Effects of low-impact logging on understory birds in the Brazilian Amazon
iForest - Biogeosciences and Forestry, Volume 14, Issue 2, Pages 122-126 (2021)
doi: https://doi.org/10.3832/ifor3435-014
Published: Mar 08, 2021 - Copyright © 2021 SISEF
Research Articles
Abstract
Tropical forests have a great potential for the exploitation of natural resources. Among the economic activities that depend on forest resources, timber production is the most important one. Nevertheless, these activities may negatively affect wildlife, the availability of natural resources, and ecosystem process. Here we analysed the effects of low-impact logging on understory bird species richness, number of individuals captured, species composition, and assemblage structure in central Brazilian Amazon. We compared logged and unlogged areas over a period of three years (from August 2014 to May 2017). We captured a total of 180 birds and 42 species (20 families) in the logged area and 226 birds and 49 species (20 families) in the unlogged area. Bird assemblage structure in the logged area changed more intensely over the three years of study and became more similar to the assemblage found in the unlogged area. The degree of similarity (Jaccard’s index) in species composition between logged and unlogged areas increased from 18% in the third year to 39% in the fifth year after logging. The results suggest that the minor effects of low-impact logging were reduced a few years after the disturbance, probably due to ecological succession. The proximity of logged and unlogged areas and the reduced impact in the study site may facilitate the recovery of the bird assemblage after the disturbance.
Keywords
Biodiversity, Conservation, Environmental Disturbance, Forest Resources, Sustainable Development
Introduction
Tropical forests are the most diverse biomes on Earth, which makes them valuable for human society and fundamental to biodiversity conservation ([25]). However, human activities are promoting biodiversity loss by the destruction of these forests ([37], [18]). Agricultural expansion, fires, logging, and unplanned exploitation of forest resources are among the major threats to tropical forests ([15], [21]). In this sense, sustainable forest management (SFM) is proposed as a way of using forest resources without compromising their availability for future generations ([28]).
Low-impact logging is one of the mostly approached techniques of SFM and consists in planning the construction of roads, drag lines, stockyards, and trails, as well as managing the direction of treefall ([33], [23]). This planned and carefully controlled system of timber harvesting aims to minimize the negative impacts of logging by allowing the recovery of forests after logging ([33]). However, the sustainability of this type of activity may be questioned as the productivity may intensely drop after the first cutting cycle to less than 70% of the initial yield ([13], [35]). The association with other silvicultural techniques, such as control of lianas and non-commercial competing species, can contribute to keep the forests healthy and productive after the first logging ([33]). Nevertheless, the consequences of low-impact logging in tropical forests may still be devastating ([34]).
After logging, changes in the forest structure consequently affect animal species. The intensity of the impacts on wildlife is related to the extension of the logged area, the number and volume of trees removed from the natural ecosystems, and the elapsed time after logging ([19]). The main effects are the reduction in the number of species and individuals, and changes in species composition ([8], [21], [26]). These effects can also alter the structure of animal assemblages ([11], [22]).
Bird species play an important role in ecological processes, such as in pollination, seed dispersion, and seed predation ([40], [30]). The effects of low-impact logging on birds are often less intense than those of other types of disturbances ([2]). Nevertheless, this type of activity may affect bird species, especially cavity-nesters and understory birds ([1], [19], [11], [20]). In tropical forests, low-impact logging may affect bird species composition ([41], [9]), population density and habitat use ([8]), inter-species interactions in mixed flocks ([6]), and phylogenetic diversity ([29]). These negative impacts call into question the adequacy of SFM practices for economic and social development in the Tropics.
Logging is one of the main threats to biodiversity and also one of the most important economic activities in tropical forests ([28]). Thus, understanding the impacts of low-impact logging on biodiversity allows an evaluation of this type of activity as an alternative for sustainable development. To detect the effects of SFM on metrics of biodiversity, we captured and identified birds in logged and unlogged forests in Central Brazilian Amazon. We searched for evidence of the recovery of the bird assemblage in the logged area after the disturbance due to ecological succession. For that purpose, we tested whether species richness, number of individuals captured, bird diversity, species composition, and assemblage structure changed over the three years of study similarly in low-impact logged and unlogged areas.
Material and methods
Study site
We studied birds in two areas owned by Precious Woods Amazon (PWA) Ltd. in the municipality of Silves (02° 43′ 12.3″ S, 58° 31′ 44.73″ W), state of Amazonas, Brazil. Vegetation is typical of low-land rainforests (Floresta Pluvial Tropical de Terra Firme) with large, emergent trees reaching 35 to 52 m above the ground and with the presence of woody lianas ([32]). The forest management system in the study site is polycyclic and allows the regeneration of the native forest after exploitation (i.e., CELOS Management System - [14]). Low-impact logging techniques include: the selection and mapping of all trees to be harvested (only a subset of all trees with diameter at breast height ≥ 50 cm); planning the construction of roads, stockyards, and dragging trails; and directing tree fall to minimize environmental impact ([39]).
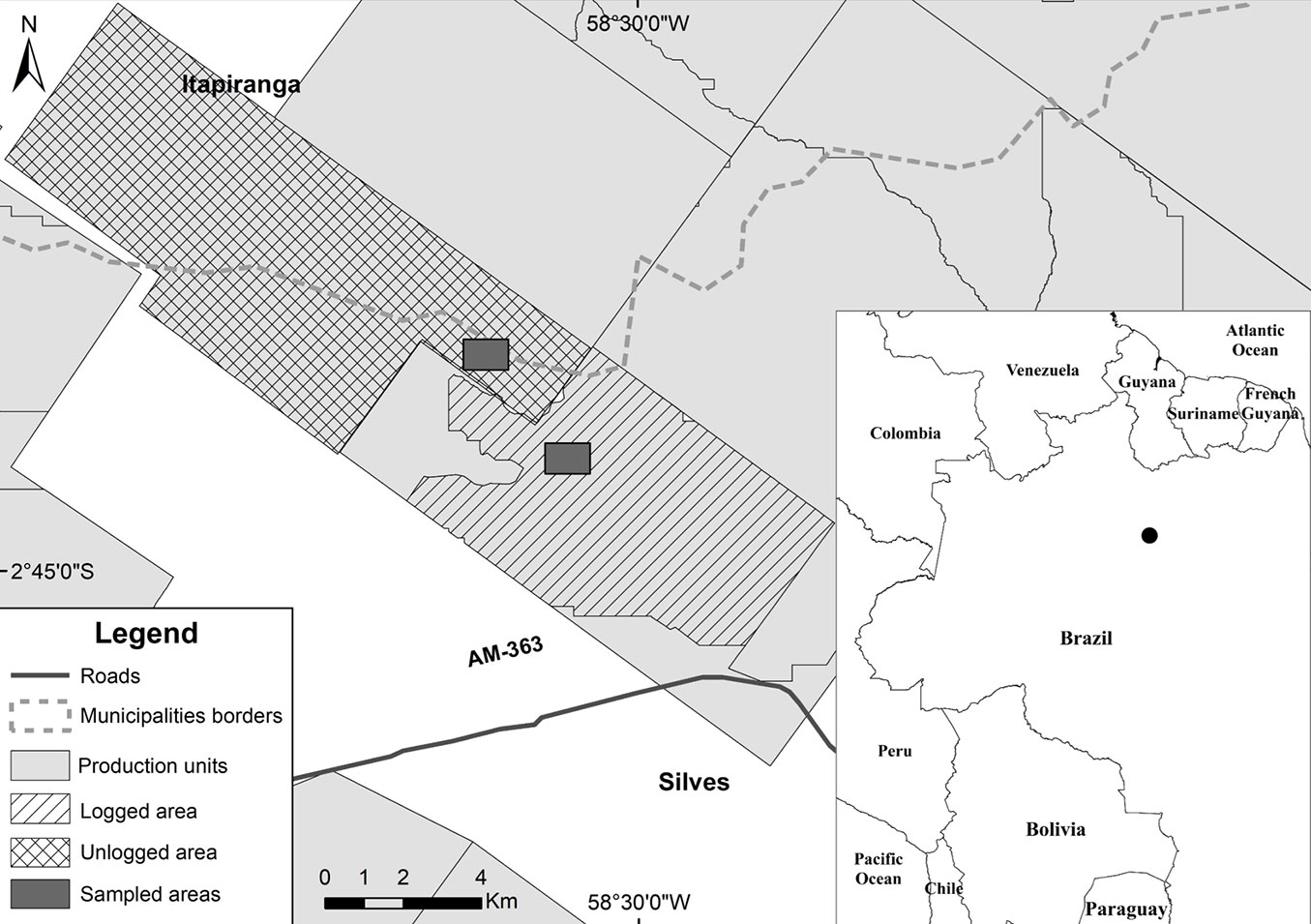
We had access to the results of the inventory of all trees with diameter at breast height ≥ 40 cm performed by PWA in both sampled areas before exploitation. One of the areas (hereafter, logged area - 4535 ha) was logged during 2012 with intensity of 20.36 m³ ha-1, approximately 30% of forest production capacity. The second area (hereafter, unlogged area - 7164 ha) was not used for low-impact logging activities before or during the study. Similarity between the logged area (87,318 individuals, 80 species, Shannon Diversity Index H: 3.6) and the unlogged area (95.177 individuals, 66 species, H: 3.51) in tree species composition (Jaccard’s index) was 78% and in tree assemblage structure (1 - Bray-Curtis dissimilarity) was 73%. Both areas sum up 9751.78 ha and are part of a mosaic of areas destined to low-impact logging exploited since 1995, in a total of more than 200,000 ha. The distance between sampled areas is 3 km (Fig. 1).
Fig. 1 - Location of the logged and the unlogged areas in the study site in Central Brazilian Amazon.
Data collection
As the interval between the end of the disturbance and the beginning of field sampling may affect the results ([5]), bird sampling in both areas started two years after exploitation to avoid detecting effects not related to ecological succession. We sampled birds between August 2014 and May 2017, in March, May, September, and November, totalling four samples per year in each area.
Each sample was conducted during two consecutive days by using twelve mist-nets (3 m height, 12 m length, mesh size 20 × 20 mm) concomitantly along 150 m long transects. Sampling started at sunrise and stopped at sunset (11 hours per day), totalling 264 mist-nets per hours per sample (12 mist-nets × 11 hours per day × 2 days) and 6336 mist-nets per hours in the study (264 mist-nets per hours per sample × 12 samples × 2 areas). Captured birds were identified at the species level and banded with metal rings from the Brazilian Bird Banding Agency (Centro Nacional de Pesquisa para a Conservação das Aves Silvestres - CEMAVE).
Statistical analysis
As our objective was to determine short-term temporal changes in bird assemblage, we calculated biodiversity metrics for each sample. Species richness was determined as the number of species and bird diversity as the Shannon-Wiener index (H’). To determine assemblage structure, we performed a non-metric multidimensional scaling (NMDS) based on the Bray-Curtis dissimilarity and calculated from a Hellinger transformed matrix with the number of individuals captured for each species. We started reducing the variability into two NMDS components and increased the number of components until a solution with stress < 20% was reached. As different groups of species may respond differently to the environmental fluctuations, we considered each NMDS component as a representative of a part of the bird assemblage structure.
We used Analysis of Covariance (ANCOVA) to test the effects of the time elapsed after logging on bird biodiversity metrics. We built covariance models with the number of days after logging for each sample and the identity of the area (logged vs. unlogged) as explanatory variables, and one of the biodiversity metrics (species richness, number of individuals captured, bird diversity, and bird assemblage structure) as the response variable. For bird assemblage structure, we tested each of the NMDS components separately. Significant results were based on F-tests with p < 0.05.
We also evaluated changes in bird species composition by calculating Jaccard’s dissimilarity index between the logged and the unlogged areas in different years of sampling. Bird assemblages of the four samples per year were gathered for this analysis. Results are reported as percentage of similarity.
Results
During the three years of study, we captured 406 birds, 63 species, and 24 families (Tab. 1, Tab. S1 in Supplementary material). From all species, 35 (56%) were detected in only one of the areas, 14 were only captured in the logged area, and 21 species only in the unlogged area. In the logged area, we captured 180 individuals and 42 species (20 families), while in the unlogged area we captured 226 individuals and 49 species (20 families).
Tab. 1 - Spearman correlation between the number of individuals captured for each species and NMDS components of bird assemblage structure in logged and unlogged areas in central Brazilian Amazon. (*): indicate that the absolute value of correlation (rho) is greater than or equal to 0.4.
| Species | NMDS1 | NMDS2 | NMDS3 | Species | NMDS1 | NMDS2 | NMDS3 |
|---|---|---|---|---|---|---|---|
| Attila spadiceus | -0.26 | -0.21 | 0.23 | Mionectes macconnelli | -0.12 | 0.72* | 0.27 |
| Automolus cervicalis | 0.15 | 0.11 | 0.28 | Mionectes oleagineus | -0.33 | -0.32 | -0.13 |
| Automolus ochrolaemus | 0.60* | 0.42* | 0.2 | Momotus momota | 0.14 | 0.2 | 0.35 |
| Campylorhamphus procurvoides | -0.32 | -0.23 | -0.14 | Myiobius barbatus | 0.04 | -0.39 | -0.02 |
| Ceratopipra erythrocephala | -0.71* | 0.18 | 0.07 | Myrmoderus ferrugineus | -0.17 | 0.32 | -0.23 |
| Certhiasomus stictolaemus | -0.15 | -0.1 | 0.46* | Myrmornis torquata | 0.42* | -0.3 | -0.06 |
| Clibanornis obscurus | 0.08 | -0.35 | -0.11 | Myrmotherula axillaris | 0.44* | -0.2 | 0.35 |
| Corapipo gutturalis | -0.07 | 0.02 | 0.2 | Myrmotherula longipennis | 0.53* | 0.21 | -0.3 |
| Corythopis torquatus | 0.23 | 0.29 | 0.08 | Percnostola subcristata | -0.43* | -0.38 | -0.09 |
| Cyanoloxia rothschildii | -0.03 | -0.32 | -0.04 | Phaethornis bourcieri | 0.35 | -0.08 | 0.23 |
| Cymbilaimus lineatus | 0.26 | -0.2 | 0.26 | Phaethornis superciliosus | -0.23 | -0.19 | -0.42* |
| Deconychura longicauda | 0.1 | 0.02 | 0.02 | Pheugopedius coraya | 0.08 | -0.35 | -0.11 |
| Dendrocincla fuliginosa | -0.13 | 0.21 | 0.18 | Philydor erythrocercum | 0.54* | -0.08 | 0.25 |
| Dendrocincla merula | 0.26 | 0.35 | 0.3 | Phoenicircus carnifex | -0.02 | 0.26 | 0.03 |
| Dendrocolaptes certhia | 0.1 | -0.34 | 0.07 | Pithys albifrons | 0.59* | 0.11 | 0.3 |
| Dixiphia pipra | 0.3 | -0.17 | 0.56* | Platyrinchus saturatus | 0.29 | 0.14 | -0.08 |
| Epinecrophylla gutturalis | 0.44* | 0.17 | -0.3 | Ramphastos vitellinus | 0.35 | -0.08 | 0.23 |
| Formicarius colma | 0.09 | 0.09 | 0.19 | Schiffornis turdina | -0.35 | -0.02 | -0.2 |
| Frederickena viridis | -0.1 | -0.12 | 0.38 | Sclerurus macconnelli | 0.29 | 0.14 | -0.08 |
| Galbula albirostris | 0.33 | -0.32 | 0.16 | Selenidera piperivora | -0.46* | 0.15 | -0.07 |
| Geotrygon montana | -0.47* | 0.14 | -0.21 | Sittasomus griseicapillus | 0.08 | -0.35 | -0.11 |
| Glyphorynchus spirurus | 0.01 | -0.01 | -0.26 | Tangara varia | 0.17 | -0.17 | -0.26 |
| Gymnopithys rufigula | 0 | 0.06 | 0.62* | Terenotriccus erythrurus | 0.14 | 0.2 | 0.35 |
| Hemitriccus minor | 0.32 | 0.11 | -0.35 | Thalurania furcata | 0.2 | -0.26 | -0.05 |
| Hylexetastes perrotii | 0.31 | 0.02 | 0.11 | Thamnomanes ardesiacus | 0.49* | 0.40* | 0.17 |
| Hypocnemis cantator | -0.15 | -0.02 | -0.14 | Thamnomanes caesius | 0.32 | 0.22 | -0.16 |
| Lanio fulvus | -0.08 | -0.1 | -0.1 | Thamnophilus murinus | -0.23 | -0.09 | -0.11 |
| Lanio surinamus | 0.33 | 0.19 | -0.06 | Trogon rufus | -0.32 | -0.23 | -0.14 |
| Laniocera hypopyrra | -0.02 | -0.05 | -0.02 | Turdus albicollis | 0.79* | 0.08 | -0.31 |
| Lepidocolaptes albolineatus | 0.32 | 0.11 | -0.35 | Xenops minutus | 0.26 | -0.2 | 0.26 |
| Lipaugus vociferans | 0.14 | 0.2 | 0.35 | Xiphorhynchus pardalotus | 0.13 | -0.54* | -0.18 |
| Micrastur ruficollis | 0.14 | 0.2 | 0.35 | - | - | - | - |
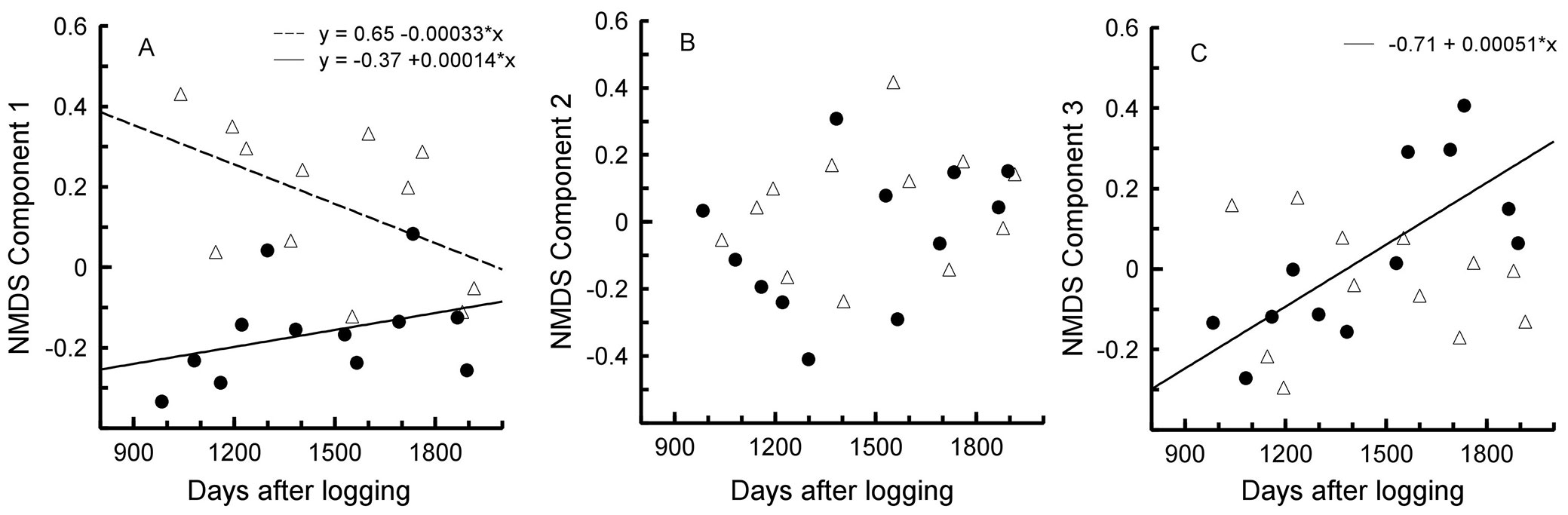
Low-impact logging did not affect species richness (F[3, 20] = 0.54, p = 0.663), number of individuals captured (F[3, 20] = 0.96, p = 0.429), and bird diversity (F[3, 20] = 0.51, p = 0.679). Variation in bird assemblage structure was reduced into three NMDS components (stress = 0.15 - see Tab. 1for species scores). ANCOVA indicated that the assemblage structure changed over time differently in logged and unlogged areas for two NMDS components (NMDS1: F[3, 20] = 11.31, p = 0.0001; NMDS2: F[3, 20] = 1.18, p = 0.342; NMDS3: F[3, 20] = 4.53, p = 0.014). The first component (NMDS1) represents a group of species in the assemblage structure that became more similar between the logged and the unlogged areas over time (Fig. 2A). The second component represents a group of species that were not affected by low-impact logging (Fig. 2B). The third component (NMDS3) represents a group of species that changed over time in the logged area only (Fig. 2C).
Fig. 2 - Changes in bird assemblage composition as determined by Non-metric Multidimensional Scaling (NMDS) from three to five years after logging in logged (filled circles, solid line) and unlogged (open triangles, hatched line) areas.
Jaccard’s similarity index between the logged and unlogged areas increased over time, from 18% in the third year, to 25% in the fourth year, and to 39% in the fifth year after logging.
Discussion
Our results show an increase in similarity in bird assemblage structure between the logged and the unlogged areas over a short period of time. However, responses of the bird assemblage to the effects of low-impact logging were complex, and some species contributed to an increase in dissimilarity between the areas (Fig. 2C). The logged area was more intensely affected by changes in the bird assemblage during the study, probably due to ecological succession after disturbance ([31]). Changes in forest structure may result from the opening of clearings, stockyards, roads, and drag lines ([16]).
Species richness, number of individuals captured, and bird diversity were similar between the logged and unlogged areas, although observed total values were slightly lower in logged forests (14% less rich and 20% less abundant - see Tab. S1 in Supplementary material). After logging, bird species richness and abundance may decrease ([17], [19]). Ecological succession increases the complexity of forest structure, which in turn may lead to a greater diversity of birds ([4]). The slight observed reduction in bird richness and number of individuals captured may be a consequence of low-impact changes in microclimatic conditions (e.g., temperature, humidity, luminosity) and in vegetation structure that happened only in the logged area.
Changes in species composition due to low-impact logging may be a result of the substitution of mature forest specialists by disturbed forests opportunists ([11]). This may explain why assemblage structure (NMDS1) changed over time both in the logged and in the unlogged forests and became more similar during the fifth year after logging. Among those sensitive species, we detected forest specialists found in the understory and on the ground level (e.g., Thamnomanes ardesiacus, Thamnomanes caesius, Epinecrophylla gutturalis, and Myrmotherula longipennis). The population of these species is usually reduced in disturbed environments ([1], [19]). Other species (e.g., Terenotriccus erythrurus, Schiffornis turdina, Mionectes oleagineus, and Pheugopedius coraya) are usually found in disturbed forests and may benefit from environmental changes ([10], [7]). These changes may result in differences in the assemblage structure in logged and unlogged forests.
The increasing similarity in assemblage structure between logged and unlogged areas suggests a decrease of environmental impacts in a relatively short time period (five years after logging). Thus, the results may indicate the positive effects of ecological succession on bird biodiversity by the substitution of species by those typically found in undisturbed forests ([27]). This may explain why in the fifth year after logging forest specialist species were captured in the logged area, and those were absent in previous years (e.g., Pithys albifrons, Gymnopithys rufigula, and Automolus ochrolaemus). These species forage on the ground and in the understory, and are susceptible to environmental disturbances ([10], [24], [36]). However, low-impact logging areas may also function as sink in a metapopulational system, in such a way that species spill-over may be the cause of the increased similarity between logged and unlogged areas ([38]). In this case, the return of species after disturbance may not necessarily indicate the recovery of the forest ecosystem and environmental quality.
Variation in assemblage structure over time was less intense in the unlogged area. This may be a result of the conservation of important ecological processes and factors that help maintaining wildlife communities at equilibrium ([3], [15]). Thus, the increase in similarity between logged and unlogged areas indicates that time after logging may be the most important factor for recovering wildlife communities in areas under SFM ([12]).
Conclusions
Our results suggest that the effects of low-impact logging altered bird assemblage structure and that the similarity between logged and unlogged areas gradually increased over a short period of time after harvesting. Species richness and the number of individuals captured were similar between logged and unlogged areas. The observed impacts were not intense and changes in the assemblage suggest an ongoing process of recovery, probably facilitated by the proximity of logged and unlogged areas and by the reduced impact of logging in the study area. Long-term effects of SFM on bird assemblages remain to be tested.
Acknowledgements
We would like to thank all the people who helped in the fieldwork. We thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for providing financial support to the postgraduation programs of the authors. This work was supported by the Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM) for granting a scientific initiation scholarship to JCRS and for supporting the work of RASC (FIXAM 062.01637/2018).
References
Online | Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Adriene O Amaral 0000-0001-7608-8107
Programa de Pós-Graduação em Ciências Florestais e Ambientais, Universidade Federal do Amazonas, Manaus, Amazonas, 69067-005 (Brazil)
Ricardo AS Cerboncini 0000-0002-8140-1417
Louri Klemann Junior 0000-0002-3532-9805
Universidade do Estado do Amazonas, Centro de Estudos Superiores de Itacoatiara, Itacoatiara, Amazonas, 69100-000 (Brazil)
Louri Klemann Junior 0000-0002-3532-9805
Programa de Pós-Graduação em Ciência e Tecnologia para Recursos Amazônicos, Instituto de Ciências Exatas e Tecnologia, Universidade Federal do Amazonas, Itacoatiara, Amazonas, 69100-000 (Brazil)
Corresponding author
Paper Info
Citation
Soares JCR, Amaral AO, De Moura RS, Cerboncini RAS, Klemann Junior L (2021). Effects of low-impact logging on understory birds in the Brazilian Amazon. iForest 14: 122-126. - doi: 10.3832/ifor3435-014
Academic Editor
Mirko Di Febbraro
Paper history
Received: Apr 04, 2020
Accepted: Jan 05, 2021
First online: Mar 08, 2021
Publication Date: Apr 30, 2021
Publication Time: 2.07 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2021
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 36368
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 30545
Abstract Page Views: 2818
PDF Downloads: 2446
Citation/Reference Downloads: 1
XML Downloads: 558
Web Metrics
Days since publication: 1796
Overall contacts: 36368
Avg. contacts per week: 141.75
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2021): 1
Average cites per year: 0.20
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Review Papers
The forest biodiversity artery: towards forest management for saproxylic conservation
vol. 9, pp. 205-216 (online: 26 October 2015)
Research Articles
Impact of management practices on habitat use by birds in exotic tree plantations in northeastern Argentina
vol. 19, pp. 38-44 (online: 06 February 2026)
Research Articles
Networking sampling of Araucaria araucana (Mol.) K. Koch in Chile and the bordering zone of Argentina: implications for the genetic resources and the sustainable management
vol. 2, pp. 207-212 (online: 22 December 2009)
Technical Reports
Conservation and use of elm genetic resources in France: results and perspectives
vol. 13, pp. 41-47 (online: 03 February 2020)
Research Articles
Biodiversity conservation and wood production in a Natura 2000 Mediterranean forest. A trade-off evaluation focused on the occurrence of microhabitats
vol. 12, pp. 76-84 (online: 24 January 2019)
Research Articles
Community based forest management and its impact on vegetation: a case study
vol. 2, pp. 93-98 (online: 10 June 2009)
Research Articles
Forest and tourism: economic evaluation and management features under sustainable multifunctionality
vol. 2, pp. 192-197 (online: 15 October 2009)
Research Articles
Effects of forest management on bird assemblages in the Bialowieza Forest, Poland
vol. 8, pp. 377-385 (online: 02 October 2014)
Editorials
Workshop COST E52 “Evaluation of beech genetic resources for sustainable forestry”
vol. 2, pp. 104 (online: 10 June 2009)
Review Papers
Perspectives of plantation forests in the sustainable forest development of China
vol. 14, pp. 166-174 (online: 09 April 2021)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword