Effects of forest management on bird assemblages in the Bialowieza Forest, Poland
iForest - Biogeosciences and Forestry, Volume 8, Issue 3, Pages 377-385 (2015)
doi: https://doi.org/10.3832/ifor1212-007
Published: Oct 02, 2014 - Copyright © 2015 SISEF
Research Articles
Abstract
We examined the effects of different intensities of forest management on bird communities in the Bialowieza Forest, eastern Poland. Stands that had been managed for more than 100 years (cutting, planting, removal of dead wood) and stands that were partially protected in nature reserves (sporadic sanitary cutting, removal of dead wood until 2008) were compared with unmanaged stands in the Bialowieza National Park by surveying the bird community during three breeding seasons (2010-2012). Surveys were conducted within three forest habitats: spruce-pine (Pino-Quercetum), lime-hornbeam (Tilio-Carpinetum) and ash-alder (Fraxino-Alnetum). Results showed that habitat structure significantly affected the avian community. The basal area of live trees had a positive effect on abundance of birds, while the density of live trees had negative significant effect on bird abundance and species diversity. We also found significantly lower abundance of insectivorous birds and cavity-nesters in managed compared to unmanaged stands. Birds’ assembly in the spruce-pine and ash-alder stands were most sensitive to management. These results show both that management can be used to sustain bird communities, including species of conservation concern, and that inappropriate management may harm them.
Keywords
Bialowieza Forest, Breeding Bird Communities, Forest Biodiversity, Primeval Stands, Forest Management
Introduction
Birds are often used as indicators of the quality of forest environments ([30], [16], [23]). Forest management practices can affect the conservation value of forests for birds ([24], [17], [36], [61]). Forest bird species are particularly sensitive to habitat changes, especially habitat loss due to logging ([9], [45], [15]), forest fragmentation ([27]), stand age and tree species composition ([39]) and the amount of dead wood ([49], [51], [31], [45], [6]). Knowledge of how these forest elements affect bird assemblages is essential for avian conservation ([9], [32], [15]). These relationships have been studied extensively in the managed forests of Europe and North America ([20], [31], [34], [32]), but seldom managed forest stands have been compared with unmanaged, primeval forest to such purposes.
The Bialowieza Forest located in central Europe is one of the last remaining temperate lowland forests that has survived to the present in primeval condition ([43]). By primeval forest we define a forest of great age without significant disturbance which exhibits unique ecological features. The best preserved stands are protected in the Bialowieza National Park (BNP), presenting a unique opportunity to study organisms under primeval conditions. However, for over a hundred years, a large part of the Bialowieza Forest, outside of the BNP, has been subjected to human activities including clear-cuts, selective cutting and tree planting ([3]).
Since 1975, the breeding bird communities of the BNP have been monitored in three forest types: spruce-pine (Pino-Quercetum), lime-hornbeam (Tilio-Carpinetum) and ash-alder (Fraxino-Alnetum - [43], [41], [42], [60], [59], [57]). However, each of these studies focused on the avifauna of the strictly protected, primeval stands. Only a few papers describe the status of selected species, e.g., the Chiffchaff Phylloscopus collybita and woodpeckers, in managed stands near the BNP ([26], [52], [49], [51], [56], [5], [6]).
We present the results of a three-year study of the breeding avifauna in three different parts of the Bialowieza Forest that have experienced different levels of forest management: (1) no intervention (“natural”); (2) partially managed (“semi-natural”); and (3) intensive forestry (“managed”). Although forest management (especially removal of dead wood) in the Bialowieza Forest is known to strongly influence some bird species such as woodpeckers ([52], [49], [51], [5], [6]), this is the first study to attempt a wider assessment of the effects of forest management on the forest breeding bird community.
We focus specifically on how forest management has altered bird community structure (avian richness, abundance, diversity, foraging and nesting groups) in the three major types of forest found in the BNP: spruce-pine, lime-hornbeam, ash-alder stands. We assumed that the BNP represents natural conditions, and we hypothesized that simplification of the spatial structure, the felling and replanting of trees and the removal of the dead wood ([3], [53]) would simplify the structure of the bird community, as it has been demonstrated for woodpeckers ([49], [51], [6]). An additional aim of this study was to identify the forest habitat types that are most sensitive to current forest management practices.
Material and methods
Study area
The study was conducted in the Polish part of the Bialowieza Forest (52° 29′ - 52° 57′ N and 23° 31′ - 24° 21′ E). This forest remnant survived in primeval condition because the whole Bialowieza Forest was protected as a royal hunting grounds beginning in the XV century. Logging started in 1914 but a few legacy trees remain even in heavily logged stands. The dominant forest type is deciduous lime-hornbeam (Tilio-Carpinetum) composing approximately 49% of total forest area on the fertile, mainly brown soils, with moderate wetness. Mixed coniferous spruce-pine (Pino-Quercetum) occurs on approx. 37% of the total forest area, exclusively on the driest sandy soils with low fertility. Alder and ash-alder forests (Carici elongatae-Alnetum, Fraxino-Alnetum) represent about 14% of forest area and grow along forest streams or in depressions with standing water.
Approximately 16% of the Bialowieza Forest has been strictly protected as the Bialowieza National Park since 1921. In addition, the Bialowieza Forest includes 21 “nature reserves” outside the national park (19% of the total forest area) where different kinds of “sanitary” treatments (selective removal of some dead trees, small-scale plantations) are undertaken. These represent areas with relatively low intensity management. The rest of the forest is managed by State Forests that carry out logging, planting, dead wood removal, and control of forest pests (often by cutting infected trees), that represent intensive forest management. Overall, forest management in the Polish part of the Bialowieza Forest during the XX century consisted mainly of cutting trees (150 000 m3 per year), although in last four years logging was reduced approximately threefold. Logging during the XX century led to a significant reduction in the average age of tree stands outside the BNP and nature reserves. In logged areas only a few of the oldest trees are protected as nature monuments.
Sampling design
Study plots were selected to represent the three main forest types: spruce-pine (SP), lime-hornbeam (LH) and ash-alder (AA). Plots were established in areas differing in the intensity of forest management: (1) natural, near-primeval stands of the Bialowieza National Park (NAT); (2) semi-natural nature reserves with low-intensity management (SEMI); and (3) managed stands with high intensity forest management (MAN). Labels of the plots represent the different combinations of forest type and management intensity in each plot: SP-NAT, SP-SEMI, SP-MAN; LH-NAT, LH-SEMI, LH-MAN; AA-NAT, AA-SEMI, AA-MAN. The characteristics of each plot are reported in Tab. 1.
Tab. 1 - Characteristics of the study plots in the Bialowieza Forest grouped by stand type (AA: ash-alder stands; LH: lime-hornbeam stands; SP: spruce-pine stands) and management intensity (NAT: unmanaged stands in the Bialowieza National Park; SEMI: low intensity management in nature reserves; MAN: intensively managed stands).
| Study plot | Main tree species | Stand age | Structure of the tree stand | Human activity |
|---|---|---|---|---|
| SP-NAT | Pinus sylvestris, Picea abies, Quercus robur, Carpinus betulus | Uneven aged 0 to 300 years old |
Old growth with much dead wood | Very limited human activity for centuries, research only, no forest management |
| SP-SEMI | Pinus sylvestris, Picea abies, Quercus robur, Carpinus betulus | Some trees 80-150 years old but most are much younger | Old growth with some gaps filled with younger trees | Tourist traffic on the trails; management limited to sanitary cutting of infested spruces. Removal of dead wood from 1921 until 2008 |
| SP-MAN | Pinus sylvestris, Picea abies,some Quercus robur | 60-80 years old | Mostly even aged stands with a few old trees | Tourist traffic on the trails; intensive forest management: clear-cuts, tree planting within fenced areas |
| LH-NAT | Tilia cordata, Carpinus betulus, Picea abies, Quercus robur, Acer platanoides | Uneven aged 0-200 (300) years old |
Old growth, multi-story stand | Only limited tourist traffic restricted to the main trail, research only; no management |
| LH-SEMI | Carpinus betulus, Tilia cordata, Picea abies, Quercus robur, Acer platanoides | Uneven aged 0-200 (300) years old |
Old growth, multi-story stand with some gaps filled with younger trees | Tourist traffic on the trails; sanitary cutting of infested spruces and some thick trees, removal of dead wood from 1921 until 2008 |
| LH-MAN | Carpinus betulus, Picea abies, Quercus robur, Tilia cordata | 30-40 years old | A few large old trees in regenerating stands | Tourist traffic on the trails; intensive forest management: clear-cuts, tree planting within fenced plots |
| AA-NAT | Alnus glutinosa, Fraxinus excelsior, Picea abies | Uneven aged 0-200 (300) years old |
Old growth, semi-open, with dense shrub layer in some places | Very limited human use for centuries, research only; no management |
| AA-SEMI | Alnus glutinosa, Fraxinus excelsior, Picea abies | Uneven aged 0-150 (200) years old |
Single big trees; semi-open, with dense bush layer in some places |
Limited to sanitary cutting of infested spruces and big trees; c. 30 years ago most large trees were removed, removal of dead wood from 1921 until 2008 year, no planting only natural regeneration of trees |
| AA-MAN | Alnus glutinosa, Fraxinus excelsior, Picea abies | 30-60 years old | More open, younger stand compared to NAT and SEMI | Selective cutting of trees every 40-50 years and most of the oldest trees are removed, permanent removal of dead wood, no planting, only natural regeneration of trees |
Within each of the nine plots, eight points were selected randomly with a minimum distance of 250 m each other and marked on the field. In total, 72 sites were marked. This design includes no replicates of the forest type/management intensity treatments. Consequently, point count locations were spatially correlated, but this did not affect results of our analyses, because we tested only for the main effects of forest and management type. No significant autocorrelation was found among plots (Moran’s I test) for three bird indices: abundance (I = -0.146, p = 0.64), diversity (I = -0.116, p = 0.87) and richness (I = -0.127, p = 0.78).
Bird surveys
During three breeding seasons (April-May, 2010-2012) point counts ([19]) of all birds were carried out at each of 72 sites. Bird surveys were conducted from dawn to approximately 09:00 a.m. Three visits per season were made at each site. Surveys were conducted by experienced observers only on days when the weather was good, no wind or rain ([2]). During each survey at each site, the number of individuals all bird species heard and/or seen within a 50 m radius and greater than 50 m, were recorded during a 10-min period. Overflying birds and wintering visitors (Bohemian waxwing, Bombycilla garrulus and Brambling, Fringilla montifringilla) were not included in analyses. At every point the maximum number of each bird species from the three counts in each year was used in analyses. Bird species detected outside of the 50-m radius circle were used only for qualitative comparisons (e.g., assemblage composition).
Although the point method is less accurate than mapping bird territories, not allowing the estimation of the absolute density of birds ([2]), the composition and relative abundance of bird species in different habitats in the BNP as revealed by point counts were similar to those obtained by the mapping territories ([57]). In addition, because the community indexes used are based on relative abundance in each habitat type, the potential influence of differences in detectability of the species among habitats was mitigated ([7], [46]).
Habitat variable
At each point count site, tree measurements were made once during the study. Measures were taken within a square of 0.25 ha centered on the point count site. The species, diameter at the breast height (DBH) and condition (live or dead) of each standing tree (≥ 10 cm DBH) was recorded within the sampled square, and the total basal area for each tree species in the plot was calculated. We analyzed six continuous variables: (1) the total basal area of all live trees (expressed as m2 per ha); (2) density of live trees (number of stems per ha); (3) density of live conifers; (4) species diversity of live trees (Shannon-Wiener’s index based on the number of live trees with Betula spp. and Ulmus spp. being treated at generic level); (5) basal area of standing dead trees; and (6) density of standing dead trees.
Data analysis
Bird indes
The following indexes were calculated:
S: bird richness was calculated as the number of bird species detected at every point count site.A: bird abundance was calculated as the number of all individuals detected at each point count site.H′: bird diversity per plot was calculated using Shannon-Wiener index ([37]).
For each site, we also calculated abundance indexes for groups of birds with common foraging and nesting habits, i.e., for foraging or nesting guilds, respectively. These guilds were distinguished following Tomialojć et al. ([43]), Tomialojć & Wesolowski ([40]) with some changes by Wesolowski et al. ([57]):
Foraging guilds
Birds foraging outside the forest were grouped together independently of the type of food taken, while within-forest foragers were classified as: (i) predators (hunting mainly vertebrates); (ii) herbivores (birds feeding on plants including seed eaters); and (iii) “insectivores”. The last group was further subdivided into those foraging on the ground (“ground-feeders”), on the bark (“bark-feeders”) or on the crown (“crown-feeders”).
Nesting guilds
Three guilds were defined to reflect an increasing nest vulnerability to predators: (i) birds nesting on the ground or in low vegetation (up to 1.5 m above the ground), labeled as “ground-nesters”; (ii) birds building open or domed nests on high bushes or trees, labeled as “crown-nesters”; and (iii) those that nest in cavities, called “cavity-nesters”. Because there were no nest-boxes on our plots, cavity-nesting birds used only natural cavities. To calculate an index value for each guild (e.g., an abundance index) for a single season, we summed the number of individuals of all species in each group recorded at each site.
Statistical analyses
Data on tree-stand characteristics and abundance in nesting and foraging guilds was log10-transformed before analyses, because the distribution of some variables was right-skewed. A linear mixed-effects model fitted by REML was applied. Initial analyses included only plot ID as random variable. Because these analyses revealed that the sampling year was not a significant factor, we also included the year as a random variable in further analyses. In the subsequent models, all habitat characteristics, forest type and management intensity were used as fixed variables. Separate models were run using each index (bird richness, bird abundance and bird diversity) as the dependent variable. To reduce the number of analyses for foraging and nesting guilds, we used only the abundance index as dependent variable. The significance of fixed effects in the model was determined using likelihood ratio tests - LR (-2 times the difference in log-likelihoods between hierarchical models estimated using maximum likelihood, tested against a χ2 distribution with the number of degrees of freedom equal to the difference in the number of terms estimated). The post-hoc Tukey’s HSD test was applied to estimate the significance of differences between means of indices obtained for the three consecutive years, the three main forest types (SP, LH and AA) and the three levels of management intensity (NAT, SEMI and MAN stands). All analyses were performed using the software package R ([29]). Means and model coefficients are presented ± standard error or standard deviation.
Results
Bird abundance (LR = 4.97, df = 2, p = 0.08) and bird diversity (LR = -1.31, df = 2, p = 0.52) did not differ significantly among years after adjusting for the effect of management intensity and forest type. Only the index of avian richness differed significantly among years (LR = 7.15, df = 2, p = 0.03), due to slightly higher values for the year 2011 in comparison with the year 2010 (Tukey’s HSD z = 2.39, p = 0.05). For the sake of simplicity we used the year as a random variable in all further analyses, assuming that the difference in richness index was small enough and did not substantially affected final results.
Forest management intensity affected the bird community, with lowest S, A and H′ indexes occurring typically in intensively managed stands (Tab. 2); semi-natural and natural stands usually did not differ significantly in these indexes. The composition of the bird community was similar in all study plots. Species lists for each plot are given in Tab. S1-S9 of Appendix 1. Bird abundance was significantly higher in NAT and SEMI forests than in MAN stands (Tukey’s HSD, p < 0.001 for both comparisons), but NAT and SEMI forests did not differ significantly (p = 0.86 - Tab. 3). Species richness (p < 0.001 for both comparisons) and species diversity (p < 0.001 for both comparisons) were also higher in NAT and SEMI forests than in MAN stands. Again the difference between NAT and SEMI stands was not significant (p = 0.97 and p = 0.99 for index of species richness and species diversity, respectively - Tab. 3).
Tab. 2 - Mean (± SD) and sample size of bird indices: richness (S), abundance (A), diversity (H′) in all study plots representing three forest types (spruce-pine, lime-hornbeam, ash-alder) and three levels of management intensity (NAT: natural, near-primeval forest, Bialowieza National Park; SEMI: semi-natural forest, nature reserves; MAN: intensively managed forest).
| Forest type | Bird indices | NAT | SEMI | MAN | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | N | Mean ± SD | N | Mean ± SD | N | ||
| Spruce-pine | Species richness (S) | 12.0 ± 1.69 | 8 | 11.0 ± 1.87 | 8 | 9.9 ± 0.83 | 8 |
| Birds abundance (A) | 15.2 ± 2.02 | 8 | 15.0 ± 1.91 | 8 | 13.3 ± 1.23 | 8 | |
| Species diversity (H′) | 3.4 ± 0.20 | 8 | 3.3 ± 0.26 | 8 | 3.1 ± 0.11 | 8 | |
| Lime-hornbeam | Species richness (S) | 12.4 ± 1.50 | 8 | 14.2 ± 1.50 | 8 | 12.1 ± 2.01 | 8 |
| Birds abundance (A) | 17.0 ± 2.64 | 8 | 18.5 ± 1.33 | 8 | 15.0 ± 3.17 | 8 | |
| Species diversity (H′) | 3.5 ± 0.17 | 8 | 3.7 ± 0.16 | 8 | 3.5 ± 0.24 | 8 | |
| Ash-alder | Species richness (S) | 16.9 ± 1.59 | 8 | 15.8 ± 2.13 | 8 | 12.2 ± 2.15 | 8 |
| Birds abundance (A) | 22.6 ± 1.40 | 8 | 20.1 ± 2.69 | 8 | 14.6 ± 2.29 | 8 | |
| Species diversity (H′) | 3.9 ± 0.14 | 8 | 3.9 ± 0.20 | 8 | 3.5 ± 0.31 | 8 | |
Tab. 3 - Summary of statistics (z-values of Tukey’s HSD post-hoc test) for the effect of management regime and forest type on bird indices - richness (S), abundance (A), diversity (H′) and index of abundance for different feeding and nesting guilds. (AA): ash-alder stands; (LH): lime-hornbeam stands; (SP): spruce-pine stands; (NAT): natural, near-primeval areas of the Bialowieza National Park; (SEMI): semi-natural areas in nature reserves; (MAN): intensively managed areas); (′): p<0.10; (*) p<0.05; (**): p<0.01; (***): p<0.001.
| Effects | Stands | Species richness (S) |
Birds abundance (A) |
Species diversity (H’) |
Index of abundance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Birds feeding outside forest |
Insect ground- feeders |
Insect bark- feeders |
Insect crown- feeders |
Predators | Herbivores | Crown- nesters |
Ground- nesters |
Cavity- nesters |
|||||
| Management regime |
NAT-SEMI | 0.23 | 0.53 | 0.13 | 1.47 | -0.11 | -0.02 | 0.81 | -0.32 | -0.53 | -1.12 | 0.01 | 2.06′ |
| NAT-MAN | 4.34*** | 5.64*** | 3.75*** | 2.20′ | 3.31** | 4.13*** | 3.75*** | 0.32 | 0.27 | 0.48 | 2.52* | 5.94*** | |
| SEMI-MAN | 4.12*** | 5.11*** | 3.88*** | 0.73 | 3.41** | 4.15*** | 2.94** | 0.63 | 0.80 | 1.60 | 2.51* | 3.88*** | |
| Forest type | AA-LH | 3.79*** | 3.22** | 3.01** | 0.99 | 3.74*** | 2.81* | 1.31 | -0.20 | -0.79 | -0.87 | 5.34*** | 1.74 |
| AA-SP | 7.27*** | 6.48*** | 7.89*** | 3.60*** | 7.04*** | 3.73*** | 2.27′ | 0.85 | 0.43 | 1.58 | 3.36** | 5.18*** | |
| LH-SP | 3.48** | 3.26** | 4.89*** | 2.61* | 3.30** | 0.92 | 0.96 | 1.05 | 1.22 | 2.45* | -1.98 | 3.44** | |
Over 80% of breeding birds in all study plots feed on invertebrates (including crown-foragers accounting for 42-49% of individuals), while herbivores were only 10-16% of the community assemblages (Tab. 4). The abundance of birds classified to the different foraging guilds did not differ significantly among the forest management levels, except for insectivorous species. Mean abundance of insectivorous ground-feeders, bark-feeders and crown-feeders was higher both in NAT and SEMI forest than in intensively managed forest (Tukey’s HSD, p < 0.001 for both comparisons), but abundance of insectivorous birds did not differ between NAT and SEMI forests (p = 0.91 - Tab. 3).
Tab. 4 - Total bird abundance (mean ha-1 ± SE) in foraging and nesting guilds for birds sampled during 2010-2012 in the Bialowieza Forest, Poland. (AA): ash-alder stands; (LH): lime-hornbeam stands; (SP): spruce-pine; (NAT): natural, near-primeval stands of the Bialowieza National Park; (SEMI): semi-natural stands in nature reserves; (MAN): managed stands.
| Groups | Variables | Spruce-pine (SP) | Lime-hornbeam (LH) | Ash-alder (AA) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NAT | SEMI | MAN | NAT | SEMI | MAN | NAT | SEMI | MAN | ||
| Foraging groups | Forage outside forest |
0.09 ± 0.08 | 0.02 ± 0.00 | 0 | 0.28 ± 0.04 | 0.41 ± 0.08 | 0.38 ± 0.08 | 0.96 ± 0.30 | 0.36 ± 0.08 | 0.13 ± 0.00 |
| Predators | 0.15 ± 0.08 | 0.08 ± 0.02 | 0.10 ± 0.03 | 0.08 ± 0.03 | 0.26 ± 0.08 | 0.13 ± 0.02 | 0.18 ± 0.07 | 0.12 ± 0.05 | 0.14 ± 0.06 | |
| Herbivores | 1.63 ± 0.23 | 2.06 ± 0.38 | 2.09 ± 0.60 | 2.16 ± 0.44 | 2.25 ± 0.28 | 2.24 ± 0.73 | 2.32 ± 0.40 | 2.18 ± 0.69 | 1.59 ± 0.24 | |
| Ground insectivores |
4.53 ± 0.42 | 4.64 ± 0.64 | 3.79 ± 0.67 | 5.35 ± 0.37 | 5.69 ± 0.23 | 4.94 ± 0.11 | 7.21 ± 0.72 | 6.85 ± 0.26 | 5.33 ± 0.33 | |
| Bark insectivores |
1.39 ± 0.05 | 1.13 ± 0.21 | 0.82 ± 0.26 | 1.35 ± 0.27 | 1.91 ± 0.16 | 0.66 ± 0.16 | 2.48 ± 0.51 | 2.20 ± 0.19 | 1.07 ± 0.10 | |
| Crown insectivores |
7.42 ± 0.50 | 7.10 ± 0.60 | 6.50 ± 0.54 | 7.78 ± 0.68 | 7.98 ± 0.49 | 6.60 ± 0.62 | 9.43 ± 0.65 | 8.43 ± 0.31 | 6.32 ± 0.60 | |
| Nesting groups | Ground-nesters | 5.28 ± 0.33 | 4.45 ± 0.60 | 3.75 ± 0.99 | 3.65 ± 0.50 | 4.01 ± 0.19 | 3.75 ± 0.14 | 5.90 ± 0.79 | 6.37 ± 0.32 | 4.71 ± 0.42 |
| Crown-nesters | 4.83 ± 0.36 | 6.53 ± 0.58 | 6.08 ± 0.67 | 6.88 ± 0.77 | 7.70 ± 0.51 | 6.65 ± 0.99 | 7.48 ± 0.15 | 6.70 ± 0.86 | 5.71 ± 0.48 | |
| Cavity-nesters | 5.00 ± 0.62 | 4.02 ± 0.48 | 3.35 ± 0.35 | 6.33 ± 0.23 | 6.55 ± 0.82 | 4.38 ± 0.37 | 8.91 ± 0.55 | 6.75 ± 0.27 | 4.07 ± 0.38 | |
The proportion of ground-nesters ranged from 22 to 35% and was the highest in SP-NAT and the lowest in LH-NAT and in LH-SEMI. The proportion of cavity-nesters ranged from 25 to 40% and was the highest in AA-NAT and the lowest in SP-MAN (Tab. 4). The abundance of birds nesting in the crowns was not significantly affected by the management zone. Cavity-nesters were less abundant in MAN forests than in NAT and SEMI forests (Tukey’s HSD, p < 0.001 for both comparisons). There was no significant difference between NAT and SEMI forest in the abundance of birds belonging to this nesting group (p = 0.10 - Tab. 3).
Forest types differed in the number of bird species detected, with highest S, A, H′ indexes typically recorded in ash-alder stands. Lowest values of these parameters occurred in spruce-pine stands (Tab. 2). Bird abundance was significantly higher in AA forest than in LH and SP forests (Tukey’s HSD, p = 0.004 and p < 0.001, respectively), and also in LH forest than in SP forest (p = 0.003, Tab. 3).
The abundance of birds feeding outside the forest was significantly higher in LH and AA forest types than in SP forest (Tukey’s HSD, p = 0.02 and p < 0.001, respectively - Tab. 3). It was related mainly to presence of the Starling Sturnus vulgaris in deciduous stands (see Tab. S6-S8 in Appendix 1). The guild of insectivorous ground-feeders was least abundant in spruce-pine forest, significantly more numerous in lime-hornbeam forest (p = 0.002) and most abundant in ash-alder forest (p < 0.001, for difference between AA and SP forest and AA and LH forest). Also, insectivorous bark-feeders were more abundant in AA forest than in SP forest (p < 0.001) and LH forest (p = 0.01), but there was no significant difference between LH and SP forests (p = 0.62 - Tab. 4). The abundance of crown-feeders, predators and herbivores did not differ significantly among forest types (Tab. 3).
The abundance of ground-nesters was significantly higher in AA forest than in LH forest (Tukey’s HSD, p < 0.001) and SP forest (p = 0.002). The abundance of cavity-nesters was also lower in SP forest than in LH forest and AA forest (p = 0.002 and p < 0.001, respectively), but not in lime-hornbeam forest compared with the ash-alder forest (p = 0.19 - Tab. 3). The abundance of birds nesting in the crowns was not significantly affected by the forest type.
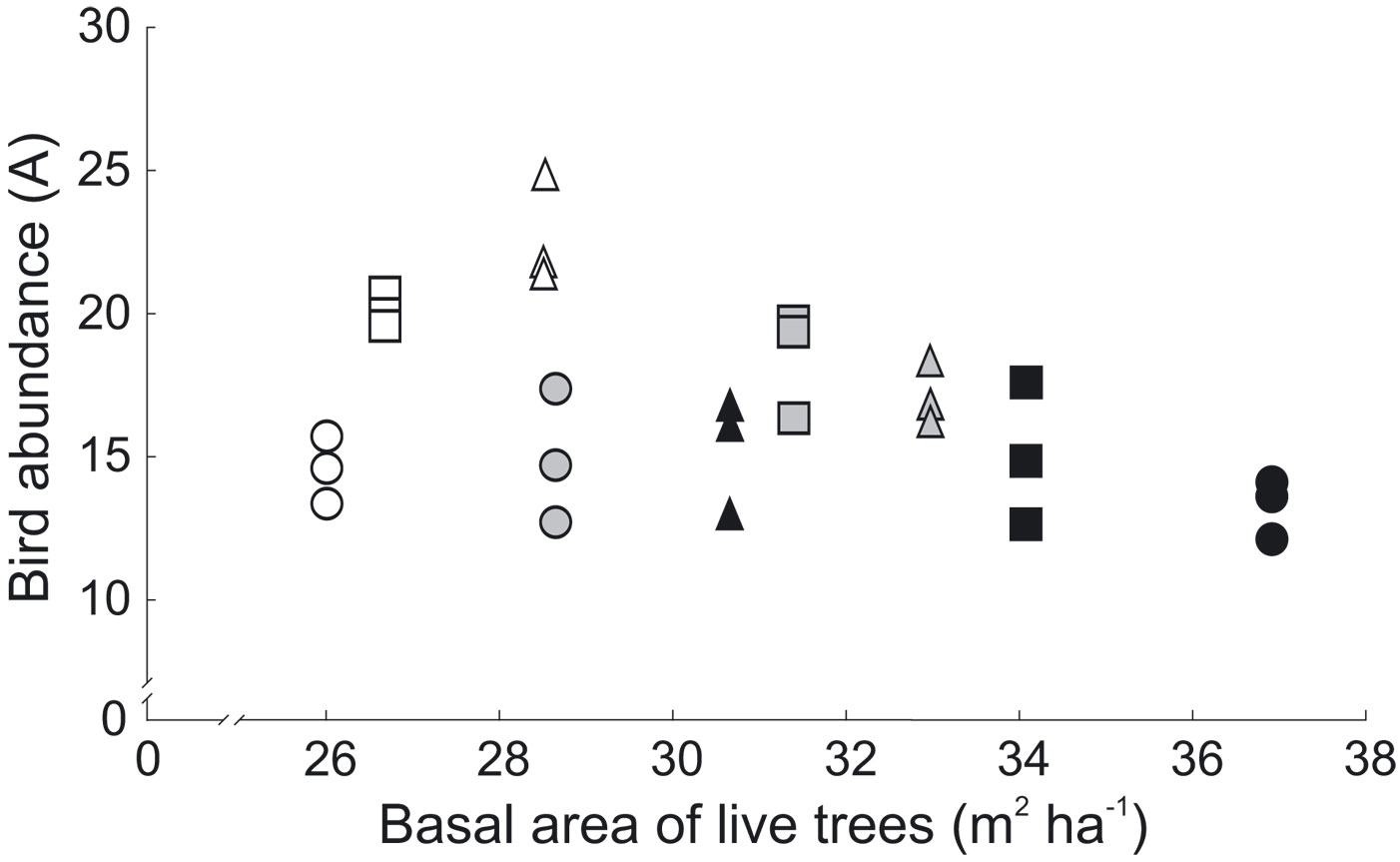
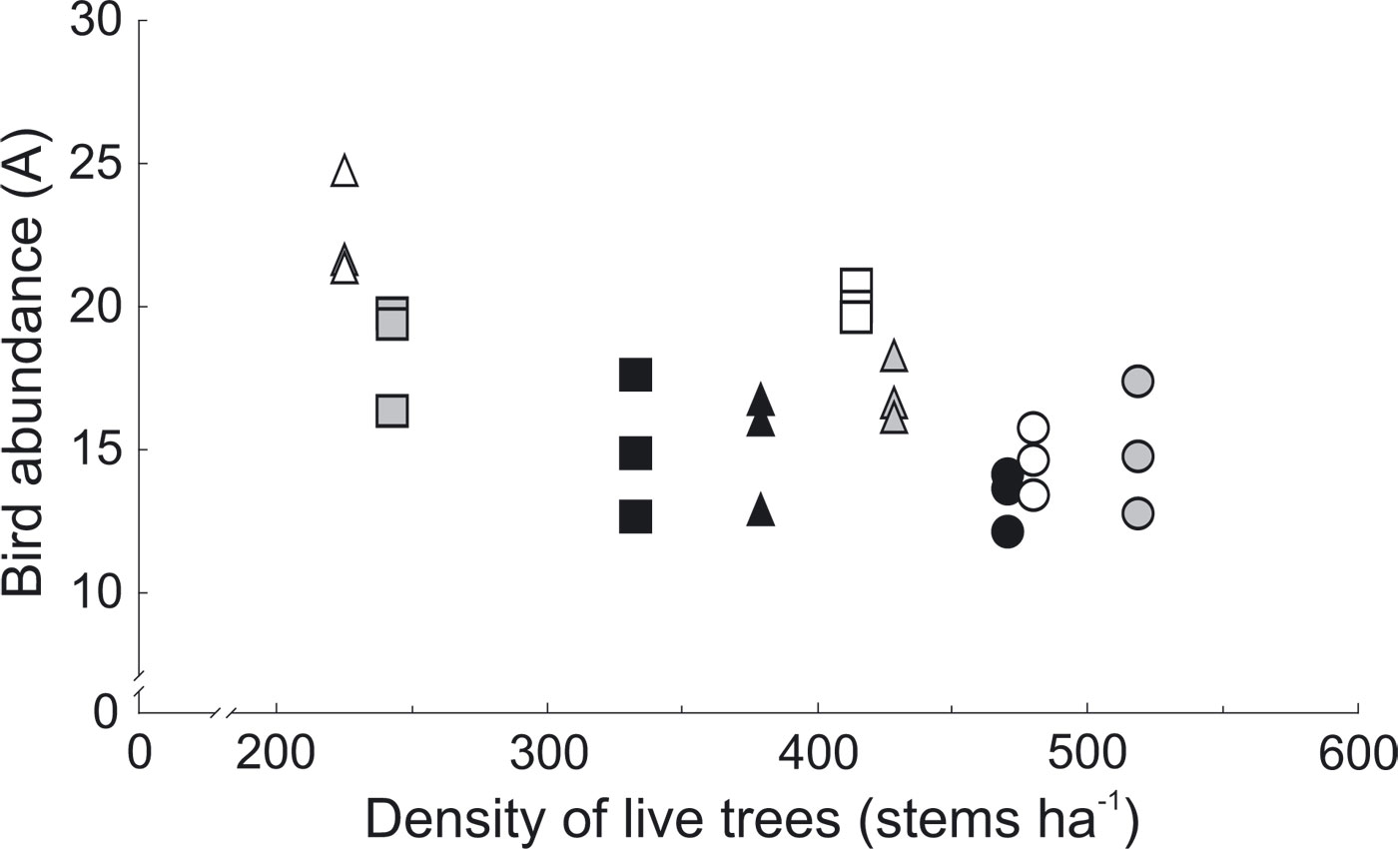
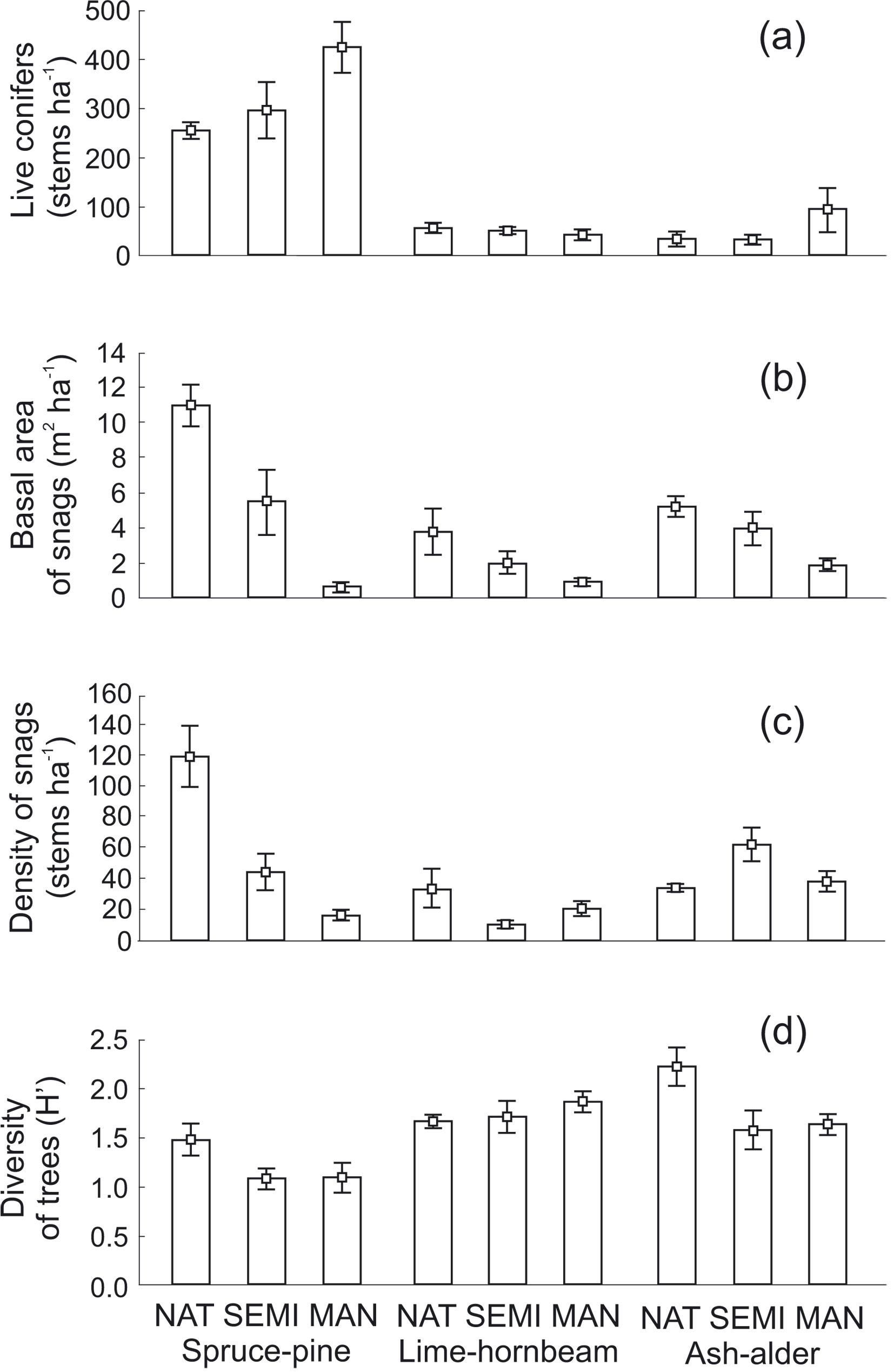
The effect of vegetation structure was estimated after adjusting for the effect of main factors (forest type and management intensity). Among them, only total basal area of live trees and tree density significantly affected bird abundance. Basal area of live trees had positive effect on abundance of birds (LR = 9.87, df = 1, p = 0.002 - Fig. 1). The density of live trees negatively affected bird abundance (LR = 17.01, df = 1, p < 0.001 - Fig. 2). The density of live trees also negatively affected species richness (LR = 9.52, df = 1, p = 0.002), but not species diversity (LR = -2.03, df = 1, p = 0.15). Tree density effects may have been underlying the observed differences in tree species composition and management intensity, as the density of conifers was significantly higher in MAN stands and SEMI stands in comparison with NAT stands (Tukey’s HSD, p < 0.001, for both comparisons - Fig. 3a, Tab. 5).
Fig. 1 - Relationship between bird abundance and basal area of live trees in 2010-2012. Different shapes represent different intensity of forest management: circles - MAN (intensively managed stands); squares - SEMI (semi-natural areas); triangles - NAT (natural, near-primeval stands of the Bialowieza National Park). Colors represent forest stand types: black - SP (spruce-pine); grey - LH (lime-hornbeam); white - AA (ash-alder).
Fig. 2 - Relationship between bird abundance and density of live trees in 2010-2012. Different shapes represent different intensity of forest management: circles - MAN (intensively managed stands); squares - SEMI (semi-natural areas); triangles - NAT (natural, near-primeval stands of the Bialowieza National Park). Colors represent forest stand types: black - SP (spruce-pine); grey - LH (lime-hornbeam); white - AA (ash-alder).
Fig. 3 - Structural parameters of the three forest stands across three levels of forest management. (a): Density of coniferous trees; (b) basal area of dead trees; (c): density of standing dead trees; (d) diversity of live trees. Forest types: (SP) spruce-pine; (LH) lime-hornbeam; (AA) ash-alder. Management intensities: (NAT) natural; (SEMI) semi-natural; (MAN): managed.
Tab. 5 - Summary of statistics (z-values of Tukey’s HSD post-hoc test) for the effect of management regime and forest type on tree species composition. (AA): ash-alder stands; (LH): lime-hornbeam stands; (SP): spruce-pine stands; (NAT): natural, near-primeval areas of the Bialowieza National Park; (SEMI): semi-natural areas in nature reserves; (MAN): intensively managed areas; (′): p<0.10; (*) p<0.05; (**): p<0.01; (***): p<0.001.
| Effects | Stands | Live trees density |
Live trees basal area |
Conifers density |
Dead trees density |
Dead trees basal area |
Live trees diversity |
|---|---|---|---|---|---|---|---|
| Management | NAT-SEMI | -9.23*** | -8.82*** | -6.55*** | 0.34 | 9.54*** | -0.27 |
| NAT-MAN | -12.08*** | -6.70*** | -6.44*** | 2.81* | 12.34*** | -0.56 | |
| SEMI-MAN | -3.00** | 1.93 | -0.15 | 2.40* | 2.52* | -0.28 | |
| Forest type | AA-LH | -0.07 | -3.09** | 0.01 | 5.93*** | 4.87*** | 2.26′ |
| AA-SP | 2.13′ | -2.14′ | -14.28*** | 3.79*** | 1.52 | 12.26*** | |
| LH-SP | 2.20′ | 0.91 | -14.29*** | -2.40* | -3.69*** | 9.89*** |
Basal area of standing dead trees differed significantly among all the three levels of management intensity, being higher in NAT and SEMI stands than in MAN stands (Tukey’s HSD, p < 0.001 and p = 0.03, respectively), and also was significantly higher in NAT stands than in SEMI stands (p < 0.001 - Fig. 3b, Tab. 5). The density of dead trees was lower in MAN stands than in NAT stands (p = 0.01) and SEMI stands (p = 0.04), but there was no significant difference between NAT and SEMI stands (p = 0.94 - Fig. 3c, Tab. 5).
Regarding forest type, density of conifers was significantly higher in SP forest than in LH and AA forests (Tukey’s HSD, p < 0.001 for both comparisons - Tab. 5). In addition, species diversity of trees was lower in SP forest when compared with the other two forest types (p < 0.001 for both comparisons with LH and AA forests, respectively - Fig. 3d, Tab. 5). Basal area of standing dead trees was higher in SP forest than in LH forest (p < 0.001) and AA forest than LH forests (p < 0.001), but not in AA forest in comparison with SP forests (p = 0.28 - Fig. 3b). The density of dead trees was also significantly higher in SP and AA forests than in LH forest (p = 0.04 and p < 0.001, respectively), but was also higher in AA than in SP forest (p < 0.001 - Fig. 3c, Tab. 5).
Discussion
Forest management affected stand characteristics that are important to birds. The density of trees, their total basal area and the tree species diversity each influenced one or more bird community indexes. The density of dead trees was significantly higher in NAT in comparison with SEMI and MAN stands. The loss of dead wood was the most visible in SP-MAN plots in comparison to SP-NAT stands (Fig. 3b, Fig. 3c). The highest number of dead trees (mostly spruces) were found in SP-NAT stands because of the mortality associated with the climate warming that has been ongoing at BNP since many years ([22], [1], [47]). Outside BNP forest managers try to minimize the effects of these changes by removing all infected and dying trees. Although the density of dead trees did not directly influence bird community indexes, it is known that some bird species are strongly dependent on dead wood (e.g., White-backed Woodpecker Dendrocopos leucotos, Three-toed Woodpecker Picoides tridactylus). In addition, the presence of snags shapes the forest structure, as dying trees create canopy gaps where natural regeneration takes place. Dead wood in various stages of decay, including single dead trees of pioneer species (e.g., birch, Betula spp. and aspen, Populus tremula), are important nesting spots for many bird species including woodpeckers. The root disk of fallen trees also provides nesting sites for many bird species ([43]). Consequently, bird abundance and species richness within treefall gaps is higher than in close-canopy forest ([14]). Such gaps are fairly common in BNP (NAT plots in contrast to MAN plots), where older trees are regularly cut by foresters and replaced mainly by pines and oaks, often planted in fenced gaps. Such planting is the reason underlying the highest density of coniferous trees occurring in the SP-MAN plots.
Bird community indexes are also affected by the forest structure. The richness, abundance and diversity of bird communities was the highest in natural or in semi-natural stands and the lowest in managed stands. In semi-natural forests where management impacts are very moderate, bird indexes did not differ significantly from those found in the BNP (NAT plots). This indicates that silvicultural practices (logging, planting trees, etc.) led to the decline of some specialized bird species ([52]), but some common species, e.g., Chaffinch Fringilla coelebs in coniferous managed stands, seem to have taken advantage of forest management (see Tab. S1-S3 in Appendix 1).
Analyses of nesting guilds revealed that forest management negatively influenced the abundance of cavity-nesters. Significantly lower tree cavity density occurred in managed stands compared to natural stands of the BNP (unpublished data, [50]). The high total basal area of deciduous trees in NAT plots suggests a much higher proportion of large, old trees, where typically more cavities may be found. The Collared Flycatcher Ficedula albicollis, the most common cavity-nester in BNP, chooses nesting cavities in trees with an average DBH of 43 cm ([48]). Consequently, the conservation of large deciduous trees and snags is important for maintaining the current and future resources for cavity-nesters ([21]). Tree cavities are at risk in harvested forests, where large, old trees are eliminated by logging, and it takes more than a century to be replaced ([11]).
Analysis of foraging guilds showed that forest management mostly affected insectivorous bird species, whereas the abundance of the other foraging groups did not differ significantly between natural and semi-natural forests. Most of the breeding bird species in each study plot were insectivorous, and insects and other invertebrates such as snails can affect breeding density of birds ([32]). Typically caterpillar biomass is much higher in BNP compared with other temperate deciduous forests ([33]), and the density of winter moth Operophtera brumata (the most important food for birds during breeding - [4]) was much higher in primeval forest than in intensively managed stands ([58]). Older trees also create a more diverse forest structure in comparison to younger ones (more dead and dying parts, more cracks and irregularities, etc.), leading to a higher invertebrate species richness in the unmanaged BNP with many old trees. Forest management, therefore, can also negatively affect various groups of invertebrates ([18], [35], [38]).
The highest values of the avian community indexes (richness, abundance, diversity) were found in AA-NAT and the lowest in SP-MAN managed spruce-pine forest. Bird communities in AA stands usually show the highest species richness and coniferous stands the lowest ([43], [57]). However, this difference was not apparent in intensively managed stands, where the avian indexes were the highest in lime-hornbeam (LH) and the lowest in spruce-pine (SP) stands. This suggests that ash-alder stands in the Bialowieza forest were more affected by forest management than lime-hornbeam stands, and the primary effect of management was to reduce the basal area of live trees, that was the lowest in AA-MAN compared with other investigated plots (Fig. 2d). The basal area of live trees was significantly associated with bird densities. AA stands are found in swampy areas and typically support a low density of large trees. Logging even a small number of these large trees may result in a remarkable change in basal area and forest structure compared with other forest types (Tab. 4).
Since early 1990s the ash-alder stands were seriously affected by ash dieback disease (Chalara fraxinea) in the Bialowieza Forest, and many ash trees are currently dead or dying ([28]). It is likely that the proportion of such species will be significantly reduced in the near future, also affecting bird communities. Ash is one of the most important cavity tree for cavity-nesters in ash-alder stands ([54], [55]). On the other hand, some bird species may benefit from increased resources of dead wood, at least where snags are not removed as in managed stands.
The basal area of live trees had a significant positive effect on the total abundance of birds in the stand, but the effect of live tree density was negative, i.e., both the species richness and diversity of birds were lower where the density of live trees was high. This suggests that intensive management practices, such as rotational cutting, planting and also partially natural regeneration, may lower habitat quality for many bird species. It is obvious that younger trees offer less diverse nest sites (e.g., cavities) and foraging possibilities (lack of rough bark, dead branches, big crowns). Rare woodpeckers prefer foraging on large diameter trees, with dead parts or trees with rough, richly-tectured bark. Results of many studies support the importance of mature large trees for sustaining the richness of bird communities ([25], [12], [31], [15]), and bird species richness has been reported to decline with intensive forest management ([10], [44]).
It is worth stressing that since the 1920s through the 1980s forest managers often clear-cut stands (mostly coniferous stands) in the Bialowieza forest, though such practice is no longer allowed. In the 1990s, several 2-3 ha clearings were created in the Bialowieza Forest in order to sustain some bird species that were scarce or absent in old-growth stands ([13]).
In summary, our results show that some stand types (e.g., ash-alder) are more sensitive to forest management than others, and the bird community of these ecosystems may be deeply affected by the currently intensive management practices. However, almost all bird community indexes in SEMI stands were similar to those obtained in NAT forest, indicating that silvicultural practices targeted at sustaining forest health have a limited effect on local bird communities.
Conservation implications
Although we found significant differences among the stand types and levels of management intensity in the amount of dead wood, no direct effect of these differences on the avian communities of these stands were observed. However, it is well known that some endangered species of birds are susceptible to the removal of dead trees ([56], [5], [31]) and study from forests in Estonia showed that the abundance of coarse woody debris was the main factor affecting the bird community ([32]).
Forestry practices in the Bialowieza Forest have caused noticeable changes in tree species composition and decreased snag density. Our results emphasize the importance of mature forests for multiple bird species, highlighting how intensive forest management prcatices (e.g., removal of dead wood, tree planting) may reduce the forest conservation value. Nonetheless, the Bialowieza forest still show a high bird diversity and abundance, and is currently considered one of the main biodiversity hotspot in Europe (e.g., all but one European woodpeckers breed there). However, a significant portion of old-growth stands has not been currently excluded from management activities in the 2012-2021 plans.
Our results suggest the lack of intensive management activities (no cutting, semi-commercial thinning or planting trees) as an effective strategy to maintain the natural forest biodiversity. In most Polish national parks, naturalization practices often result in the removal of pioneer tree species (aspen and birch) that are essential for the foraging and breeding of different bird species, e.g., White-backed Woodpecker. Removal of these pioneer species leads to a decline in biodiversity of protected areas ([8]). Although long-lasting directional changes in spruce-pine and lime-hornbeam stands are inevitable due to natural and anthropogenic climate changes, we did find no basis for either inhibiting or accelerating such changes by silvicultural practices in any of the three different forest types analyzed.
Acknowledgements
This research was funded by the National Center of Science in Poland (National Science Center Grant NN304 218437). We heartily thank to R. J. Fuller, N. L. Rodenhouse and one anonymous reviewer for valuable comments on an earlier version of the manuscript.
References
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Tomasz Stanski
Anna Kapusta
Wieslaw Walankiewicz
Department of Zoology, Siedlce University of Natural Sciences and Humanities, Prusa 12, 08-110 Siedlce (Poland)
Mammal Research Institute, Polish Academy of Sciences, 17-230 Bialowieza (Poland)
ul. Lecha 7a/91, 05-400 Otwock (Poland)
Corresponding author
Paper Info
Citation
Czeszczewik D, Zub K, Stanski T, Sahel M, Kapusta A, Walankiewicz W (2015). Effects of forest management on bird assemblages in the Bialowieza Forest, Poland. iForest 8: 377-385. - doi: 10.3832/ifor1212-007
Academic Editor
Massimo Faccoli
Paper history
Received: Dec 23, 2013
Accepted: Jul 24, 2014
First online: Oct 02, 2014
Publication Date: Jun 01, 2015
Publication Time: 2.33 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 56769
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 45798
Abstract Page Views: 4650
PDF Downloads: 4906
Citation/Reference Downloads: 25
XML Downloads: 1390
Web Metrics
Days since publication: 4162
Overall contacts: 56769
Avg. contacts per week: 95.48
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 42
Average cites per year: 3.82
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Bird response to forest structure and composition and implications for sustainable mountain forest management
vol. 19, pp. 18-27 (online: 11 January 2026)
Research Articles
Bird composition and diversity in oak stands under variable coppice management in Northwestern Turkey
vol. 11, pp. 58-63 (online: 25 January 2018)
Research Articles
Impact of management practices on habitat use by birds in exotic tree plantations in northeastern Argentina
vol. 19, pp. 38-44 (online: 06 February 2026)
Research Articles
Effects of low-impact logging on understory birds in the Brazilian Amazon
vol. 14, pp. 122-126 (online: 08 March 2021)
Review Papers
Prospects for evolution in European tree breeding
vol. 17, pp. 45-58 (online: 06 March 2024)
Research Articles
Variability of ant community composition in cork oak woodlands across the Mediterranean region: implications for forest management
vol. 10, pp. 707-714 (online: 27 July 2017)
Research Articles
Saproxylic beetles in non-intervention and coppice-with-standards restoration management in Meerdaal forest (Belgium): an exploratory analysis
vol. 9, pp. 536-545 (online: 25 March 2016)
Research Articles
The impact of seed predation and browsing on natural sessile oak regeneration under different light conditions in an over-aged coppice stand
vol. 9, pp. 569-576 (online: 04 April 2016)
Research Articles
The effects of forest management on biodiversity in the Czech Republic: an overview of biologists’ opinions
vol. 15, pp. 187-196 (online: 19 May 2022)
Research Articles
Diversity and distribution patterns of medium to large mammals in a silvicultural landscape in south-eastern Brazil
vol. 11, pp. 802-808 (online: 14 December 2018)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword