Assessment of allergenic potential in urban forests: a case study of the Royal Park of Portici in Southern Italy
iForest - Biogeosciences and Forestry, Volume 13, Issue 5, Pages 376-381 (2020)
doi: https://doi.org/10.3832/ifor3485-013
Published: Aug 25, 2020 - Copyright © 2020 SISEF
Technical Reports
Abstract
In modern cities, the abundance of allergenic plant species has contributed to making less healthy the urban environment, as on-going and direct contact of humans with the urban flora can increase the negative effects on people allergic to pollens. The allergenicity of ornamental species should be considered, and above all quantified, when designing new urban green areas. Numerous studies reported the detailed description of the flora present in cities, but only in some rare cases their allergenic power and related pollen seasons were mentioned. In the present study, starting from the existing data in literature on the vascular flora of the Royal Park of Portici, Southern Italy, tree and shrub species have been classified based on their ability to cause respiratory allergies. Thus, to estimate the allergenic potential of urban green space, two preliminary approaches were defined based on the biological characteristics of the plant species as sources of pollen emissions.
Keywords
Pollens, Allergies, Vascular Flora, Urban Green Parks, Urban Planning
Introduction
During the last century, garden and urban planning projects were bound to create urban parks specifically for the population, conceived like a livable green space with a peculiar educational and pedagogical function. These green oases are interesting both for the botanical diversity and for the implant architecture, made up largely of exotic plants, well adapted to the climatic conditions of the new sites. Still today, the ornamental function and plant management practices, as well as the range of ecosystem services, are considered very important when building gardens and planning urban green spaces ([10], [16], [11], [15]). Recent studies confirm that natural environments have positive effects on health by stimulating physical activities. Moreover, the time spent in the urban green spaces can form both emotional and cognitive psychological bonds, which shape our personal memories and therefore our life stories ([2]); hence, the need to make urban green areas safe and healthy places for citizens. However, the potential threat that some plants can pose to human health is underestimated ([14], [6]) and therefore their spread in cities must be kept under control ([20], [3]). In urban areas, the number of potentially allergenic plants has grown rapidly as the diversity of plants increased ([23]). The widespread use of anemophilous species, to which pollen of external origin carried by the wind, dust and other pollutants are added, increases the allergenicity of urban environments ([21]). Higher plants’ pollen emission during the pollination period has a significant impact, affecting the health of about 30-40% of the world population ([4]). In Italy, pollinosis, the allergy caused by pollen, affects 35% of the population with manifestations affecting the mucous membranes of the nose and eyes, while 10% are subject to respiratory disorders ([17]). Pollinosis occurs on a seasonal basis, during the pollination period, in subjects become sensitive to pollen from certain families of herbs and trees ([18]). According to World Health Organization, pollinosis ranks at the top of chronic diseases, while epidemiological studies showed that urbanization, high levels of vehicle emissions and westernized lifestyle are related to an increase in the frequency of pollen-induced respiratory allergy ([9]). This phenomenon is explained by the fact that the rhino-conjunctival mucosa of subjects affected by pollinosis is more sensitive to the irritating effects of air pollutants. Consequently, susceptibility to allergens can increase in areas characterized by intense environmental pollution due to the aggravation of the symptoms of respiratory diseases, such as rhinitis, allergic bronchial asthma and chronic broncho-pneumopathies ([12]). The pollen grain volumes dispersed in the atmosphere and the start and duration of flowering are influenced by weather, which is in turn conditioned by climate change ([1], [9]). It must be considered that plants produce different pollen volumes in different years in response to changing environmental conditions, and genetic variants of the same species can give different pollen quantities ([23]). Cariñanos et al. ([4], [5]) preliminarily studied the degree of allergenicity of urban green by specific parameters, such as the Urban Green Zone Allergenicity Index (IUGZA) applied to real situations. More recently, a further application of IUGZA allowed the classification of the pollen allergenicity risk of the urban parks in Spain ([7]). IUGZA can represent a useful tool for mitigation projects aimed at minimizing the allergenic impact. The worst case scenario occurs when one or more anemophilous species, with a high allergenic potential and a prolonged flowering period, are present in urban green spaces. Therefore, to define the urban green allergenic potential is useful to encourage the use of corrective measures. Thus, it is advisable to avoid planting male trees (for dioecious species), avoid the use of a single potential allergenic species in the hedges or along avenues and, in extreme cases, hypothesize the replacement of the allergenic species that pose a serious health risk ([4]). In fact, an important element to consider is the loss of biodiversity, which is responsible for the allergies incidence increase ([5]). Therefore, promoting controlled biodiversity and reducing the local plant species with allergenic capacity would decrease monospecific sources of allergenic pollen ([3], [13]).
A survey commissioned by the European Commission’s Directorate-General for the Environment, conducted on 25,525 citizens residing in the Member States, showed that 6 out of 10 Europeans are not informed about the air quality in their country and over 50% of respondents believe that the cause of the deterioration in air quality is attributable exclusively to emissions deriving from industrial activities and motor vehicles ([12]). It therefore appears necessary to sensitize public institutions on the activation of action plans aimed at reducing environmental risks for the health of the weaker groups and promoting the development of information systems dealing with environmental health issue.
In this work, the allergenic potential of the urban green spaces, located in the Portici Royal Park (Southern Italy), was estimated from floristic data in the literature. The vascular flora, mostly consisting of tree and shrub species, was classified on the basis of ability to cause respiratory allergies. Two preliminary approaches were defined based on the biological characteristics of the plant species present as sources of pollen emissions.
Materials and methods
Description of the study area
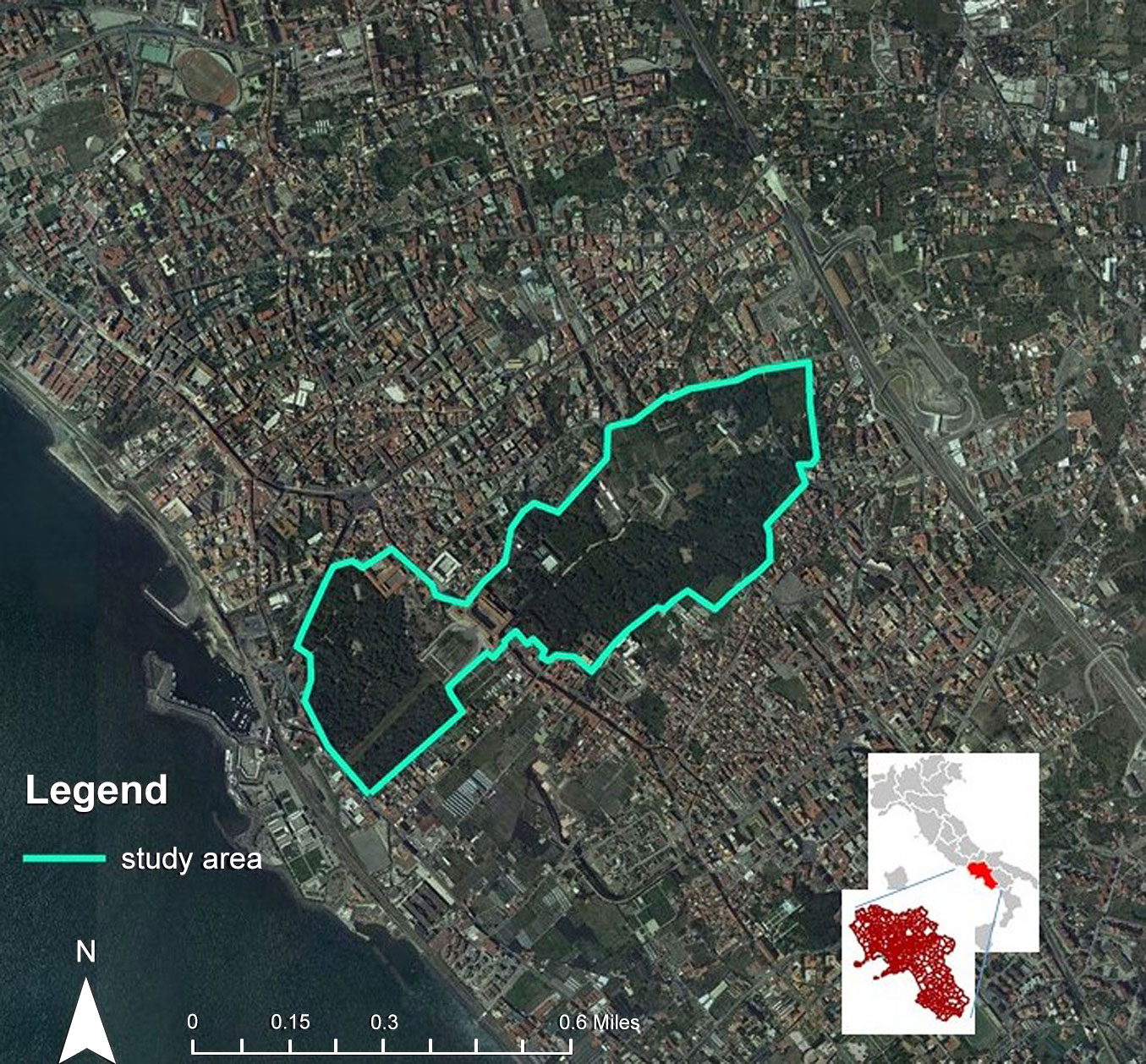
The green area under investigation is represented by the park of the Royal site located in Portici (40° 49′ 11″ N, 14° 20′ 28″ E), near Naples (Campania Region, Southern Italy), at the foot of the Southwestern slope of Mount Vesuvius, between 20 m and 90 m a.s.l. (Fig. 1). The climatic conditions are typical of the Mediterranean area, hot and temperate with the rainiest winter compared to summer. In the period 1982-2012, on average, the temperature was 15.8 °C and the rainfall was 901 mm ([8]).
The Royal Park covers an area of about 60 hectares, of which 70% covered by holm oak (Quercus ilex L.) of Sicilian origin, used for reforestation during the Bourbon reign, where some centuries-old specimens are yet well preserved ([22]). Associated with holm oak, numerous other species of trees and shrubs are found, as well as a vast herbaceous variety. Over the years, the area has undergone numerous interventions such as regular pruning, the felling of diseased trees, the cutting of the undergrowth, and the opening of new paths that have influenced the natural dynamics ([22]).
The Royal Park shows a strong polarization of the only green area in the city capable of providing a good level of hydraulic and microclimatic regulation services, of acoustic attenuation and of erosion control. The almost wild nature of the park contrasts with the structured flora of the botanical garden, which occupies 1.2% of the area. Inside it, the botanical exhibition is organized by geographical distribution and environmental types: conifers, Mediterranean flora, plants of central and southern America, Australia, South Africa and of Eurasian origin grow there. Today, the Park area, in addition to being part of the Portici Royal Palace, houses the Department of Agriculture of the University of Naples “Federico II”, the MUSA Center, born from the merge of eight Agricultural Sciences Museums, and National Research Council (CNR) research institutes, which are visited daily throughout the year by researchers, teachers and students, as well as visited by tourists and numerous school groups.
The constant permanence of people inside the park, including children particularly sensitive to diseases of the respiratory system, makes it a potentially dangerous site in terms of pollinosis.
Plant species analysis
To define the allergenic potential of the Portici Royal Park, it was necessary to draw up an inventory of plant species, and assign each species its own allergenic power. For cataloging, reference was made to the work carried out by Stinca & Motti ([22]) on the vascular flora of the Royal Park. The floristic list created by these authors is based on field surveys conducted from 2005 to 2009 and on bibliographic data. The authors found 449 species with a high incidence of Mediterranean species (41.7%) and a greater presence of alien species compared to endemics. According to the authors, the plant community of the park could be defined as a thermophilic or thermomesophilic phytocoenosis with a large representation of the Q. ilex species with an arboreal-shrubby (Mediterranean maquis) or arboreal bearing. However, the community is evolving and shows a progressive reduction of the holm oak (Q. ilex) replaced by deciduous broadleaf trees such as flowering ash (Fraxinus ornus L.), hornbeam (Carpinus betulus L.) and Mediterranean hackberry (Celtis australis L.). For an investigation aimed at defining the allergenic potential of the entire area, all the tree and some shrub species were selected from the floristic list. Among these species, Crataegus monogyna Jacq., Euonymus europaeus L., Laurus nobilis L., Ligustrum vulgare L. and L. lucidum W. T. Aiton, Pittosporum tobira (Thunb.) W. T. Aiton, Rhamnus alaternus L., and Viburnum tinus L. are normally used as hedges and subject to continuous pruning, but if left to grow in a natural habitat they take on the appearance of small trees.
Results and discussion
Allergenic species of the Portici Royal Park
In the present work, 50 plant species of the Portici Royal Park have been examined, out of which 34 arboreal, 4 shrubby/arboreal and 12 shrubby species. They were divided between 84% of deciduous trees, 10% of conifers, and 6% of palm trees. Cupressus sempervirens L. species, whose presence was verified after a field observation, was also included, even if not mentioned in the literature. It could not be omitted from research as it is a species with a high allergenic load. As shown in Tab. 1, the treated species belong to 27 different families, so providing an idea of the biodiversity involved ([24]). Among these, five families revealed to include the largest number of species: Arecaceae, Moraceae, Oleaceae, Pinaceae, and Rosaceae.
Tab. 1 - Number of species per family of the vascular flora studied at Royal Park of Portici, Southern Italy.
| No | Botanical family | Plant species (N) |
|---|---|---|
| 1 | Aceraceae | 1 |
| 2 | Anacardiaceae | 1 |
| 3 | Arecaceae | 3 |
| 4 | Caesalpiniaceae | 1 |
| 5 | Caprifoliaceae | 2 |
| 6 | Celastraceae | 1 |
| 7 | Corylaceae | 2 |
| 8 | Cupressaceae | 1 |
| 9 | Ericaceae | 1 |
| 10 | Euphorbiaceae | 1 |
| 11 | Fabaceae | 2 |
| 12 | Fagaceae | 2 |
| 13 | Ginkgaceae | 1 |
| 14 | Lauraceae | 1 |
| 15 | Malvaceae | 1 |
| 16 | Mimosaceae | 1 |
| 17 | Moraceae | 3 |
| 18 | Myrtaceae | 2 |
| 19 | Oleaceae | 6 |
| 20 | Pinaceae | 5 |
| 21 | Pittosporaceae | 1 |
| 22 | Rhamnaceae | 1 |
| 23 | Rosaceae | 5 |
| 24 | Rutaceae | 1 |
| 25 | Simaburaceae | 1 |
| 26 | Taxaceae | 1 |
| 27 | Ulmaceae | 2 |
A floristic dataset was created that specifies the plant species, the botanical family to which it belongs, the flowering period with indication of the allergenic degree, the type of pollen diffusion, its frequency in the park and, finally, the bibliography consulted about the allergenic power and flowering. In the dataset, the plant species is classified by alphabetical order of its scientific name. The degree of allergenicity of each species, as shown in Tab. S1 (Supplementary material), was indicated by a color code on the base of its risk: yellow for low, orange for medium, and red for high pollinosis evidence; the species that do not cause allergies are in blue. The data related to the adverse effects of the analyzed species were not easy to retrieve and, for many ornamental species, there are no detailed reports on the subject in the bibliography. In addition, the introduction of species from other continents made it difficult to predict their possibility of flowering and the possible release of pollen in our areas ([17]). From the bibliography and the accredited websites consulted ([19]) dealing with plant species and defining their allergenicity (European Journal of Aerobiology, Regional Environmental Information System of Tuscany, ARPAE, ARPA Molise etc.), in some cases, conflicting data on the flowering (and therefore of the pollen dispersion period) and the degree of allergenicity were found. Given that the objective of the study is to estimate the allergenic potential of the green area to avoid high risk for visitors, the highest allergological risk and the widest flowering interval were considered in the controversial situations. We must also account that plants are influenced by climatic conditions, in particular local ones; in fact, the pollen release phase is controlled by humidity and air temperature, while the wind speed and its direction influence the pollen dispersion and diffusion ([9]). Weather conditions affect the amount of pollen emitted by plants and the start and duration of flowering (pollen season). Consequently, the symptoms of sensitized subjects are more acute depending on how high the pollen concentration is in the air ([17]). All this considered, and referring to works carried out in different geographical contexts, the consulted data showed variations in terms of the flowering beginning and end of each species. In doubtful situations, the values provided by the European Aerobiology Society (EAS) were considered, as they are based on systematic review and recommendations of the evidence of pollinosis caused by tree and shrub plants used in urban green.
The allergenic potential of the Portici Royal Park
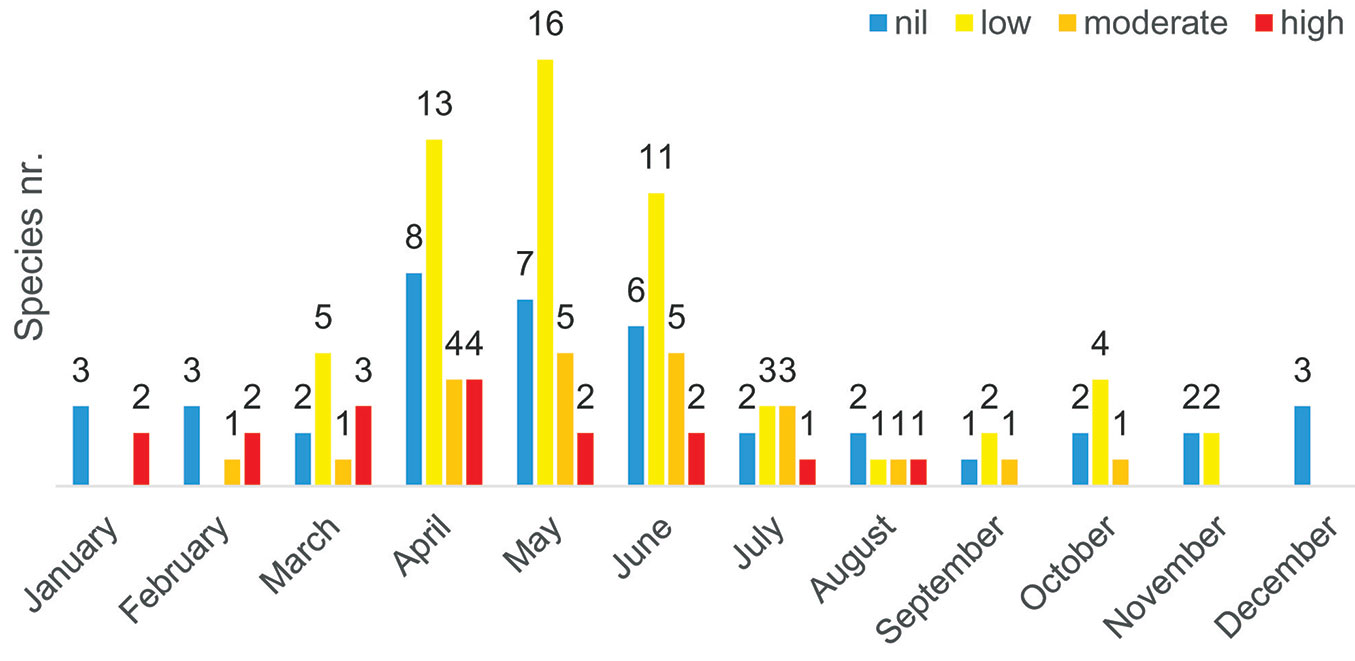
Observing the data cataloged in Tab. S1 (Supplementary material), it is clear that the period of greatest risk for those suffering from respiratory disorders related to pollinosis falls between January and June, where there is the greatest grouping of species with a high degree of allergenicity, whose presence is common or very common (C or CC). More in detail, the allergenic effect of approximately 58% and 60% of the species is concentrated in April and May, respectively, with a higher incidence in April of the species with the highest degree of allergenicity (Fig. 2).
Fig. 2 - The diagram shows the number of plant species present in the Royal Park of Portici (Southern Italy) by their degree of allergenicity (nil, low, medium, high) and flowering period. April and May have the greatest number of allergenic species with a medium and high degree of allergenicity.
Out of the 50 identified species (Fig. S1 in Supplementary material), 5 were highly allergenic (10%), 7 moderately allergenic (14%) and 22 showed a low allergenic power (44%), while for 16 species (32%) there was no evidence that pollen might be responsible for allergic sensitization with or without clinical relevance, and therefore their use does not seem to constitute a risk for respiratory allergies.
The increase in allergenicity was proportional not only to the simultaneous presence of multiple allergenic species and the duration of their flowering, but also to their diffusion throughout the territory. This latter information, obtained from the work of Stinca & Motti ([22]), if put in relation to the other data, showed that the most allergenic species, such as hazelnut, Ginkgo biloba L. (one specimen in the study area) and olive trees, were limited to few rare specimens and therefore their influence was limited. Some species have a more prolonged flowering, which extends to the summer period, too (e.g., Ailanthus altissima (Mill.) Swingle, Eucalyptus camaldulensis Dehnh., Ricinus communis L.), but being entomophilous they have a more contained effect on pollinosis. It should be borne in mind that the anemophilous trees and shrubs are those of greatest allergological interest ([17]).
Tab. 2 - Parameters and values assigned for the determination of the potential allergenicity value (PAV).
| Parameter | Value | Description |
|---|---|---|
| Type of pollination (tp) | 1 | Plants of primarily or exclusively biotic pollination with low pollen emission |
| 2 | Mixed pollination system plants, which display moderate-high pollen emission | |
| 3 | Wind-pollinated plant species that produce and release large amounts of pollen | |
| Duration of pollination (dpp) | 1 | Pollen emissions last 1 month |
| 2 | Pollen emissions last 2 month | |
| 3 | Pollen emissions last more than 2 month | |
| Allergenic potential (ap) | 0 | Non allergenic or not reported as allergenic |
| 1 | Low allergenicity | |
| 2 | Moderate allergenicity, with moderate effect on population | |
| 3 | High allergenicity, with marked effect on population |
Among the studies done to evaluate the allergological risk of urban green, the method introduced by Cariñanos et al. ([4]) is particularly interesting. They used an index that takes into account the biological characteristics of each species, i.e., sexual reproduction, pollination, flowering, size and number of individuals present by species. This index reflects the composition, development and abundance of allergenic and non-allergenic species present in a given green area. However, this type of work requires a very detailed study with related field activities and mapping, besides a time-consuming data processing. The approach pursued in the present work allows to define the allergenic potential of the urban green taking into account the flowering period, the degree of allergenicity, the type of pollen emission as well as the frequency of the species (Tab. 2). These elements are the same that Cariñanos et al. ([4]) identified to determine the potential allergenicity value (PAV), i.e., species-specific allergenicity, expressed by the following formula (eqn. 1):
where tp is the type of pollen emission, dpp is the duration of the pollination period, and ap is the allergenic potential.
To check whether the results obtained following the method used in the present study, linked to data found in the bibliography, are comparable with the analytical data obtained by the method used by Cariñanos et al. ([4]), in Tab. 2 the parameters and values attributed for determination of PAV for the species analyzed in the Portici Royal Park are reported. Once the PAV was determined for each species, with values between 0 and 27, allergenicity was classified according to four ranges of values that define its risk (Tab. 3).
Tab. 3 - Ranks of potential allergenicity value (PAV) applicable to urban tree species in the Mediterranean region.
| PAV | Allergenicity |
| 0 | Nil (N) |
| 1-6 | Low (L) |
| 8-12 | Moderate (M) |
| 18-27 | High (H) |
Comparing the results of the two methods (Tab. 4 and Tab. S1 in Supplementary material), we observed that the data were coincident, with some exceptions. A first group of three species (Pinus halepensis Mill., Pistacia lentiscus L., and Q. ilex), considered less allergenic in the bibliography, was classed as moderately allergenic (PAV 8-12) for the abundant pollen production and for wide (anemophilous) and prolonged (3-4 months) dispersion periods. Conversely, four species (A. altissima, E. camaldulensis, L. vulgare, and R. communis), reported with moderate allergenicity being entomophilous, were considered with low PAV (1-6). Finally, Taxus baccata L., moderately allergenic according to MiPAAF data, was considered highly allergenic due to anemophilous dispersion and prolonged flowering, and the higher values were attributed to the factors tp and dpp. To precisely define the allergenicity of the park, field analyses should be done, so acquiring data about the size and surface occupied by each tree and their number. Nevertheless, the available data showed a low allergenic potential for almost the whole year, with a medium/high peak in the spring-summer period (March-July) due to the higher concentration of medium and high allergenic species. The latters, however, were mostly represented by anemophilous species rarely spread on the territory, with the exception of Ostrya carpinifolia Scop., which is a highly allergenic species widespread in the study area (CC). The limit of this analysis lies in the fact that, lacking a precise mapping of the size and number of the trees, it was not possible to numerically determine the allergenicity of the entire study area.
Tab. 4 - Potential allergenicity value (PAV) and allergenicity of the 50 plant species in study. (tp): type of pollination; (dpp): duration of the pollination period; (ap): allergenic potential. Rank: (N) nil, (L) low, (M) moderate, and (H) high PAV (see Tab. 3).
| Species | Family | tp | dpp | ap | tp·dpp·ap | PAV | Rank |
|---|---|---|---|---|---|---|---|
| Acer negundo | Aceraceae | 2 | 3 | 1 | 2 · 3 · 1 | 6 | L |
| Ailanthus altissima | Simaburaceae | 1 | 3 | 2 | 1 · 3 · 2 | 6 | L |
| Albizzia julibrissin | Mimosaceae | 1 | 2 | 0 | 1 · 2 · 0 | 0 | N |
| Arbutus unedo | Ericaceae | 1 | 3 | 0 | 1 · 3 · 0 | 0 | N |
| Broussonetia papyrifera | Moraceae | 3 | 2 | 1 | 3 · 2 · 1 | 6 | L |
| Cedrus deodara | Pinaceae | 3 | 2 | 1 | 3 · 2 · 1 | 6 | L |
| Celtis australis | Ulmaceae | 3 | 2 | 0 | 3 · 2 · 0 | 0 | N |
| Ceratonia siliqua | Caesalpiniaceae | 1 | 2 | 0 | 1 · 2 · 0 | 0 | N |
| Cercis siliquastrum | Fabaceae | 1 | 2 | 1 | 1 · 2 · 1 | 2 | L |
| Chamerops humilis | Arecaceae | 1 | 2 | 1 | 1 · 2 · 1 | 2 | L |
| Citrus sinensis | Rutaceae | 2 | 3 | 1 | 2 · 3 · 1 | 6 | L |
| Corylus avellana | Corylaceae | 3 | 3 | 3 | 3 · 3 · 3 | 27 | H |
| Crataegus monogyna | Rosaceae | 1 | 2 | 0 | 1 · 2 · 0 | 0 | N |
| Cupressus sempervirens | Cupressaceae | 3 | 3 | 3 | 3 · 3 · 3 | 27 | H |
| Eriobotrya japonica | Rosaceae | 1 | 2 | 0 | 1 · 3 · 0 | 0 | N |
| Eucalyptus camaldulensis | Myrtaceae | 1 | 3 | 2 | 1 · 3 · 2 | 6 | L |
| Euonymus europaeus | Celastraceae | 3 | 3 | 0 | 3 · 3 · 0 | 0 | N |
| Ficus carica | Moraceae | 1 | 2 | 0 | 1 · 2 · 0 | 0 | N |
| Fraxinus ornus | Oleaceae | 2 | 3 | 2 | 2 · 3 · 2 | 12 | M |
| Ginkgo biloba | Ginkgaceae | 3 | 3 | 3 | 3 · 3 · 3 | 27 | H |
| Hibiscus mutabilis | Malvaceae | 1 | 3 | 0 | 1 · 3 · 0 | 0 | N |
| Laurus nobilis | Lauraceae | 3 | 2 | 1 | 3 · 2 · 1 | 6 | L |
| Ligustrum lucidum | Oleaceae | 1 | 3 | 1 | 1 · 3 · 1 | 3 | L |
| Ligustrum vulgare | Oleaceae | 1 | 3 | 2 | 1 · 3 · 2 | 6 | L |
| Malus domestica | Rosaceae | 1 | 1 | 0 | 1 · 1 · 0 | 0 | N |
| Morus alba | Moraceae | 3 | 2 | 2 | 3 · 2 · 2 | 12 | M |
| Myrtus communis | Myrtaceae | 1 | 2 | 1 | 1 · 2 · 1 | 2 | L |
| Olea europea | Oleaceae | 2 | 3 | 3 | 2 · 3 · 3 | 18 | H |
| Ostrya carpinifolia | Corylaceae | 3 | 3 | 3 | 3 · 3 · 3 | 27 | H |
| Phillirea angustifolia | Oleaceae | 3 | 2 | 1 | 3 · 2 · 1 | 6 | L |
| Phillirea latifolia | Oleaceae | 3 | 2 | 1 | 3 · 2 · 1 | 6 | L |
| Phoenix canariensis | Arecaceae | 3 | 2 | 1 | 3 · 2 · 1 | 6 | L |
| Pinus halepensis | Pinaceae | 3 | 3 | 1 | 3 · 3 · 1 | 9 | M |
| Pinus pinaster | Pinaceae | 3 | 2 | 1 | 3 · 2 · 1 | 6 | L |
| Pinus pinea | Pinaceae | 3 | 2 | 1 | 3 · 2 · 1 | 6 | L |
| Pinus wallichiana | Pinaceae | 3 | 2 | 1 | 3 · 2 · 1 | 6 | L |
| Pistacia lentiscus | Anacardiaceae | 3 | 3 | 1 | 3 · 3 · 1 | 9 | M |
| Pittosporum tobira | Pittosporaceae | 1 | 3 | 0 | 1 · 3 · 0 | 0 | N |
| Prunus persica | Rosaceae | 2 | 2 | 0 | 2 · 2 · 0 | 0 | N |
| Quercus ilex | Fagaceae | 3 | 3 | 1 | 3 · 3 · 1 | 9 | M |
| Quercus pubescens | Fagaceae | 3 | 2 | 1 | 3 · 2 · 1 | 6 | L |
| Rhamnus alaternus | Rhamnaceae | 1 | 3 | 0 | 1 · 3 · 0 | 0 | N |
| Ricinus communis | Euphorbiaceae | 1 | 3 | 2 | 1 · 3 · 2 | 6 | L |
| Robinia pseudoacacia | Fabaceae | 1 | 3 | 1 | 1 · 3 · 1 | 3 | L |
| Sambucus nigra | Caprifoliaceae | 1 | 2 | 0 | 1 · 2 · 0 | 0 | N |
| Sorbus domestica | Rosaceae | 3 | 2 | 0 | 3 · 2 · 0 | 0 | N |
| Taxus baccata | Taxaceae | 3 | 3 | 2 | 3 · 3 · 2 | 18 | H |
| Ulmus minor | Ulmaceae | 3 | 2 | 1 | 3 · 2 · 1 | 6 | L |
| Viburnum tinus | Caprifoliaceae | 1 | 3 | 0 | 1 · 3 · 0 | 0 | N |
| Washintonia filifera | Arecaceae | 1 | 2 | 1 | 1 · 2 · 1 | 2 | L |
Conclusions
The analysis of the 50 plant species belonging to 27 families that populate the Portici Royal Park made a contribution on the identification of the allergenic species and knowledge their allergenicity level. It would be interesting to verify how much the results highlighted by the present study agree with those obtained by systems that use numerical and field methods, and it will be necessary perform further researches to extend also to herbaceous species.
It is essential that public administrations adopt an effective policy for the prevention of allergic respiratory diseases in cities, based not only on reducing the rate of the main air pollutants, but also on containing the load of allergenic pollen. This is possible during the green areas’ design phase with the technical advice of an experts’ team, implementing all the strategies necessary to limit the use of allergenic plants: to increase urban biodiversity, choose female individuals of dioecious species, use entomophilous species with low-to-moderate pollen production, limit the presence of spontaneous species and weed producing allergenic pollen by maintenance of green areas, respect minimum planting distances limiting the proximity pollinosis, reduce cross-species reactivity among pollens.
While waiting for the local administrations of urban green parks to establish guidelines and implement all measures to reduce their allergenic potential, it should be strongly recommended that the subjects at risk of pollinosis are immediately informed of the allergenic plant species present in the attended green areas and their degree of allergenicity. This information can be provided by equipping urban green spaces with signs highlighting the potential allergological risk and the periods of higher risk of exposure.
Acknowledgments
The authors are grateful to Prof. Maria Palumbo for her help on English translation.
References
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Online | Gscholar
Authors’ Info
Authors’ Affiliation
Università degli Studi di Napoli Federico II - Dipartimento di Medicina Clinica e Chirurgia, v. Pansini 5, I-80131 Napoli (Italy)
Consiglio Nazionale delle Ricerche - Istituto di Bioscienze e BioRisorse (IBBR), v. Università 133, I-80055 Portici, NA (Italy)
Consiglio Nazionale delle Ricerche - Istituto di Ricerca sugli Ecosistemi Terrestri (IRET), v. P. Castellino 111, I-80131 Napoli (Italy)
Corresponding author
Paper Info
Citation
Rispo M, De Masi L, Calandrelli MM (2020). Assessment of allergenic potential in urban forests: a case study of the Royal Park of Portici in Southern Italy. iForest 13: 376-381. - doi: 10.3832/ifor3485-013
Academic Editor
Werther Guidi Nissim
Paper history
Received: Apr 28, 2020
Accepted: Jun 22, 2020
First online: Aug 25, 2020
Publication Date: Oct 31, 2020
Publication Time: 2.13 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2020
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 38483
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 32316
Abstract Page Views: 2908
PDF Downloads: 2556
Citation/Reference Downloads: 2
XML Downloads: 701
Web Metrics
Days since publication: 1994
Overall contacts: 38483
Avg. contacts per week: 135.10
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2020): 3
Average cites per year: 0.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
The concept of green infrastructure and urban landscape planning: a challenge for urban forestry planning in Belgrade, Serbia
vol. 11, pp. 491-498 (online: 18 July 2018)
Review Papers
Green Infrastructure as a tool to support spatial planning in European urban regions
vol. 6, pp. 102-108 (online: 05 March 2013)
Research Articles
Green oriented urban development for urban ecosystem services provision in a medium sized city in southern Italy
vol. 7, pp. 385-395 (online: 19 May 2014)
Research Articles
LIFE-CLIVUT, ecosystem benefits of urban green areas: a pilot case study in Perugia (Italy)
vol. 15, pp. 133-140 (online: 09 April 2022)
Research Articles
Evaluation of urban forest landscape health: a case study of the Nanguo Peach Garden, China
vol. 13, pp. 175-184 (online: 02 May 2020)
Research Articles
Microclimate regulating functions of urban forests in Changchun City (north-east China) and their associations with different factors
vol. 11, pp. 140-147 (online: 07 February 2018)
Research Articles
The willingness of inhabitants in medium-sized city and the city’s surroundings settlements to pay for recreation in urban forests in Poland
vol. 14, pp. 483-489 (online: 27 October 2021)
Research Articles
Evaluation of urban forest spatial distribution characteristics in Guangdong - Hong Kong - Macao Greater Bay Area
vol. 16, pp. 136-143 (online: 16 May 2023)
Research Articles
Communicating spatial planning decisions at the landscape and farm level with landscape visualization
vol. 7, pp. 434-442 (online: 19 May 2014)
Research Articles
The analytic hierarchy process for selection of suitable trees for Mexico City
vol. 13, pp. 541-547 (online: 18 November 2020)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword