days of incubation, DOM biodegradation was higher for broadleaf trees than coniferous trees, although DOM molecular weight became convergent among tree species. Moreover, the interspecific patterns of DOM biodegradation was primarily driven by DOC: DTN:DTP stoichiometry rather than DOM aromaticity and molecular weight. These findings imply that, compared with coniferous trees, broadleaf trees can provide labile litter-derived DOM for microbial growth and metabolism, and thus facilitate soil organic matter formation and accumulation via the DOM-microbial path in subtropical plantations ([3]). Therefore, our results will help explain and forecast the spatial patterns of C and nutrient cycles between broadleaf and coniferous tree plantations, and also suggest that broadleaf tree species are preferentially recommended for afforestation and reforestation programs in subtropical regions of China.
Acknowledgments
We thank two anonymous reviewers for their constructive comments on earlier drafts of this paper, and Jian-Jun Li, Jia-Wen Xu, and Shan-Shan Liu for their help in the field sampling and laboratory analyses. This study was financed by the National Natural Science Foundation of China (Nos. 32060295 and 31800524) and the Double Thousand Plan of Jiangxi Province (jxsq2018106044).
References
Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Yi-Dong Ding
Su-Li Li
Yun Zhang
Rong Mao 0000-0002-0469-2281
Key Laboratory of National Forestry and Grassland Administration on Forest Ecosystem Protection and Restoration of Poyang Lake Watershed, College of Forestry, Jiangxi, Agricultural University, Nanchang 330045 (China)
Key Laboratory of Sustainable Forest Ecosystem Management - Ministry of Education, School of Forestry, Northeast Forestry University, Harbin 150040 (China)
Corresponding author
Paper Info
Citation
Wu P-P, Ding Y-D, Li S-L, Sun X-X, Zhang Y, Mao R (2021). Carbon, nitrogen and phosphorus stoichiometry controls interspecific patterns of leaf litter-derived dissolved organic matter biodegradation in subtropical plantations of China. iForest 14: 80-85. - doi: 10.3832/ifor3609-013
Academic Editor
Claudia Cocozza
Paper history
Received: Aug 06, 2020
Accepted: Dec 16, 2020
First online: Feb 19, 2021
Publication Date: Feb 28, 2021
Publication Time: 2.17 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2021
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 37286
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 31181
Abstract Page Views: 3055
PDF Downloads: 2483
Citation/Reference Downloads: 7
XML Downloads: 560
Web Metrics
Days since publication: 1818
Overall contacts: 37286
Avg. contacts per week: 143.57
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2021): 11
Average cites per year: 2.20
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Changes in organic compounds during leaf litter leaching: laboratory experiment on eight plant species of the Sudano-guinea Savannas of Ngaoundere, Cameroon
vol. 1, pp. 27-33 (online: 28 February 2008)
Research Articles
Exploring machine learning modeling approaches for biomass and carbon dioxide weight estimation in Lebanon cedar trees
vol. 17, pp. 19-28 (online: 12 February 2024)
Research Articles
Nutrients (N, P and K) dynamics associated with the leaf litter of two agroforestry tree species of Bangladesh
vol. 2, pp. 183-186 (online: 15 October 2009)
Research Articles
Fine root morphological traits and production in coniferous- and deciduous-tree forests with drained and naturally wet nutrient-rich organic soils in hemiboreal Latvia
vol. 16, pp. 165-173 (online: 08 June 2023)
Research Articles
Spectral reflectance properties of healthy and stressed coniferous trees
vol. 6, pp. 30-36 (online: 14 January 2013)
Research Articles
Soil drench of ethylenediurea (EDU) protects sensitive trees from ozone injury
vol. 4, pp. 66-68 (online: 05 April 2011)
Research Articles
Soil stoichiometry modulates effects of shrub encroachment on soil carbon concentration and stock in a subalpine grassland
vol. 13, pp. 65-72 (online: 07 February 2020)
Technical Notes
Comparative analysis of students’ attitudes toward implementation of genetically modified trees in Serbia
vol. 8, pp. 714-718 (online: 08 January 2015)
Research Articles
Determination of differences in temperature regimes on healthy and bark-beetle colonised spruce trees using a handheld thermal camera
vol. 14, pp. 203-211 (online: 02 May 2021)
Short Communications
Seasonal change in soil nitrogen mineralization in young Chamaecyparis obtusa stands at the upper and lower positions on a slope in central Japan
vol. 18, pp. 197-201 (online: 20 July 2025)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword